Summary
The presence of tumor-associated macrophages (TAMs) in melanomas is correlated with a poor clinical prognosis. However, there is limited information on the characteristics and biological activities of human TAMs in melanomas. In this study, we developed an in vitro method to differentiate human monocytes to macrophages using modified melanoma-conditioned medium (MCM). We demonstrate that factors from MCM-induced macrophages (MCMI-Mϕ) express both M1-Mϕ and M2-Mϕ markers, and inhibit melanoma-specific T cell proliferation. Furthermore, microarray analyses reveal that the majority of genes up-regulated in MCMI-Mϕ are associated with tumor invasion. The most strikingly up-regulated genes are CCL2 and MMP-9. Consistent with this, blockade of both CCL-2 and MMPs diminish MCMI-Mϕ-induced melanoma invasion. Finally, we demonstrate that both MCMI-Mϕ and in vivo TAMs express the pro-invasive, melanoma-associated gene, GPMNB. Our study provides a framework for understanding the mechanisms of crosstalk between TAMs and melanoma cells within the tumor microenvironment.
Keywords: Melanoma, macrophages, invasion, tumor microenvironment, GPMNB
Introduction
Malignant melanoma is the deadliest form of skin cancer, with an overall survival rate of 25% at one year after diagnosis. Recent studies have indicated that tumor-associated macrophages (TAMs) provide an inflammatory microenvironment and play essential roles in tumor progression and metastasis (Mantovani and Sica, 2010; Porta et al., 2007; Qian and Pollard, 2010; Solinas et al., 2009). Major factors that contribute to tumor metastasis include chemokines, which attract tumor cells to the metastasis site, as well as proteinases, such as MMPs, which degrade the extracellular matrix and result in tissue remodeling, invasion and metastasis (Kessenbrock et al., 2010).
Macrophages are the most abundant leukocytes in melanoma lesions (Brocker et al., 1988). Accumulating evidence indicates that increased numbers of TAMs infiltrating melanomas are associated with poor prognosis (Bernengo et al., 2000; Brocker et al., 1987; Makitie et al., 2001; Varney et al., 2005). Furthermore, elevated expression of CD68 and CD163, two markers of TAMs, in melanoma tissues and serum are poor prognostic markers for early stage melanomas (Jensen et al., 2009). TAMs appear to be involved in every stage of melanoma progression and metastasis. Macrophage-derived IFN-γ plays a critical role in melanoma transformation and melanoma cell survival in an ultraviolet-induced melanoma mouse model (Zaidi et al., 2011). TAMs also produce TNF-α and IL-1α, which promote melanoma angiogenesis, and targeting TAMs by blockade of CCL-2 inhibits melanoma angiogenesis and growth in mice (Gazzaniga et al., 2007; Torisu et al., 2000). In addition, TAMs inhibit tumor-specific CD8+ T cell-mediated cytotoxicity in melanomas resulting in an immunosuppressive phenotype (Kono et al., 1996). Finally, it has been shown in a melanoma mouse model that urokinase-type plasminogen activator receptor (uPAR) and MMP-9 produced by tumor cells and by TAMs increase tumor invasion and metastasis (Marconi et al., 2008).
TAMs are derived from blood monocytes and differentiate within the tumor microenvironment due to factors produced by tumor cells. Experimentally, TAMs can be differentiated from peripheral blood monocytes by factors secreted from tumor cells and by stroma cells (Mantovani and Sica, 2010). A major factor that differentiates monocytes to TAMs is M-CSF. Solinas et al. reported that pancreatic cancer-conditioned media (PCM) was able to differentiate monocytes to PCM-induced macrophages (PCMI-Mϕ), while neutralization of M-CSF totally inhibited PCMI-Mϕ differentiation (Solinas et al., 2010). However, other factors, such as VEGFA, CCL2, IL-6, LIF and GM-CSF, have also been reported to be involved in the differentiation of monocytes to macrophages (Bennicelli and Guerry, 1993; Duluc et al., 2007; Lazar-Molnar et al., 2000; Paglia et al., 1995; Richmond et al., 2009).
Macrophages have been classified as activated macrophages (M1-Mϕ) and “alternatively activated macrophages” (M2-Mϕ), largely based on factors they produce. M1-Mϕ are induced by proinflammatory factors, produce a lower level of IL-10 and high levels of IL-12, IL-6 and TNF-α, and have anti-tumor activity. Conversely, M2-Mϕ produce high levels of IL-10, TGFβ, CCL1 and CCL-22 and a lower level of IL-12, and promote tumor growth and metastasis. Most TAMs characterized to date demonstrate an M2-Mϕ phenotype. However, current evidence suggests that TAMs are a mixed population bearing both M1 and M2 phenotypes (Umemura et al., 2008). It has also been proposed that M-CSF and GM-CSF induce macrophages as M2 and M1 macrophages, respectively (Sierra-Filardi et al., 2011; Svensson et al., 2011).
In order to better characterize TAMs, we developed a highly efficient in vitro method to differentiate macrophages using modified melanoma conditioned-media (MCM). Microarray analysis on these MCMI-Mϕ showed that many genes associated with melanoma cell invasion and metastasis were up-regulated, such as CCL-2 and MMP-9. MCMI-Mϕ were able to increase melanoma cell invasion in vitro. Blocking both CCL2 and MMPs significantly inhibit MCMI-Mϕ induced melanoma invasion, even though there was no inhibitory effect by either factor alone. Finally, through microarray analysis and tissue staining, we show that TAMs present in human melanomas highly express the pro-invasion gene, Glycoprotein Non-Metastatic Melanoma Protein B (GPMNB). These results provide a valuable tool to further understand the roles of TAMs in melanoma progression and metastasis.
Results
Differentiation of human MCMI-Mϕ in vitro with MCM
To differentiate monocytes to MCMI-Mϕ, we concentrated 3 day MCM with a Centrifugal Filter Device from Millipore (pore size, 10 kD), and added the MCM to RPMI medium supplemented with 10% fetal bovine serum (FBS) at a 50% ratio of the original MCM. After 7 days of incubation, monocytes differentiated to MCMI-Mϕ, based on cell morphology and the pattern of expression of macrophages/TAM markers (see below). This effect was elicited by MCM from 2 non-metastatic melanoma lines, WM35 and WM793 (Figure S1A, and data not shown), as well as from 2 metastatic melanoma cell lines, 1205Lu and C8161.
To test whether this approach has a broad application, we tested whether TCM from one ovarian cancer line, Ovca42, and 2 breast cancer cell lines, T47D and MD-MB-231, also differentiated monocytes to MCMI-Mϕ. Similar to the MCM, TCM from these other cell lines also differentiated monocytes to macrophages (Figure S1B, and data not shown). These data indicate that this is a reliable method to differentiate monocytes to MCMI-Mϕ.
