Abstract
Purpose
The present study aims to investigate apoptosis of U937 cells induced by hematoporphyrin monomethyl ether (HMME)-mediated sonodynamic therapy (SDT).
Materials
HMME concentration was kept constant at 10 μg/mL. Tumor cells suspended in serum-free RPM1640 were exposed to ultrasound at 1.1 MHz for up to 60 seconds with an intensity of 1 W/cm2 in the presence and absence of HMME. The viability of cells was determined by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltertrazolium bromide tetrazolium (MTT) test. Apoptosis was analyzed using a flow cytometer with Annexin V-PE/7-ADD staining as well as fluorescence microscopy with 4′-6-diamidino-2-phenylindole (DAPI) staining. The DNA damage of U937 cells, intracellular reactive oxygen species (ROS), and mitochondria membrane potential (MMP) were also analyzed by a flow cytometer after exposures. Western blotting and reverse transcriptase–polymerase chain reaction were used to analyze the protein and mRNA expression level of caspase-3 and poly(ADP-ribose) polymerase (PARP).
Results
Fluorescent imaging revealed that HMME mainly localized in the mitochondria. MTT assay showed 55.6% of cell survival at 4 hours post-SDT. Flow cytometric analysis displayed a significant increase in the early- and late-apoptotic cell populations (35.6%) of U937 cells by HMME-mediated SDT. Compared with the control, ultrasound-alone, and HMME-alone groups, the intracellular ROS and the MMP loss were greatly increased in the combined SDT group. Obvious nuclear condensation was also found with DAPI staining, and the DNA fragment increased to 33.9% at 2 hours post-SDT treatment. Immunofluorescent staining indicated obvious Bax translocation after SDT. Western blot showed visible enhancement of caspase-3 and PARP cleavage. In addition, caspase-3 and PARP mRNA expression of U937 cells increased remarkably after SDT treatment.
Conclusions
The findings demonstrated that HMME-mediated sonodynamic action (HMME-SDT) significantly induced apoptosis of U937 cells, suggesting that HMME may be a good sonosensitizer, and HMME-SDT might be a potential therapeutic strategy for cancer treatment.
Key words: apoptosis, HMME, leukemia U937 cells, sonodynamic therapy
Introduction
Sonodynamic therapy (SDT) is a relatively new approach for cancer treatment based on photodynamic therapy, which involves the synergistic effect on cell killing by the combination of a drug (sonosensitizer) and ultrasound. SDT has been proven to be a potential tool for cancer therapy; it is based on preferential uptake and/or retention of the sonosensitizer in tumor tissues and subsequent activation of the sensitizer by ultrasound.1 Umemura et al. first reported that ultrasound-activating hemotoporphyrin derivatives (HpD) probably produced excessive singlet oxygen (1O2) accumulation, which can destroy the structure of tumor cells and eventually cause cell death.2
Recent studies have shown that low-intensity ultrasound could efficiently activate some sensitizers to produce cytotoxic reactive oxygen species (ROS), directly or indirectly damaging tumor cells.3,4 Lagueaux et al. found that ultrasound exposure can induce apoptotic response in human leukemia cells such as HL-60, Kgla, and Nalm-60. They proposed that the ultrasound-induced sonochemical reaction decreased the intracellular glutathione level, suggesting that oxidative stress may play a role in ultrasound-induced apoptosis. Further, they presumed that the ultrasonic treatment could be a promising tool for the ex vivo elimination of leukemic cells by means of apoptosis.5 Firestein et al. reported that apoptosis induced by ultrasound in human malignant lymphoid cells was time related, depending on the mitochondrion–caspase pathway activation.6
Sonosensitizer is a key component affecting the efficacy of SDT. Hematoporphyrin monomethyl ether (HMME) is a second-generation, porphyrin-related photosensitizer that has been developed recently.7 HMME is a synthesized mixture of the two positional isomers of 3-(1-methyloxyethyl)-8-(1-hydroxyethyl) deuteroporphyrin IX and 8-(1-methyloxyethyl)-3-(1-hydroxyethyl) deuteroporphyrin IX. Experimental studies and clinical trials have shown that HMME has higher selective uptake by tumor tissue, stronger photodynamic effect, lower toxicity, and shorter-term skin photosensitizations than HpD (the first generation of photosensitizer), and is a promising sensitizer for PDT.8–10 HMME is also utilized as a helpful diagnostic tool for detection of neoplastic tissue based on the target delivery character.11 Recently, HMME has been primarily studied in SDT research. Hua Jin et al. showed that upon SDT, different concentrations of HMME caused distinct types of CNE-2 cell death: apoptosis was induced by a low concentration of HMME, while necrosis was triggered by a higher concentration of HMME. Their findings also indicated that the membrane damage and the cytoskeleton disruption may be key factors for HMME-SDT-induced cell apoptosis, and the disturbance of mitochondrial transmembrane potential and calcium channels played important roles in the process.12 Jianhua Li et al. reported that HMME-mediated SDT could kill C6 glioma cells in vitro and possibility through induction of apoptosis and necrosis, and 1O2 may play an important role in the action.13 Due to the unclear mechanism of SDT and the advantages of HMME as a sensitizer in PDT or SDT, this study was to investigate the sonodynamic effect of HMME in human leukemia U937 cells; the obtained findings may provide further detailed information about HMME as an effective sonosensitizer in SDT-mediated cancer therapy.
Materials and Methods
Chemicals
HMME was purchased from Red-Green Photosensitizer Company, with a purity of 98% (HPLC). HMME was dissolved in dimethyl sulfoxide (DMSO) at 5 mg/mL, and stored in the dark at −20°C. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltertrazolium bromide tetrazolium (MTT), rhodamine-123 (Rh123), 4′-6-diamidino-2-phenylindole (DAPI), and propidium iodide (PI) were purchased from the Sigma Chemical Company. Mito Tracker Green (MTG) and 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) were supplied by Molecular Probes, Inc. (Invitrogen). Guava Nexin Assay kit (4500-0450) was obtained from Millipore Company (Guava Technologies, Inc.).
Cell culture
The human acute myeloid leukemia cell line U937 was obtained from the Institute of Chinese Academy of Medical Sciences. The cell line was cultured in an RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 mM l-glutamine. The cells were passaged 1–2 days. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere. Cells in the exponential phase of growth were used in each experiment (cell viability was above 98% using trypan blue exclusion test).
SDT treatment protocols
U937 cells in the exponential phase were collected and divided randomly into four groups: (1) control, (2) HMME alone, (3) ultrasound alone, and (4) ultrasound plus HMME. For the HMME and ultrasound plus HMME groups, the cells incubated with 10 μg/mL HMME involved a 3-hour drug-loading time in the serum-free RPMI 1640 medium, allowing sufficient time for cell uptake of the sensitizer to reach a maximum level. Instead of HMME, an equivalent quantity of DMSO was used for the control and ultrasound-alone groups. The cells in the ultrasound and ultrasound plus HMME groups were exposed to ultrasound at a frequency of 1.1 MHz and an intensity of 1 W/cm2 for 60-second duration. After the treatment procedure, cells were resuspended in a fresh medium and cultured for an additional time as specified in the text and then subjected to different analyses.