Characterization of MCMI-Mϕ in melanomas
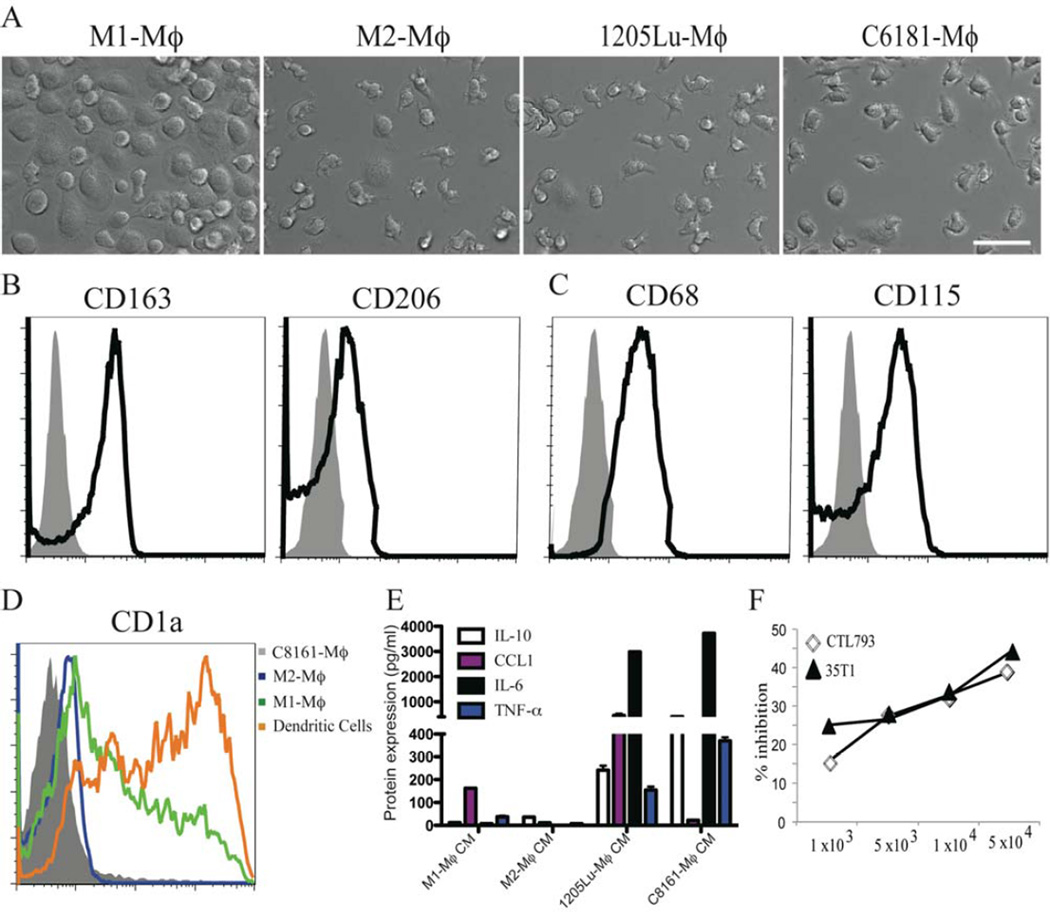
We characterized the MCMI-Mϕ by analyzing their morphology, expression of surface markers, cytokine/chemokine profile and function. After 7 days of incubation, MCMI-Mϕ that were differentiated by C8161 or 1205Lu MCM (C8161-Mϕ and 1205Lu-Mϕ) showed elongated shapes and typical spindle-like macrophage morphology, which is similar to the M2-Mϕ, whereas M1-Mϕ typically show a round, fried egg-shape as previously described (Figure 1A) (Svensson et al., 2011; Waldo et al., 2008).
Figure 1. Differentiation of monocytes to MCMI-Mϕ.
Monocytes from healthy donors were cultured in the presence of GM-CSF (10 ng/ml), M-CSF (10 ng/ml), 1205Lu-MCM or C8161-MCM for 7 days. (A) Morphological analysis of M1-Mϕ, M2-Mϕ, 1205Lu-Mϕ and C8161-Mϕ using a Nikon inverted microscope. Scale bar = 50 µm. (B) & (C) Expression of M2-Mϕ and macrophage surface markers in C8161-Mϕ. FACS analysis was performed for cell surface expression of the M2-Mϕ markers (CD163, CD206) in C8161-Mϕ (B), macrophage markers (CD68 and CD115) (C). Gray shadow fills = isotype matched control; black lines = primary antibodies. (D) FACS analysis of CD1a expression in M2-Mϕ, M1-Mϕ, DCs and C8161-Mϕ. Each experiment is representative of at least 6 independent experiments from 6 different healthy donors. (E) Conditioned medium from M1-Mϕ, M2-Mϕ, 1205Lu-Mϕ and C8161-Mϕ were harvested. Production of M2-Mϕ cytokine and chemokines IL-10, CCL2, and M1-Mϕ cytokines IL-6 and TNFα were measured by Luminex analysis. (F) MCMI-Mϕ inhibit the proliferation of anti-CD3-induced proliferation of human anti-melanoma specific T cells. Anti-melanoma specific T cell clones were co-cultured with increased numbers of 1205Lu-Mϕ in the presence of anti-CD3 (1 µg/m) for seven days, 3H-TdR was added 16 h before the cells were harvested.
We then characterized the expression of macrophage surface markers by flow cytometry analysis. C8161-Mϕ expressed the M2-Mϕ markers, CD163 and CD206 (Figure 1B). In addition, CD68 and CD115, which are expressed by both M1 and M2 macrophages, are also expressed by C8161-Mϕ (Figure 1C). Furthermore, C8161-Mϕ also expressed other M2-Mϕ markers, such as CXCR4, CD16 and CD36 (Figure S1C). Neither C8161-Mϕ nor 1205Lu-Mϕ expressed the dendritic cell marker CD1a (Figure 1D, and data not shown), indicating that C8161-Mϕ and 1205Lu-Mϕ are macrophage and not dendritic cell lineage.
To further characterize MCMI-Mϕ based on factors they produce, we analyzed the production of cytokines/chemokines previously implicated to be expressed in M1-Mϕ and M2-Mϕ (Ilkovitch and Lopez, 2008), (Payne and Cornelius, 2002). Both 1205Lu-Mϕ and C8161-Mϕ secreted high levels of the M2-Mϕ cytokines, IL-10 and CCL1, as well as the M1-Mϕ cytokines, IL-6 and TNF-α. No production of IL-12 (p40) was detected in 1205Lu-Mϕ or C8161-Mϕ (Figure 1E, and data not shown). Of note, 1205Lu-Mϕ and C8161-Mϕ produced more cytokines/chemokines than M2-Mϕ, and there are significant differences in cytokines/chemokines produced by 1205Lu-Mϕ and C8161-Mϕ, further suggesting that MCMI-Mϕ may be heterogenous and bear both M1 and M2 phenotypes.