Ultrasound exposure setup
The experiment setup for insonation was similar as previously described.14 Briefly, the focused ultrasound transducer with a circular ceramic plate of 15 mm in diameter, manufactured by the Institution of Applied Acoustics, Shaanxi Normal University (Xi'an, China), was submerged in degassed water in the tank facing directly upward. The electrical signal was generated and amplified by a multifunctional generator (AG1020; T&C Power Conversion, Inc.) before feeding the transducer. The ultrasound field distribution was recorded by the Ultrasonics C-Scan image System (3560C; Physical Acoustics Corporation). The central focal spot was shown at about 26 mm away from the piezoelectric sound source. The produced focus area was about 6–8 mm in width, and the depth of focus was about 1.6 cm. Ultrasound irradiation was conducted with a frequency of 1.1 MHz in a continuous wave mode for 60-second duration. About 0.5 mL sample contained in a polypropylene test tube was placed into the center of the focal zone for irradiation. Samples were submerged entirely in degassed water, and the test tube was rotated at 20 rpm by a micromotor to improve mixing and to provide a uniform exposure. In a 1640 serum-free medium, suppose in the free field conditions, we previously evaluated that the acoustic intensities in the tube were about 1 W/cm2 (ISATA, spatial average time average intensity) when the load power by the AG1020 apparatus indicated 3 W, which was used throughout the experiment.
For all experiments, the coupling water was degassed before ultrasound treatment and was maintained at the room temperature during irradiation. Temperature increase inside the test tube was measured before and after ultrasound treatment with a digital thermometer, and no significant variation of temperature was detected (±1°C).
Cell uptake of HMME detection by flow cytometry
U937 cells were harvested in an exponential growth phase, washed once with a serum-free medium, and incubated with various concentrations of HMME at 37°C in a six-well microplate for different time intervals. The serum-free medium was used to avoid HMME interacting with serum and excreting from the cells.
For determining the HMME content, cells were collected after different incubation time points and immediately detected with flow cytometry. The mean fluorescence intensity of HMME was recorded at the same measurement conditions.
Subcellular localization of HMME
After 3 hours of incubation with HMME (10 μg/mL), cells were costained with 2.5 nM MTG, a well-established fluorescent probe for mitochondria. After being washed twice with cold PBS (PBS, in g/L: NaCl 8.0 g, KCl 0.2 g, Na2HPO4 1.44 g, and KH2PO4 0.24 g, pH 7.2), the cells were observed for the subcellular localization patterns of HMME under a laser-scanning confocal microscope (LSCM; Model TCS SP5). Fluorescence images were taken at the emission wavelength of 396 nm for HMME and 490 nm for MTG, respectively.
Cell survival assay
MTT assay relies primarily on the mitochondrial metabolic capacity of viable cells and reflects the intracellular redox state. The MTT assay was used to monitor the cytotoxicity of HMME-mediated SDT on U937 cells. At 4 hours after ultrasound exposure, cells in 100 μL were added to 96-well culture plates, and the viability was determined by adding 10 μL MTT solution (5 mg/mL in PBS) to each well, and the mixture was incubated for 4 hours at 37°C in a CO2 incubator. The formazan crystals were dissolved in 100 μL of 10% sodium dodecyl sulfate (SDS) and 0.01 M HCl solution, and the absorbance at 570 nm was recorded using a microplate reader (BIO-TEKELX800) against the reference value at 690 nm. The cell survival of treated samples was then obtained by comparing the results of the incubated, but nonexposed, control sample.
Apoptosis detection by flow cytometry
In the present study, after 4 hours of incubation post-SDT, cell apoptosis was analyzed using a flow cytometer with Annexin V-PE and 7-AAD staining. Annexin V-PE can recognize the externalization of phosphatidylserine on the external of apoptotic cells, which usually indicates both early and later apoptosis; the cell-impermeant dye, 7-AAD, is also used as an indicator of cell membrane integrity. Double-negative staining cells were considered as viable. Briefly, 100 μL cells from each sample was suspended in a mixture of 100 μL Annexin V-PE and 7-ADD-binding buffer and then incubated at room temperature for 20 minutes. The samples were analyzed using a flow cytometer (Guava easyCyte 8HT; Millipore). The population was separated into three groups: live cells with a low level of fluorescence; apoptotic cells in the earlier period with green fluorescence; and the advanced-stage apoptotic cells with both red and green fluorescence.
DAPI staining
DAPI is a fluorescence probe that binds to natural double-stranded DNAs and represents the nuclear morphology. U937 cells were incubated for 24 hours in a six-well microplate after the treatment of ultrasound and HMME (10 μg/mL). Cells were then stained with 4 μg/mL DAPI for 30 minutes at 37°C. The stained cells were washed three times in PBS and observed using a fluorescence microscopy (excitation wavelength was 364 nm, and emission wavelength was 454 nm).
Analysis of DNA fragmentation by flow cytometry
Krysko et al. describe an easy and quantitative way to analyze DNA fragmentation based on flow fluorocytometric detection of DNA hypoploidy after adding PI to the dying cells and permeabilizing them by freeze–thawing. PI intercalates in the DNA, and the size of DNA fragments appears as a hypoploid DNA histogram.15 To investigate the effect of HMME-mediated SDT on DNA damage of U937 cells, we performed oligonucleosomal DNA fragmentation by flow fluorocytometry. At 2 hours post-treatment, U937 cells were stained with 5 μg/mL PI and analyzed for DNA content by using flow cytometry.
Determination of intracellular ROS and the MMP
Intracellular ROS was analyzed using a flow cytometer with DCFH-DA. DCFH-DA, a nonfluorescent cell-permeant compound, is cleaved by endogenous esterases within the cell, and the de-esterified product can be converted into the fluorescent compound DCF upon oxidation by intracellular ROS. To estimate intracellular ROS, immediately after treatment, both the control and treated cells were loaded with 100 nM DCFH-DA for 10 minutes at 37°C, washed with PBS, and immediately analyzed using flow cytometry.
Fluorescence probe Rh123 was used to evaluate perturbations in the mitochondrial membrane potential (MMP). Rh123 selectively enters mitochondria with an intact membrane potential and is retained in the mitochondria. Once the MMP is lost, Rh123 is subsequently washed out of the cells. At 0.5 hours post-treatment, cells were stained with 2 μg/mL Rh123 in an incubator for 20 minutes with gentle shaking, followed by washing with PBS; after that, the samples were immediately analyzed using flow cytometry.
Immunocytochemistry
To further provide the evidence of SDT-induced apoptosis in U937 cells, we examined the ability of SDT to induce the translocation of Bax from the cytosol onto the mitochondria.
The control and SDT-treated cells were harvested and then fixed with 3.5% paraformaldehyde for immunofluorescence assays. Cells preincubated with 100 nM MTG were stained for the detection of colocalization of damaged mitochondria and Bax (a rabbit polyclonal antibody, 1:400; Santa Cruz). The corresponding anti-rabbit specific secondary antibodies were performed by immunoglobulin TRITC conjugates (ZSGB-BIO). Cells were imaged with an LSCM.
Western blot staining
SDS–polyacrylamide gel electrophoresis and immunoblotting were performed according to the standard procedures. Briefly, cells were lysed by the RIPA buffer on ice. The protein samples were separated on a 10% SDS polyacrylamide gel, and then the gel was transferred to nitrocellulose membranes (Millipore) and blotted with primary antibodies (caspase-3 and Cleaved-RARP were from Cell Signaling Technology) overnight at 4°C. The bound primary antibodies were then tagged with IRDye-68-Conjugated IgG (Li-cor Biosciences) at room temperature for 1 hour. Moreover, the infrared fluorescence was detected with the Odyssey infrared imaging system (Li-Cor Bioscience).
RNA isolation and reverse transcriptase–polymerase chain reaction
At the indicated time points, total RNA was isolated with the Biozol total RNA Kit (Bioer technology) and spectrophotometrically quantified, and the reverse transcriptase–polymerase chain reaction (RT-PCR) was performed with the Bio RT-PCR Kit (Bioer technology) according to manufacturer's instructions. The reactions were heated at 94°C for 5 minutes and then immediately cycled 30–35 times through a 30-second denaturating step at 94°C, a 30-second annealing step at 55°C, and a 30-second extension step at 72°C. After the cycling procedure, a final 5-minute elongation step at 72°C was performed. All amplifications were done in the linear range of the assay. The reaction products were separated on a 1.0% agarose gel, and imaged by a Gel Imaging Analysis system (Beijing Junyi-Dongfang Electrophoresis Instrument Co., Ltd.). The following primers were used: GAPDH (F) 5′-CGCTCTCTGCTCCTCCTGTT-3′ and (R) 5′-CCA TGGTG T- CTGAGCGATGT-3′; caspase-3, (F) 5′-CAAATGGACCT GTTGACCTGA-3′ and (R) 5′-ATGGCACAAAGCGACTGG ATG-3′; PARP, (F) 5′-AGGGCAAGCAC AGTGTCAAA-3′ and (R) 5′-TACCCATCAGCAACTT- A GCG-3′. Reagents were routinely checked for contamination. Results were confirmed by at least four replicate experiments.
Statistical analysis
All values were expressed as means±SD of at least four independent experiments, and the differences among the groups were analyzed of variance (one-way ANOVA); p<0.01 and p<0.05 were considered to be significant.
Results
Uptakes and intracellular localization of HMME in U937 cells
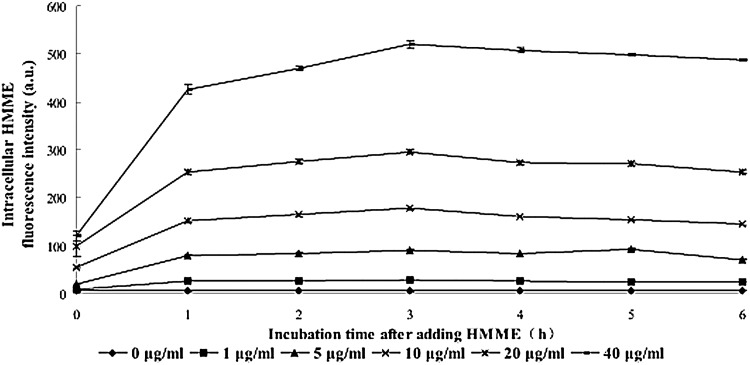
The intracellular concentration changes of HMME at different time points after addition to U937 cells were evaluated by the mean fluorescence intensity as determined by flow cytometry. As shown in Figure 1, immediately after administration, the intracellular HMME increased quickly for the first 1 hour, then slightly increased and peaked at 3 hours, followed by a slight decrease and almost sustained the same level at 4–6 hours after adding of HMME. The intracellular HMME accumulation curves at different concentrations showed similar trends.
FIG. 1.
Kinetics of the intracellular HMME level in U937 cells after incubation with different concentrations of HMME. Data are presented as mean±SD from four independent experiments. HMME, hematoporphyrin monomethyl ether.
To assess the subcellular localization pattern of HMME in U937 cells, we coloaded cells with the mitochondrial-specific dye MTG after a 3-hour incubation with HMME. The fluorescence distributions of HMME and MTG were captured using laser-scanning confocal microscopy. In dual-channel imaging, photomultiplier sensitivities and offsets were set to a level at which bleed-through effects from one channel to another were negligible. The subcellular localization pattern of HMME in U937 cells is shown in Figure 2. The red fluorescence pattern of HMME corresponded well with that of MTG green fluorescence, indicating that HMME mainly localized to the mitochondria of U937 cells.
FIG. 2.
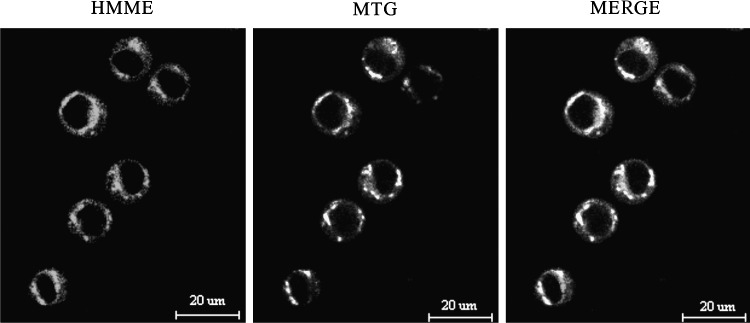
Intracellular localization of HMME in U937 cells. After 3 hours of incubation, HMME displayed a red fluorescence pattern that corresponded well with that of the mitochondrial probe MTG (mitochondrion special dye).