One of the major activities of TAMs is their ability to suppress anti-tumor immunity (Biswas and Mantovani, 2010). For example, it has been shown that macrophages were able to inhibit T cell proliferation due to expression of indoleamine 2, 3-dioxygenase (Munn et al., 1999).. To determine the potential ability of MCMI-Mϕ to inhibit T cell proliferation, we co-cultured 1205Lu-Mϕ with 2 different anti-melanoma reactive T cell clones: a CD4 T cell clone, 35Th1, and a CD8 T cell clone, CTL793, each established as described earlier from peripheral blood lymphocytes of melanoma patients (Somasundaram et al., 2002). C8161-Mϕ significantly inhibited T cell proliferation to anti-CD3 stimulation both in CD4 and in CD8 T cell clones in a dose-dependent manner (Figure 1F). These data suggest that MCMI-Mϕ are able to inhibit T-cell proliferation.
Differentiation of MCMI-Mϕ in melanomas is not dependent on M-CSF
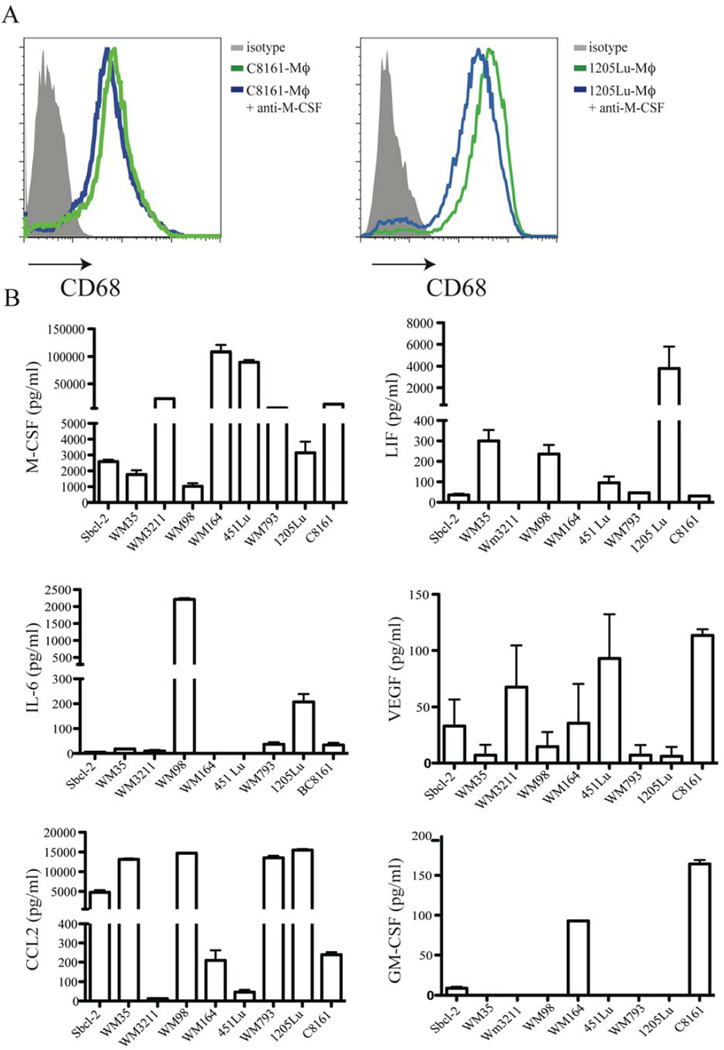
The differentiation of monocytes to PCMI-Mϕ has been reported to be dependent on M-CSF (Solinas et al., 2010). To investigate whether MCMI-Mϕ differentiation is also dependent on M-CSF, we incubated monocytes in the presence of C8161 MCM or 1205Lu MCM in the presence of anti-human M-CSF (10 µg/ml) or an isotype control antibody for 7 days. We observed a slightly decreased expression of CD68 in both C8161-Mϕ and in 1205Lu-Mϕ (Figure 2A). These data indicate that the differentiation of MCMI-Mϕ is not only dependent on M-CSF, but that other factors may also play roles in MCMI-Mϕ differentiation.
Figure 2. Differentiation of melanoma MCMI-Mϕ is independent of M-CSF.
(A) Monocytes were incubated in the presence of 1205Lu-MCM with or without anti-M-CSF (10 µg/ml) for 7 days. Expression of CD68 was analyzed by flow cytometric analysis. (B) Melanoma cells from RGP (Sbcl-2, WM35, WM3211), VGP (WM98, WM164, WM793) and metastatic (451Lu, 1205Lu, C8161) melanomas were seeded in 6-well plates, and incubated for 3 days. Culture media were harvested and the production of M-CSF, CCL-2, IL-6, LIF, VEGF and GM-CSF was measured using Luminex analysis.
Melanoma cells produce factors in addition to M-CSF that are related to macrophage differentiation, including CCL2, GM-CSF, VEGFA, LIF and IL-6. It is possible that melanomas at different stages of development may produce very different TAMs based on their unique cytokine patterns. Therefore, we characterized cytokine/chemokine production in melanoma cell lines derived from melanomas at different stages: 3 radial growth phase melanoma lines (RGP): Sbcl-2, WM35 and WM3211, 3 vertical growth phase melanoma lines (VGP): WM98, WM793, WM164, and 3 metastatic melanoma cell lines, 1205Lu, 451Lu and C8161. All melanoma cell lines produced M-CSF, CCL2 and VEGFA, but at different levels. Seven of the 9 cell lines produced LIF and IL-6, and only 3 of the 9 cell lines produced GM-CSF, which is a major M1ϕ differentiation factor. Of note, there was no pattern of cytokine production specific for different stages of melanomas (Figure 2B) and, therefore the production of the different types of macrophage is not likely correlated with melanoma progression.
Gene profiling of MCMI-Mϕ in melanomas
To further characterize novel factors expressed in MCMI-Mϕ, we performed microarray analyses to characterize the molecular gene signature of C8161-Mϕ by comparing them with the normal monocyte gene profile. A total of 1,912 genes were differentially regulated (1,019 up-regulated and 893 down-regulated) in a total of 47,000 probes in C8161-Mϕ (Excel S1, 2).
Next, we compared the gene expression profiles using microarray data that are publically available in the GEO database (GSE). We found that 16.4% and 16% of up-regulated genes in C8161-Mϕ overlapped with genes expressed in GSE for M1-Mϕ and M2-Mϕ, respectively (Figure S2A), while 17.9 and 7% of down-regulated genes overlapped between M1-Mϕ and M2-Mϕ, respectively (Figure S2B). Therefore, MCMI-Mϕ have a gene expression profile that is not characteristic of either M1-Mϕ or M2-Mϕ.
An invasive signature in melanoma MCMI-Mϕ
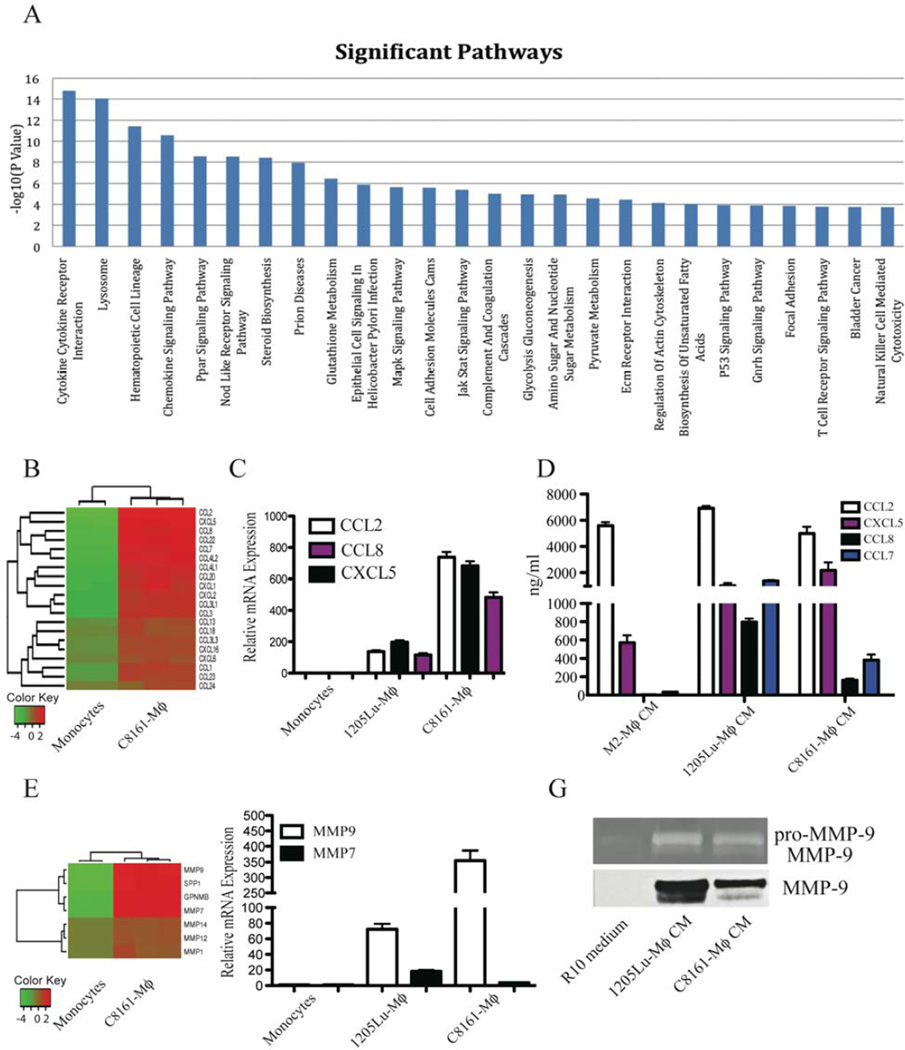
We performed pathway analysis of significantly up-regulated genes that were revealed by the gene expression profiling. Twenty-six pathways were found to be significant under a family-wise error rate level of 0.05 (Figure 3A). Among those pathways, 7 are linked to cell metabolism, such as glutathione metabolism. Strikingly, nearly all other pathways have been implicated to play roles in tumor invasion and metastasis, such as cytokine/cytokine receptor interactions, chemokine signaling pathways, cell adhesion molecules, the Jak-Stat signaling pathway, ECM receptor interactions, regulation of the actin cytoskeleton and focal adhesion molecules (Figure 3A, Excel S3).
Figure 3. Gene profiling reveals an invasive signature in MCMI-Mϕ.
(A) Gene Set Enrichment Analysis over 186 KEGG pathways. With Bonferroni correction, 26 pathways were identified to be significantly expressed under a family-wise error rate (FWER) level of 0.05. (B) Heatmap of chemokines in C8161-Mϕ compared to monocytes. (C) Real-time PCR was used to verify top up-regulated chemokines related to the invasive phenotype, CCL2, CCL8 and CXCL5. Data are representative of 3 independent experiments with 3 healthy donors. (D) Luminex analysis was used to verify the expression of chemokines and cytokines related to the invasive phenotype. (E) Heatmap of the invasive signature in C8161-Mϕ. (F) Real-time PCR was used to verify the top up-regulated genes (MMP9 and MMP7) identified by the microarray analysis. Data are representative of 3 independent experiments with 3 healthy donors. (G) MCMI-Mϕ were differentiated as described for Figure 1. Cells were harvested and washed with serum-free medium. 1205Lu-Mϕ and C8161-Mϕ were then cultured in the presence of RPMI-10 medium for another 48 hr. Conditioned media were harvested and subjected to gelatin substrate zymography. The electrophoretic positions of the 92-kDa pro-MMP-9 zymogen, and the 82-KDa activated forms of MMP-9 are indicated (upper panel). Western blot was used to detect expression of MMP-9 (lower panel).
A detailed analysis of the top 100 up-regulated genes revealed that most have been implicated in the promotion of tumor progression and metastasis, including 13 genes encoding chemokines and chemokine receptors (Table S2). A total of 20 chemokines are up-regulated in MCMI-Mϕ (Figure 3B), and of note, CCL2 is the highest up-regulated gene. Real-time PCR analysis confirmed that the expression of CCL2, CXCL5 and CCL8 is up-regulated in MCM (Figure 3C). Furthermore, these chemokines were found to be more highly expressed in both C8161-Mϕ and in 1205Lu-Mϕ (Figure 3D).
Other up-regulated genes in MCMI-Mϕ encoded proteases, including MMP-9, 7, 1, 12 and 14, secreted phosphoprotein 1 (SPP1, osteopontin), cathepsin L1 (CTSL1) and urokinase (uPA) (Figure 3E). In addition, a less studied molecule, GPMNB, which promotes tumor metastasis, is strongly up-regulated in MCMI-Mϕ compared to monocytes (Figure 3E). The up-regulation of MMP-9 and MMP-7 mRNA expression was verified by real-time PCR (Figure 3F). In order to confirm that MMP-9 was produced by MCMI-Mϕ rather than the melanoma cell lines, we incubated 1205Lu-Mϕ and C8161-Mϕ with fresh 10% FBS RPMI1640 medium for an additional two days, and this macrophage conditioned media supernatant was harvested for western blot analysis. As shown in Figure 3G (lower panel), 1205Lu-Mϕ and C8161-Mϕ produced high level of MMP-9. In addition, we found the activated form of MMP-9 in supernatants from 1205Lu-Mϕ and C8161-Mϕ by gelatin zymography (upper panel, Figure 3G). In summary, these data indicate that MCMI-Mϕ show an invasive signature.