Cytotoxicity of HMME-SDT on U937 cells
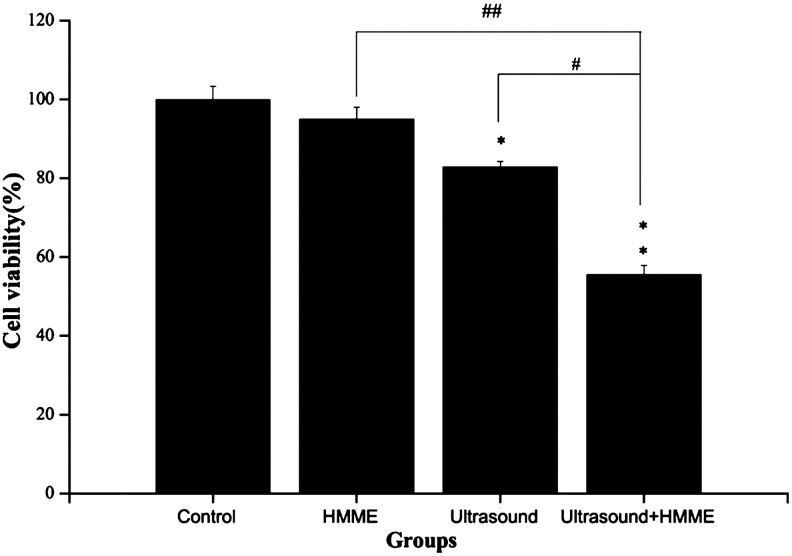
The treated cells were incubated for 4 hours to assess the cytotoxicity of HMME-mediated SDT on U937 cells. The data in Figure 3 revealed that, compared with control, there was not any cell toxicity in the HMME-alone group (95.1% cell survival), and ultrasound alone caused a slight cell damage on U937 cells (82.99% cell survival, p<0.05), while the synergistic effect of ultrasound plus HMME showed significant cell killing, which caused 45.4% cell viability loss (p<0.01). This combined cytotoxicity of HMME-SDT was much higher than that of HMME (p<0.01) or ultrasound alone (p<0.05).
FIG. 3.
The viability of U937 cells at 4 hours after HMME-SDT was assessed by MTT assay. Control, cells without any treatment; HMME, cells were treated with 10 μg/mL HMME alone; ultrasound, cells were irradiated with 1 W/cm2 ultrasound alone; Ultrasound+HMME, cells were irradiated with 1 W/cm2 ultrasound in the presence of 10 μg/ml HMME. *p<0.05, **p<0.01 versus untreated controls. ##p<0.01, versus HMME. #p<0.05, versus ultrasound.
Apoptosis induction in U937 cells after SDT
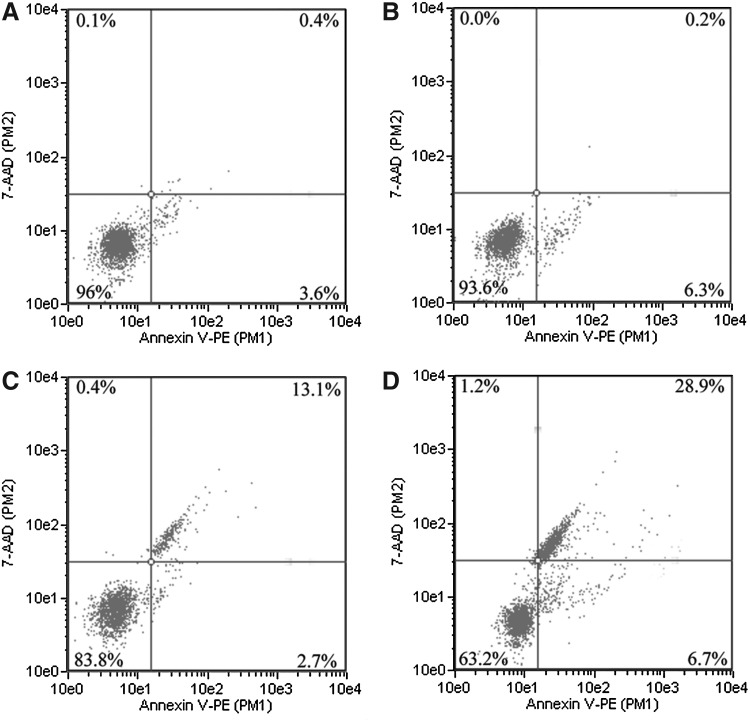
Flow cytometry with Annexin V-PE and 7-ADD staining was applied to estimate cell apoptosis at 4 hours after SDT treatment. As shown in Figure 4, the number of viable cells in the control and HMME groups was 96% and 93.6%, respectively (Fig. 4A, B). Ultrasound alone reduced the viable cells to 83.8% (Fig. 4C), whereas SDT treatment further decreased the viable cells to 63.2% (Fig. 4D). Moreover, compared with control, the number of apoptotic cells in the ultrasound-alone and SDT groups was 15.8% and 35.6%, respectively (Fig. 4C, D). These obtained data suggested that compared with control, HMME, and ultrasound alone, HMME-SDT could markedly enhance cell apoptosis in U937 cells.
FIG. 4.
Detection of apoptosis in SDT-treated U937 cells using flow cytometry. The cells were double stained with Annexin V-PE and 7-AAD as described in the Methods section after 4 hours of incubation after SDT treatment. (A) Control group with untreated cells; (B) cells were treated with 10 μg/mL HMME alone; (C) cells were irradiated with 1 W/cm2 ultrasound alone; (D) cells were irradiated with 1 W/cm2 ultrasound in the presence of 10 μg/mL HMME. SDT, mediated sonodynamic therapy.
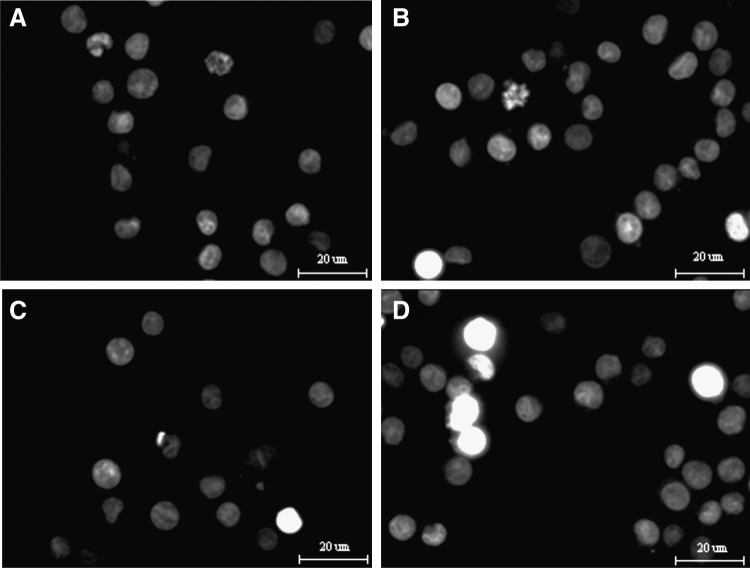
To further verify that the HMME-mediated SDT indeed induced apoptosis of U937 cells, cells were stained with DAPI at 24 hours post-treatment at an ultrasonic intensity of 1 W/cm2 in the presence and absence of 10 μg/mL HMME. As shown in Figure 5, control cells displayed a weak fluorescence and had no visible nuclear changes. Cells in the HMME-alone and ultrasound-alone groups showed slight enhancing DAPI staining, and few cells indicated damaged nuclei. While in the HMME plus ultrasound group, typical apoptosis characteristics such as nuclear condensation with more enhancing DAPI staining and altered nuclear morphology were observed.