Blockade of both MMPs and CCL2 significantly inhibit 1205Lu-Mϕ induced melanoma invasion
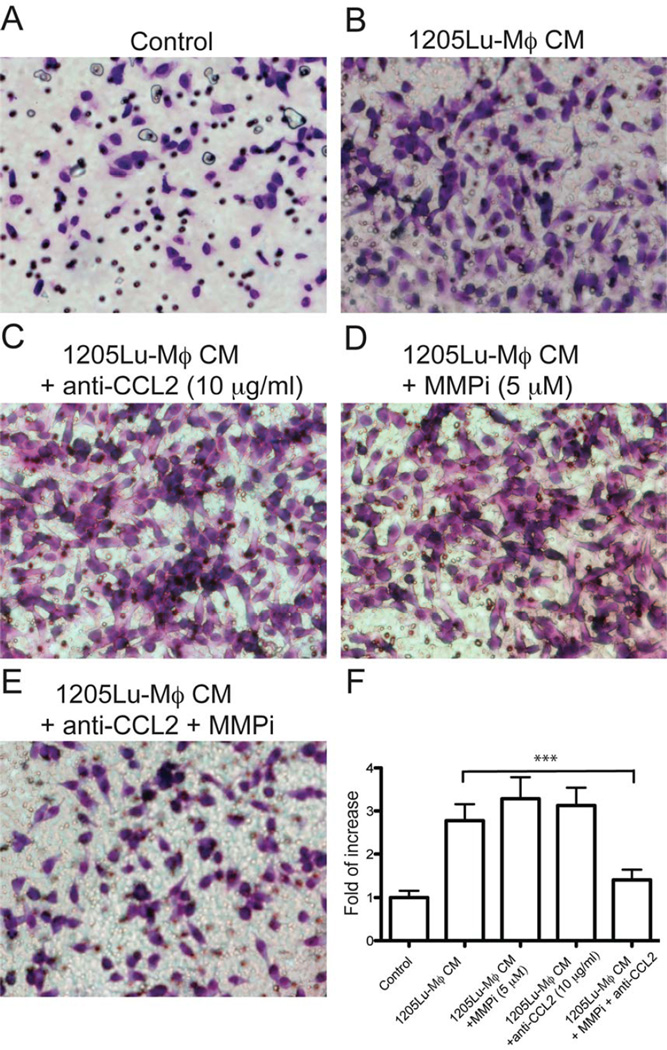
Since CCL2, MMP9 and MMP-7 are among the most up-regulated factors in supernatants of MCMI-Mϕ, and are critical for melanoma invasion, we investigated the role of CCL2 and MMPs in the supernatants of 1205Lu-Mϕ on melanoma invasion. We used a Matrigel Transwell assay, and compared invasion of melanoma cells attracted to the supernatant of 1205Lu-Mϕ alone or the supernatant with an anti-CCL2 monoclonal antibody and a pan-MMPs inhibitor GM6001 (Figure 4). The supernatant alone significantly increased melanoma cell invasion compared to control media. Surprisingly, instead of inhibiting 1205Lu-Mϕ induced invasion, treatment with either anti-CCL2 (10 µg/ml) or GM6001 (10 µM) did not have a significant effect on 1205Lu-Mϕ supernatant induced melanoma invasion, while combined treatment with both significantly inhibited 1205Lu-MCM-induced invasion. These data indicate that the combination of the anti-CCL2 antibody and the MMPs inhibitor can achieve significant inhibition of MCMI-Mϕ induced melanoma invasion in Matrigel.
Figure 4. Synergistic effect of blockade CCL2 and MMPs on MCMI-Mϕ induced melanoma invasion.
1205Lu melanoma cells were seeded into matrigel precoated Transwells and were incubated for 18 hr. Conditioned medium from 1205Lu-Mϕ or control medium were added to the bottom chamber. Migrated cells were stained (using a Diff-Quick staining kit) and photographed. (A) Control medium. (B) 1205Lu-Mϕ supernatant. Blockade of MMP or CCL-2 alone marginally increased melanoma cell invasion (C&D). Blockade of both MMPs and CCL-2 resulted in the significant inhibition of melanoma invasion (E). Data are representative of 3 independent experiments. (F) Summary of all data. *** P<0.01.
MCMI-Mϕ have an invasive signature similar to TAMs
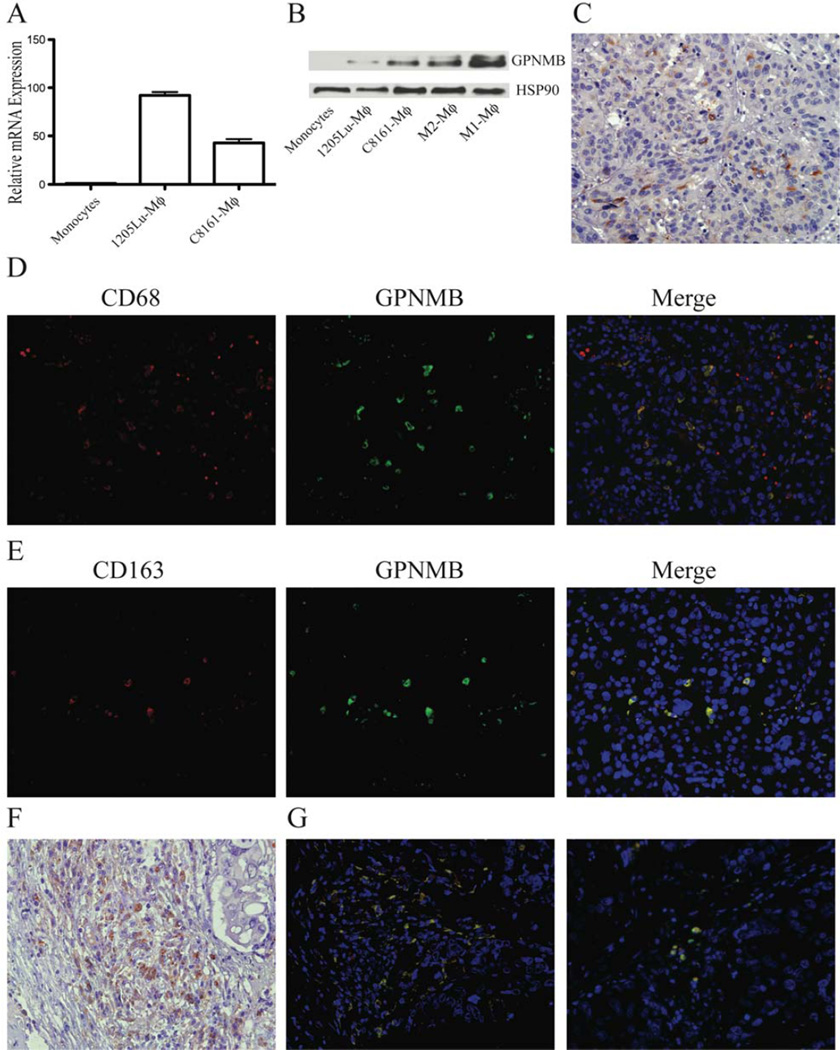
Expression of chemokines and proteinases in melanomas and macrophages has been well documented. Our studies have revealed a potentially important additional gene, GPMNB, which has not been reported to be expressed in TAMs. GPMNB is one of the top 5ranked up-regulated genes in MCMI-Mϕ (Table S2). Real-time PCR analysis revealed an 80-fold and 49-fold increased expression of GPMNB in 1205Lu-Mϕ and in C8161-Mϕ compared to monocytes, respectively (Figure 5A). Western blot analysis confirmed that GPMNB is expressed in M1-Mϕ and in M2-Mϕ, as well as in 1205Lu-Mϕ and in C8161-Mϕ (Figure 5B). To further characterize the expression of GPMNB in TAMs from melanoma lesions, we performed immunohistochemical staining with an anti-GPMNB antibody. Most GPMNB-positive cells were inflammatory cells (Figure 5C), and few were melanoma cells (Figure S3A). We further performed double staining of GPMNB with the most commonly used TAMs markers, CD68 and CD163, to confirm whether GPMNB is expressed in TAMs. As shown in Figure 5D, GPMNB was expressed in most CD68-positive cells, but there was not a complete overlap with CD68 staining. Presumably, some melanoma cells were also CD68-positive, as reported previously (Facchetti et al., 1991). As expected, most GPMNB-positive cells were CD163-positive (Figure 5E). These data identify GPMNB as a novel marker for TAMs. Since GPMNB has been implicated in the promotion of breast cancer metastasis, we evaluated whether GPMNB is expressed in TAMs in breast cancer tissues. Immunohistochemistry staining confirmed that GPMNB is expressed in breast cancer lesions with most GPMNB-positive cells having a macrophage morphology (Figure 5F), and few cancer cells stained positive (Figure S3B). Furthermore, most GPMNB-positive cells were CD68 and CD163 positive (Figure 5G). Collectively, the in vivo expression of GPMNB further supports the invasive signature we have developed for MCMI-Mϕ.
Figure 5. The pro-invasive gene, GPMNB, is induced in MCMI-Mϕ.
(A) Real-time PCR revealed that the expression of GPMNB is up-regulated in C8161-Mϕ and 1205Lu-Mϕ compared to monocytes. (B) Expression of GPMNB in monocytes, M1-Mϕ, M2-Mϕ, C8161-Mϕ and 1205Lu-Mϕ detected by Western Blot. (C) Immunohistochemistry analysis revealed GPMNB is expressed in inflammatory cells, but not in tumor cells of primary melanoma lesions. (D) & (E) Immunofluorescence analysis revealed GPMNB is expressed in infiltrated TAMs, but not in primary melanoma lesions. Scale bar = 50 µm. (F) Immunohistochemistry analysis of GPMNB in breast cancer tissues. (G) Co-staining of CD68 and CD163 in breast caner tissues. Data are representative of 8 primary melanoma and breast cancer tissues for C & F, and 3 for D, E & G.
Discussion
Using conditioned media from tumor cells is an established method to differentiate primary human monocytes to TAMs. However, there are limitations with the currently used methods. Solinas et al. (Solinas et al., 2010) reported that culture media from only 2 of 16 tumor cell lines (after 1 day of culture) were able to differentiate monocytes to macrophages. By using 3 day MCM, we are able to consistently differentiate monocytes. Under these conditions, more cytokines, including M-CSF, are produced compared with the 1-day culture media (data not shown). Also, filtration of the culture media appears to retain the growth factors needed for TAMs differentiation, while filtering out cell culture metabolites that are acidic and other small molecular weight toxic metabolites that may affect monocyte/macrophage survival (Tsunawaki and Nathan, 1986).
MCMI-Mϕ produced by this method are similar to TAMs found in cancer tissues by gene profiling in vitro and in vivo (Figures 3 and 5), and by functional studies (Figures 1F, 4). In addition to having an invasive phenotype, we have identified several genes expressed in both types of macrophages that may be important in TAM function. In particular, several MCMI-Mϕ up-regulated genes were identified, including DFNA5, that have not been previously reported to be expressed in the monocyte/macrophage lineage (Table S2), and which were also found in melanoma tissue TAMs (data not shown). Finally, we demonstrated that 3 day conditioned media from breast and ovarian cancer cells can also successfully differentiate monocytes to macrophages (Figure S1B and data not shown).
The prevailing M1-Mϕ and M2-Mϕ differentiation model probably does not fully reflect the complexity of macrophages in the tumor microenvironment. Recent studies have also demonstrated that TAMs are a heterogeneous population and share both phenotypes of M1-Mϕ and M2-Mϕ (Mosser and Edwards, 2008); (Umemura et al., 2008). In our model, we also found that TAMs in melanomas expressed both M1 and M2 markers, and secrete multiple cytokines and chemokines associated with both M1-Mϕ and M2-Mϕ. MCMI-Mϕ also produced M1-Mϕ cytokines/chemokines, such as IL-1α, IL-6 and TNF-α (Figure 1E, Table S2). Our functional studies demonstrated that MCMI-Mϕ are immunosuppressive and promote melanoma invasion, a definition of M2-Mϕ. Supporting this, GPMNB, a pro-invasion gene, is expressed in M1-Mϕ, M2-Mϕ and MCMI-Mϕ. Collectively, our data support the concept of melanoma TAMs heterogeneity with both M1 and M2 phenotypes (Biswas et al., 2008).
Previous studies have shown that melanoma cells express factors related to TAMs differentiation, but it is not known if the phenotype of TAMs is different in melanomas of different stages. Here, we show that there is no significant difference in the production of M-CSF, LIF, IL-6, VEGF, CCL2 or GM-CSF between cell lines from different stages of melanomas, consistent with the action of TAMs, which appear to be involved in every step of melanoma progression. Furthermore, multiple factors appear to be involved in TAM development, consistent with our data that neutralization of M-CSF alone has a minimal effect on TAMs differentiation in melanomas (Figure 2B). MCM from a panel of melanoma cell lines representing different stages of melanoma progression were able to differentiate monocytes similarly. While most melanoma cell lines do not express GM-CSF, some cell lines express both GM-CSF and M-CSF (Figure 2B), perhaps partially explaining the heterogeneity of the phenotype in MCMI-Mϕ.