FIG. 5.
Nuclear DAPI staining. The stained nuclei were visualized under a fluorescent microscope. (A) Control group with untreated cells; (B) cells were treated with 10 μg/mL HMME alone; (C) cells were irradiated with 1 W/cm2 ultrasound alone; (D) cells were irradiated with 1 W/cm2 ultrasound in the presence of 10 μg/mL HMME.
Analysis of DNA fragmentation by flow fluorocytometry
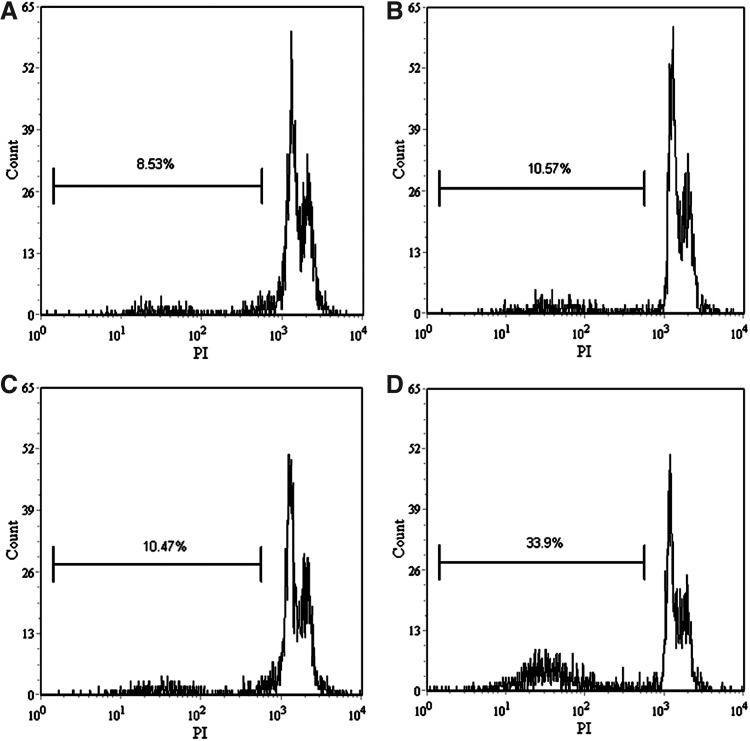
As described in the Methods section, the DNA fragment was detected by flow cytometry. The obtained results showed that after 2 hours of incubation post-treatment, the DNA fragment of control is 8.53%, while those treated by HMME alone and ultrasound alone were 10.57% and 10.47%, and the DNA fragment increased to 33.9% post-SDT treatment (Fig. 6). These results implied that HMME-mediated SDT markedly induced DNA damage of U937 cells.
FIG. 6.
DNA damage analysis by flow cytometry. (A) Control group with untreated cells; (B) cells were treated with 10 μg/mL HMME alone; (C) cells were irradiated with 1 W/cm2 ultrasound alone; (D) cells were irradiated with 1 W/cm2 ultrasound in the presence of 10 μg/mL HMME.
SDT induces mitochondrial ROS generation and the MMP loss
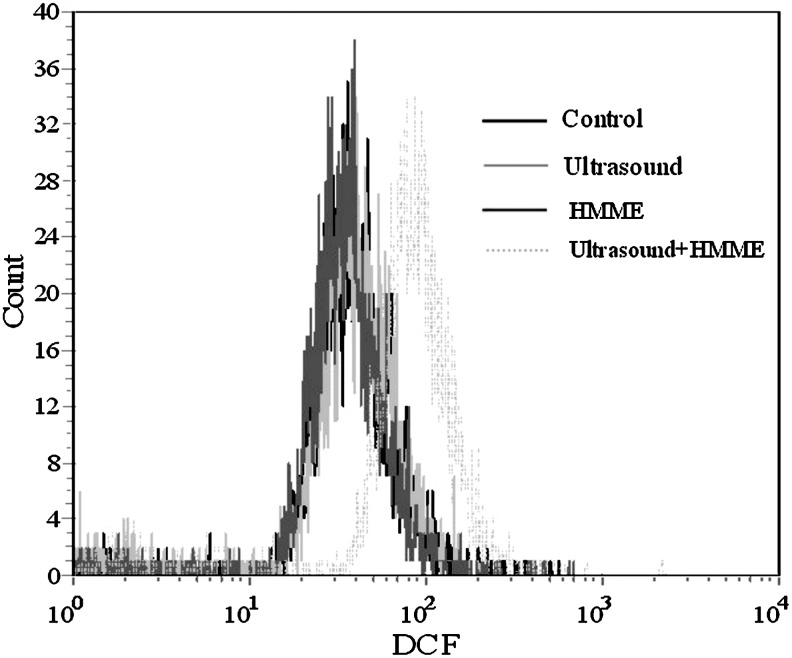
The intracellular ROS were analyzed using a flow cytometer with DCFH-DA staining immediately after a different treatment. Figure 7 shows that compared with the control, HMME, and ultrasound-alone groups, a more obvious spectral shift of DCF fluorescence curve to the right after HMME-SDT, this demonstrated that HMME-mediated SDT markedly increased the intracellular ROS level.
FIG. 7.
Measurement of intracellular ROS in U937 cells. The cells were labeled with DCFH-DA, and the fluorescence intensity of DCF in the cells was detected by flow cytometry. Control, control group with untreated cells; HMME, cells with 10 μg/mL HMME alone; ultrasound, cells were irradiated with 1W/cm2 ultrasound alone; ultrasound+HMME, cells were irradiated with 1 W/cm2 ultrasound in the presence of 10 μg/mL HMME. ROS, reactive oxygen species; DCFH-DA, 2′,7′-dichlorodihydrofluorescein-diacetate.