Metastasis is a very complex process, in part due to the influence of many components of the tumor microenvironment with the malignant cells. The tumor microenvironment is vastly different both in types and in quantities of its components, leading to dramatically different clinical outcomes for different tumors. Our findings suggest that there is heterogeneity in MCMI-Mϕ, and the differences in secreted products of tumors contribute to this heterogeneity. Despite this, we found many gene pathways that are associated with an invasive phenotype to be up-regulated in MCMI-Mϕ, especially those involving chemokines and MMPs. We also found that dual blockade of MMPs and CCL2 is required to block melanoma cell invasion promoted by MCMI-Mϕ. These data may help explain why inhibition of MMPs alone has little efficacy on the clinical outcome of cancer patients in several clinical trials. A possible explanation is that MMPs and CCL2 have a positive feedback effect on each other. For example, CCL2 has been shown to induce MMP2 and MMP9 production in macrophages (Robinson et al., 2002), while CCL2-induced melanoma invasion is dependent on MMP expression. Therefore, CCL2 may indirectly contribute to pancreatic cancer dissemination by favoring the leukocyte-mediated digestion of the extracellular matrix (Monti et al., 2003). Conversely, MMPs process and release multiple chemokines (Dean et al., 2008). Blocking one of them might not be sufficient to block this positive feedback. MMPs and CCL2 are two major drivers for TAMs induced melanoma invasion and provide a rationale to targeting both for melanoma therapy.
Previous work by Solinas et al. (2009) indicated that PCMI-Mϕ also has an invasive signature. For example, SEPP1, osteoactivin and GPMNB are among highest up-regulated genes in PCMI-Mϕ, which are also significantly up-regulated in MCMI-Mϕ. We see several differences in the phenotype of macrophages produced from PCMI compared to MCM. For example, there is a 240-fold increase in MMP-9 expression in MCMI-Mϕ, which was increased 9-fold in that other study. Other genes, up-regulated in PCMI-Mϕ, such as MMP-2, were not identified in our array list. Furthermore, unlike MCMI-Mϕ, fewer chemokines and cytokines were up-regulated in their report. This may be because the conditioned media is different between cancer types or is due to differences in the methods used to produce the different conditioned media. The efficacy in macrophage induction with our conditioned media from different tumor types suggests that this may explain the differences noted in the 2 studies.
It has been reported that GPMNB is expressed by endothelial cells, osteoclasts, osteoblasts and macrophages (Ripoll et al., 2007; Rose and Siegel, 2010; Selim et al., 2003; Sheng et al., 2008), and by a variety of cancers, including gastrointestinal cancers, glioblastomas and breast cancers (Kuan et al., 2011; Metz et al., 2007). In melanomas, GPMNB is expressed by melanocytes and by melanoma cell lines (Tse et al., 2006; Weterman et al., 1995) and by uveal melanoma tissues (Williams et al., 2010). Moreover, over-expression of GPMNB increases tumor cell invasion in vitro and promotes their metastasis in vivo (Onaga et al., 2003; Rich et al., 2003), potentially by up-regulating the expression of MMP-9 and MMP-3 (Ogawa et al., 2005), which promotes tumor metastasis (Rose and Siegel, 2010). Targeting GPMNB significantly inhibits GPMNB-positive melanoma cell growth in vivo (Tomihari et al., 2010). Even though this molecule has been proposed to be important in melanomas, there is no report of its expression in melanoma lesions in vivo. Based on the high level of up-regulated expression of GPMNB in our microarray analysis, we stained melanoma tissues for GPMNB and observed that the majority of GPMNB-positive cells are macrophages, and only a few melanoma cells express GPMNB. Similar results were also observed in breast cancer tissues (Figure 5C, 5F). This is consistent with the report (Rose et al., 2010) that 70% of GPMNB-positive cells are located in the stroma, and only 10% of GPMNB-positive cells are breast cancer cells. The inhibitory effect of treatment with an anti-GPMNB antibody on melanoma tumor growth in vivo may be explained by the fact that it targets both TAMs in the tumor stroma and tumor cells. Furthermore, Solinas et al. (2010) also found in their microarray analysis that GPMNB is expressed in PCMI-Mϕ (Solinas et al., 2010). Our data support the invasive signature of TAMs induced from MCMI-Mϕ. Future work is underway to dissect the function and gene regulation of this molecule in TAMs.
Materials and Methods
Differentiation of human monocytes to macrophages
In conformance with institutional policies regarding human experimentation, enriched monocytes were obtained from healthy volunteers by leukapheresis followed by countercurrent elutriation (AIDS Research Human Immunology Core at the University of Pennsylvania). Monocyte purity was >94% as confirmed by FACS analysis (Becton Dickinson). To produce the modified MCM, C8161 and 1205Lu melanoma cells were seeded in 10 cm plates at 50% confluence and were then cultured in melanoma media supplemented with 2% FBS for 3 days. MCM was harvested and concentrated 40-fold using Centricon concentrators (Millipore). Concentrated media were added to complete RPMI 1640 medium (R10 medium, RPMI, 10% FBS, 10 mM HEPES, 100 µM 2-mercaptoethanol, 100 IU penicillin G and 100 µg/ml streptomycin) at a 1:80 ratio to make the modified MCM.
For MCMI-Mϕ differentiation, 2×106 monocytes were seeded in tissue-culture treated 6-well plates (BD-Falcon) and were incubated in the presence of MCM derived from 1205Lu or C8161 melanoma cells for 7 days at 37°C in a humidified atmosphere of 5% CO2. 50% of media was changed in each plate on day 3. The supernatants were harvested for the detection of cytokines and chemokines.
To generate M1-Mϕ, M2-Mϕ and dendritic cells (DCs), monocytes were incubated for 7 days in the presence of GM-CSF (10 ng/mL), M-CSF (10 ng/mL) or GM-CSF plus IL-4 (10 ng/mL, R&D Systems) in R10 medium for 7 days, respectively. 50% of media was changed in each plate on day 3. For the M-CSF blocking experiment, monocytes were incubated in the presence of C8161 MCM and 1205Lu MCM with anti-human M-CSF (R&D Systems, 10 µg/ml) for 7 days.
Flow cytometric analysis
The following fluorescence conjugated antibodies were used for cell surface staining: anti-Ig mouse isotype control, anti-CD14, anti-CD68, anti-CD163, anti-CD11b, anti-CD115 and anti-CD11d (all from Biolegend). Cells were acquired using a FACSCalibur and data were analyzed by FlowJo software.
Multiplex Cell Signaling Bead-Based Luminex Assays
The production of M-CSF, CCL2, IL-6, LIF, VEGFA and GM-CSF from MCM, and of TNF-α, IL-12, IL-10, CCL1, CCL2, CXCL5 and CCL8 from 1205Lu- MCMI-Mϕ (1205Lu-Mϕ) and C8161- MCMI-Mϕ (C8161-Mϕ), was measured using the customized MILLIPLEX MAP Cytokine Kit according to the manufacturer's protocol (Millipore). Median fluorescence intensity (MFI) was calculated from duplicates of each sample. Samples were analyzed using the Bio-Plex suspension array system (Bio-Rad Laboratories).