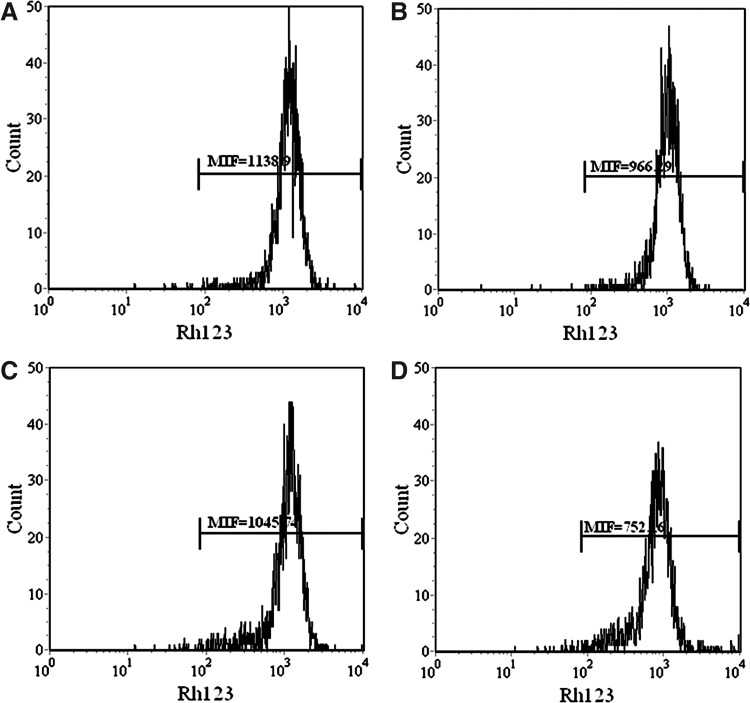
Immediately after exposure, the MMP loss as detected by flow cytometry with Rh123 staining significantly decreased in HMME-SDT-treated cells (Fig. 8). The mean fluorescent intensity of Rh123 in SDT was calculated as 752.18, which was statistically much lower than that of HMME (966.29) and ultrasound (1045.7). The results imply that the elevated ROS and decreased MMP may both act as sensitive indicators of cell injury after irradiation.
FIG. 8.
Measurement of intracellular MMP in U937 cell. The cells were labeled with Rh123, and the fluorescence intensity in the cells was detected by flow cytometry. (A) Control group with untreated cells; (B) cells were treated with 10 μg/mL HMME alone; (C) cells were irradiated with 1 W/cm2 ultrasound alone; (D) cells were irradiated with 1 W/cm2 ultrasound in the presence of 10 μg/mL HMME. MMP, mitochondrial membrane potential.
Confocal microscope observation
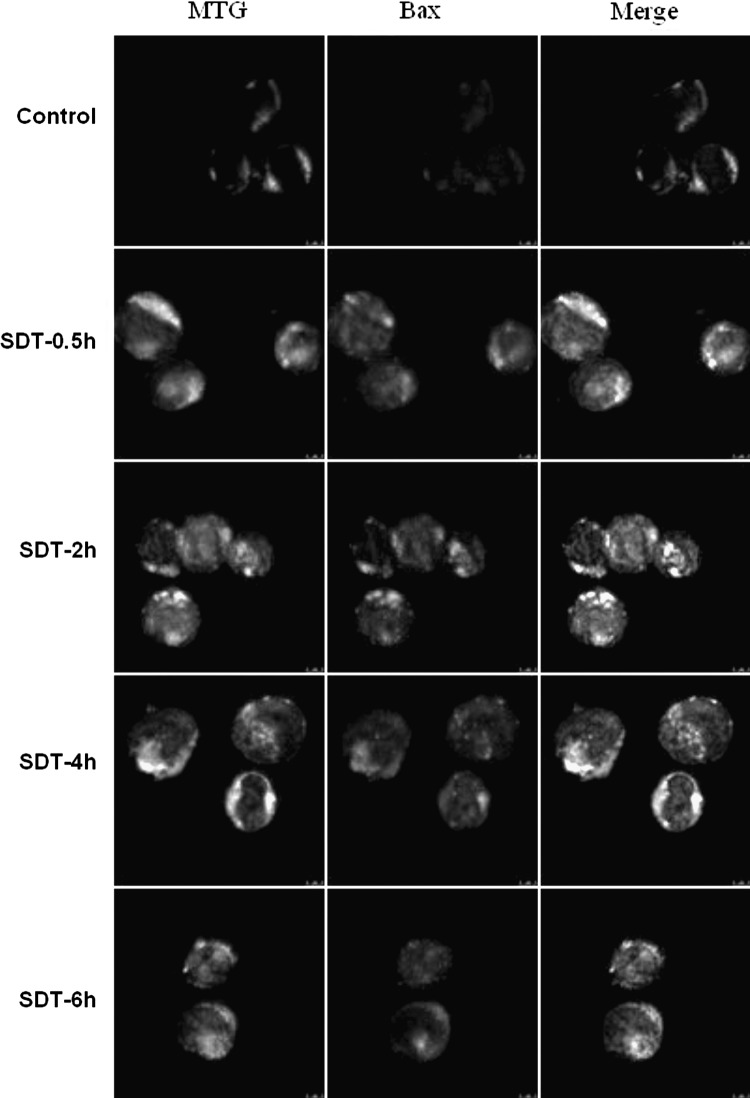
Bax is a pro-apoptotic member of the Bcl-2 family that regulates programmed cell death. On the induction of apoptosis, Bax shifts from a soluble form to a membrane-bound form, which partially colocalizes with mitochondria.16 As shown in Figure 9, in control cells, the MTG (mitochondrial special dye) green fluorescence had little overlapping with the Bax red fluorescence. In contrast, SDT-treated cells exhibited colocalization between mitochondria and Bax at 0.5 hours post-treatment, which was gradually enhanced by the incubation time and became more obvious at 2 hours post-treatment. Additionally, SDT treatment caused much more diffused distribution of mitochondria and enhancement of Bax fluorescence intensity compared with control cells.
FIG. 9.
Bax translocation of U937 cells after different incubation times after SDT treatment.
Changes of apoptosis-related protein and mRNA expression
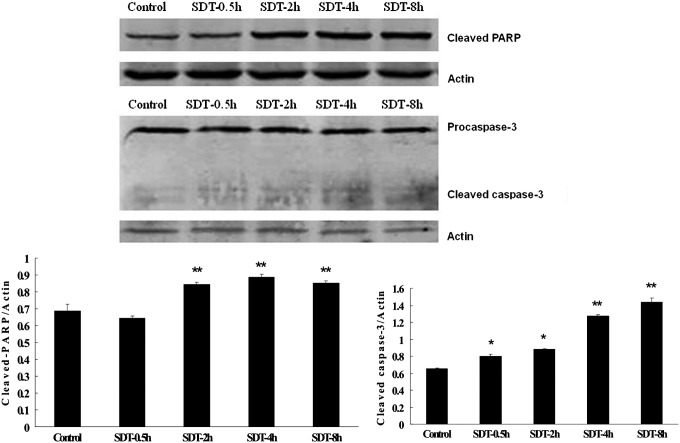
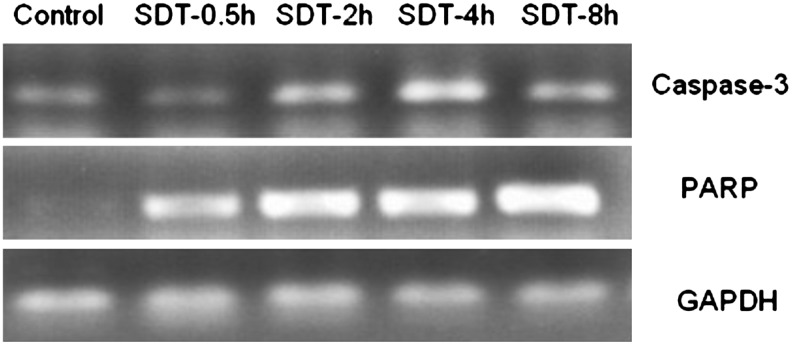
To further investigate whether the apoptotic effect was involved in HMME-SDT, we monitored the key apoptosis-related protein changes by using western blotting. Caspase-3 is a critical executioner of apoptosis.17 The applied caspase-3 antibody detects endogenous levels of full-length caspase-3 (35 kDa) and the large fragment of caspase-3 resulting from cleavage (17 kDa). As shown in Figure 10, compared with control, SDT treatment caused slight increase of typical caspase-3 cleavage at 0.5 hours post-treatment (p<0.05), which was gradually enhanced by the incubation time and became more obvious at 4 hours post-treatment (p<0.01). We know that caspase-3 is either partially or totally responsible for the proteolytic cleavage of many key proteins such as the nuclear enzyme poly(ADP-ribose) polymerase (PARP). Therefore, next, we examined PARP cleavage after treatment. The used cleaved PARP (Asp214) antibody detects endogenous levels of the large fragment (89 kDa) of human PARP produced by caspase cleavage. The result showed more obviously that the cleaved PARP fragment was detected at 2 hours post-SDT, which indirectly supported caspase activation and apoptotic response by SDT induction in U937 cells (Fig. 10). As expected, after SDT treatment, caspase-3 and PARP genes' mRNA levels were also significantly increased compared to control (Fig. 11).
FIG. 10.
Western blot analysis of cleaved PARP and caspase-3 activation in U937 cells after different incubation times after SDT. Actin was used as a loading control. Data are presented as mean±SD of four independent assessments. *p<0.05 and **p<0.01 versus untreated controls. PARP, poly(ADP-ribose) polymerase.
FIG. 11.
Reverse transcriptase–polymerase chain reaction analysis of PARP and caspase-3 genes' mRNA in U937 cells after different incubation times after SDT. GAPDH was used as a loading control.
Discussion
In cancer therapy, the induction of apoptosis by SDT is gaining interest as a preferred mode of killing cancer cells.18 The type, concentration, and subcellular localization of sonosensitizers are key factors for SDT. The subcellular localization of the sonosensitizer is of particular importance, since it determines the localization of the primary damage.19,20 The previous initial studies showed that HMME significantly enhanced the ultrasound-induced apoptosis in tumor cells, which may involve the mitochondrial damage during the process.12,13 The present study showed that HMME-mediated SDT could significantly induce apoptosis of U937 cells. To further determine whether the mitochondrion–caspase-signaling pathway was involved in HMME-SDT-induced apoptosis of U937 cells, many investigations such as uptakes and subcellular location of HMME, MMP damage, and apoptotic protein changes were performed.
As shown in Figure 1, HMME accumulated quickly in U937 cells and sustained at a relatively high level after 3 hours of incubation postadministration, when HMME mainly localized in the mitochondria of U937 cells (Fig. 2). The SDT conditions in this study were cells exposed to 1 W/cm2 ultrasound for a 60-second duration after 3 hours of incubation with 10 μg/mL HMME. The cytotoxicity was assessed by MTT assay at 4 hours post-SDT treatment; the result in Figure 3 showed that the ultrasonically induced cell damage was remarkably enhanced by 10 μg/mL, implying that HMME might be a potential good sonosensitizer, and HMME-SDT may provide an effective approach to eliminate leukemia cells.
To better understand the mechanisms of HMME-SDT-induced U937 cell death, we next examined cell apoptosis at 4 hours postirradiation. Apoptosis is a programmed cell death in response to a variety of stimuli, which is a mechanism of cellular self-destruction having uniquely defined morphological and molecular characteristics such as nuclear chromatin condensation and DNA fragmentation.21,22 The obtained results showed that at the given experimental conditions, SDT treatment could significantly increase the apoptotic cells (35.6% in SDT versus 4% in control, 6.5% in HMME, and 15.8% in ultrasound) by flow cytometry with Annexin V-PE/7-AAD double staining. DAPI staining confirmed that the typical apoptosis characteristics such as nuclear condensation with enhancing DAPI staining and altered nuclear morphology were obviously observed in HMME-SDT. Further, more significant DNA fragmentation was detected in SDT (33.9% versus 10.57% in HMME and 10.47% in ultrasound). These data suggested that an apoptotic response occurred in U937 cells after HMME-SDT.
Mitochondria play a critical role in cell apoptosis triggered by many stimuli.23,24 Many investigations suggest a mitochondrion–caspase-dependent pathway involved in SDT-induced cell apoptosis.25–27 Emerging evidence has shown that oxidative stress might be one of the major causes that initiated cell apoptosis by SDT.28,29 Low-intensity ultrasound could activate some sensitizers to produce cytotoxic ROS, and ROS might be the key factor for apoptosis.25–29 Therefore, ROS, mitochondria damage, and caspase-dependent apoptosis have complicated interactions in SDT-induced cell damaging. To confirm this, first, the generation of intracellular ROS and the MMP loss were detected. Result in Figure 7 displayed a rapid generation of intracellular ROS in the SDT group, whereas there was no occurrence in the control, HMME-alone, and ultrasound-alone groups. The MMP, as detected by flow cytometry, with Rh123 significantly decreased immediately after SDT treatment compared the other three groups (Fig. 8). These findings imply that the elevated ROS and the decreased MMP may both act as sensitive indicators of cell apoptosis after SDT. In addition, Bax translocation and insertion into the outer mitochondrial membrane are crucial for the regulation of the apoptotic process. Here, result in Figure 9 showed that SDT increased the translocation of Bax from the cytosol to mitochondria of U937 cells. Next, western blot confirmed that after SDT, the apoptotic executioner protein, caspase-3, was obviously cleaved to its active subunit, and the general substrate of caspase-3, PARP, was also cleaved to 89-kDa fragments (Fig. 10). The expression of caspase-3 and PARP mRNA also significantly increased post-SDT (Fig. 11). These results demonstrated that the caspase-dependent apoptosis pathway might be activated in HMME-SDT-induced apoptosis of U937 cells.
In conclusion, the present study demonstrated HMME-SDT significantly induced apoptosis of human leukemia U937 cells, and the generation of intracellular ROS, the MMP disruption, Bax translocation, and caspase activation might be all involved in the process. The findings may provide some information for the aforementioned ex vivo elimination of leukemic cells by means of apoptosis by SDT. More further investigations need to be performed in various human leukemia cells, to develop a potential way for leukemia treatment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81000999 and No. 10904087), the Natural Science Foundation of Shaanxi Province, China (Grant No. 2011JQ4012), and the Fundamental Research Funds for the Central Universities (Grant No. GK201102020).
Disclosure Statement
No competing financial interests exist.
References
- 1.Yumita N. Nishigaki R. Umemura K, et al. Hematoporphyrin as a sensitizer of cell damaging effect of ultrasound. Jpn J Cancer Res. 1989;80:219. doi: 10.1111/j.1349-7006.1989.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umemura S. Yumita N. Nishigaki R, et al. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J Cancer Res. 1990;81:962. doi: 10.1111/j.1349-7006.1990.tb02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuroki M. Hachimine K. Abe H, et al. Sonodynamic therapy of cancer using novel sonosensitizers. Anticancer Res. 2007;27:3673. [PubMed] [Google Scholar]
- 4.Mi N. Liu QH. Wang XB, et al. Induction of Sonodynamic effect with Protoporphyrin IX on Isolate Hepatoma-22 cells. Ultrasound Med Biol. 2009;35:680. doi: 10.1016/j.ultrasmedbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Lagneaux L. De-Meulenaer EC. Delforge A, et al. Ultrasonic low-energy treatment: A novel approach to induce apoptosis in human leukemic cells. Exp Hematol. 2002;30:1293. doi: 10.1016/s0301-472x(02)00920-7. [DOI] [PubMed] [Google Scholar]
- 6.Firestein F. Rozenszajn LA. Shemesh-Darvish L, et al. Induction of apoptosis by ultrasound application in human malignant lymphoid cells: Role of mitochondria-caspase pathway activation. Ann N Y Acad Sci. 2003;1010:163. doi: 10.1196/annals.1299.027. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JL. Liang HJ. Li QS, et al. Hematoporphyrin monomethyl ether-mediated photodynamic effects on THP-1 cell-derived macrophages. J Photochem Photobiol B. 2010;101:9. doi: 10.1016/j.jphotobiol.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y. Ma XQ. Jin P, et al. Apoptosis induced by hematoporphyrin monomethyl ether combined with He–Ne laser irradiation in vitro on canine breast cancer cells. Vet J. 2011;188:325. doi: 10.1016/j.tvjl.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Ding XM. Xu QZ. Liu FG, et al. Hematoporphyrin monomethyl ether photodynamic damage on HeLa cells by means of reactive oxygen species production and cytosolic free calcium concentration elevation. Cancer Lett. 2004;216:43. doi: 10.1016/j.canlet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZG. Song TH. Chen X, et al. Study on the interaction between hematoporphyrin monomethyl ether and DNA and the determination of hematoporphyrin monomethyl ether using the resonance light scattering technique. Spectrochim Acta A Mol Biomol Spectrosc. 2010;77:605. doi: 10.1016/j.saa.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Xu DY. Chen WH. Zhang H, et al. Hematoporphyrin 3- or 8- monomethyl ether: A promising candidate for tumor photodynamic therapy. J Med Coll PLA. 1993;8:406. [Google Scholar]
- 12.Jin H. Zhong X. Wang ZY, et al. Sonodynamic Effects of hematoporphyrin monomethyl ether on CNE-2 cells detected by atomic force microscopy. J Cell Biochem. 2011;112:169. doi: 10.1002/jcb.22912. [DOI] [PubMed] [Google Scholar]
- 13.Li JH. Song DY. Xu YG, et al. In vitro study of haematoporphyrin monomethyl ether-mediated sonodynamic effects on C6 glioma cells. Neurol Sci. 2008;29:229. doi: 10.1007/s10072-008-0972-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang XB. Liu QH. Zhao P, et al. Role of autophagy in sonodynamic therapy-induced cytotoxicity in S180 cells. Ultrasound Med Biol. 2010;36:1933. doi: 10.1016/j.ultrasmedbio.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Krysko DV. Vanden Berghe T. D'Herde K, et al. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods. 2008;44:205. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Wolter KG. Hsu YT. Smith CL. Nechushtan A, et al. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghobrial IM. Witzig TE. Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 18.Xiang JY. Xia XS. Jiang Y, et al. Apoptosis of ovarian cancer cells induced by methylene blue-mediated sonodynamic action. Ultrasonics. 2011;51:390. doi: 10.1016/j.ultras.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Achibana K. Feril LB., Jr Ikeda-Dantsuji Y. Sonodynamic therapy. Ultrasonics. 2008;48:253. doi: 10.1016/j.ultras.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal I. Sostaric JZ. Riesz P. Sonodynamic therapy- a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;1:349. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Steller H. Mechanism and genes of cellular suicide. Science. 1995;267:1445. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Apoptosis. Immunol Today. 1993;14:126. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 23.Chalah A. Khosravi FR. The mitochondrial death pathway. Adv Exp Med Biol. 2008;615:25. doi: 10.1007/978-1-4020-6554-5_3. [DOI] [PubMed] [Google Scholar]
- 24.Smith DJ. Ng H. Kluck RM, et al. The mitochondrial gateway to cell death. IUBMB Life. 2008;60:383. doi: 10.1002/iub.44. [DOI] [PubMed] [Google Scholar]
- 25.Honda H. Zhao QL. Kondo T. Effects of dissolved gases and an echo contrast agent on apoptosis induced by ultrasound and its mechanism via the mitochondria-caspase pathway. Ultrasound Med Biol. 2002;28:673. doi: 10.1016/s0301-5629(02)00509-4. [DOI] [PubMed] [Google Scholar]
- 26.Honda H. Kondo T. Zhao QL, et al. Role of intracellular calcium ions and reactive oxygen species in apoptosis induced by ultrasound. Ultrasound Med Biol. 2004;30:683. doi: 10.1016/j.ultrasmedbio.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Tang W. Liu QH. Zhang J, et al. In vitro activation of mitochondria-caspase signaling pathway in sonodynamic therapy-induced apoptosis in sarcoma 180 cells. Ultrasonics. 2010;50:567. doi: 10.1016/j.ultras.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Song W. Cui HD. Zhang R, et al. Apoptosis of SAS cells induced by Sonodynamic therapy using 5-aminolevulinic acid sonosensitizer. Anticancer Res. 2011;31:39. [PubMed] [Google Scholar]
- 29.Yumita N. Okudaira K. Momose Y, et al. Sonodynamically induced apoptosis and active oxygen generation by gallium–porphyrin complex, ATX-70. Cancer Chemother Pharmacol. 2010;66:1071. doi: 10.1007/s00280-010-1264-6. [DOI] [PubMed] [Google Scholar]