Microarray data generation and analysis
Total RNAs were extracted using the TRizol reagent (Invitrogen) from monocytes (duplicate) and from C8161-Mϕ (triplicate). cDNAs were generated, fragmented, biotinylated and hybridized to the Illumina HumanHT-12V4 expression Beadchip Arrays (Illumina). The detailed microarray data analysis procedure is noted in Supplementary Materials and Methods.
Inhibition of T-cell proliferation in co-culture assays
The ability of MCMI-Mϕ to inhibit anti-CD3 induced T-cell proliferation was determined using a co-culture assay as described before (Somasundaram et al., 2002). Briefly, inhibition of proliferation of anti-melanoma reactive CTL793, and 35Th1 (5 × 104) were determined by co-culturing T cells in the presence or absence of 1205Lu-Mϕ at various ratios. The proliferation of T cells was determined using a standard 3H-TdR incorporation assay and the % inhibition of T cell proliferation was determined as described before (Somasundaram et al., 2002).
Real-time RT-PCR
For RT reactions, 1 µg DNA-free RNA was used with oligo(dT) primers and Superscript reverse transcriptase. Transcripts of the housekeeping gene GAPDH in the same incubations were used for normalization. Oligonucleotides specific for CCL2, CCL8, CXCL5, CCL7, MMP7, MMP-9, GPMNB and GAPDH are listed in Table S1. The primers were designed according to the Roche software for quantitative real-time PCR (Roche).
Immunoblotting of MMP-9 and GPMNB
M2-Mϕ, 1205Lu-Mϕ and C8161-Mϕ were harvested and incubated in the presence of R10 medium for 2 additional days. Conditioned media were harvested and subjected to 10% SDS-PAGE electrophoresis. After the protein transfer, PDVF membranes were blocked and incubated with anti–MMP-9 or anti-GPMNB antibodies. The signals were visualized with enhanced chemiluminescence reagents (Amersham Biosciences).
Gelatin zymography
The same samples mentioned above were subjected to electrophoresis with 10% Novex® Zymogram Gels (Invitrogen). After renaturing and developing the gels according to the manufacturer’s instructions, they were stained with Collide Blue Stain Reagent (Invitrogen).
Invasion assay
The invasion assay was conducted with 24-well Transwell inserts (8 µm pore size; Corning). For the MCMI-Mϕ induced invasion assay, 4×105 1205Lu melanoma cells in 2% FBS RPMI1640 medium were added in the upper chamber pre-coated with 50 µl Matrigel (1:3 dilution, BD Biosciences). Media from 1205Lu-Mϕ were added to the lower chamber, and R10 medium was used as a control. After overnight incubation, cells that had invaded were fixed and stained with Diff-Quick staining kit (ThermoFisher). The stained cells in 4 randomly chosen fields were counted for each insert (200×). For the blocking assay, either anti-CCL2 (20 µg/ml, R&D Systems) or the MMPs inhibitor, GM6001 (10 µM, EMD Biosciences), or both combined were added to the Transwell.
Human tumor samples, immunohistochemistry and immunofluorescence
Formalin-fixed, paraffin-embedded human melanoma tumors and human breast cancer tumors were obtained from the University of Pennsylvania and from Nanjing Medical University, under an approved Institutional Review Board protocol. See the detailed staining protocol in Supplementary Materials and Methods.
Supplementary Material
Significance.
The phenotype and biological significance of macrophages in melanoma progression remain poorly characterized, especially in humans. In this study, we develop a novel method to consistently differentiate human monocytes to macrophages using melanoma-conditioned media. These macrophages share many characteristics with tumor-associated macrophages. Importantly, by using these induced macrophages, we demonstrate that combinations of blocking both CCL2 and matrix metalloproteases are able to inhibit macrophage-induced melanoma invasion. Finally, we identify GPMNB as a novel marker for TAMs. These findings provide new insights into the roles of TAMs in melanoma progression and metastasis, and the potential for targeting TAMs as novel therapeutic strategies for melanoma progression.
Acknowledgments
We thank Luis Montaner and Jose Conejo-Garcia for discussions and manuscript review; the Wistar Institute Cancer Center Flow Cytometry Core Facility for helping with instrument setup and data analysis; and the Microscopy Core Facility for imaging. We thank the Bioinformatics Core Facility for Microarray analysis and Research Supply Center for research reagents.
This work was supported by grants from the National Institutes of Health (5P30CA 010815-42), the Commonwealth Universal Research Enhancement Program of the Pennsylvania Department of Health (R.E.K), the W. W. Smith foundation, Wistar Institute Intramural grants for R.E.K and T.W., and by National Institutes of Health grants for M.H. (CA047159, CA025874, CA114046).
Footnotes
Authorship
Contribution: T.W., designed, performed research and drafted the manuscript; Y.G, A.L-C., M.X., R.A., R.S., performed experiments; Z.W and G.Z. conducted microarray analysis. X.X. provided melanoma tissues. M.H. helped organize the manuscript. R.E.K. designed the research and drafted the manuscript.
No potential conflicts of interest were disclosed.
References
- Bennicelli JL, Guerry DT. Production of multiple cytokines by cultured human melanomas. Exp Dermatol. 1993;2:186–190. doi: 10.1111/j.1600-0625.1993.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Bernengo MG, Quaglino P, Cappello N, Lisa F, Osella-Abate S, Fierro MT. Macrophage-mediated immunostimulation modulates therapeutic efficacy of interleukin-2 based chemoimmunotherapy in advanced metastatic melanoma patients. Melanoma Res. 2000;10:55–65. [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- Brocker EB, Zwadlo G, Holzmann B, Macher E, Sorg C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer. 1988;41:562–567. doi: 10.1002/ijc.2910410415. [DOI] [PubMed] [Google Scholar]
- Brocker EB, Zwadlo G, Suter L, Brune M, Sorg C. Infiltration of primary and metastatic melanomas with macrophages of the 25F9-positive phenotype. Cancer Immunol Immunother. 1987;25:81–86. doi: 10.1007/BF00199945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112:3455–3464. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–4330. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- Facchetti F, Bertalot G, Grigolato PG. KP1 (CD 68) staining of malignant melanomas. Histopathology. 1991;19:141–145. doi: 10.1111/j.1365-2559.1991.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Gazzaniga S, Bravo AI, Guglielmotti A, Van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- Ilkovitch D, Lopez DM. Immune modulation by melanoma-derived factors. Exp Dermatol. 2008;17:977–985. doi: 10.1111/j.1600-0625.2008.00779.x. [DOI] [PubMed] [Google Scholar]
- Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, Sjoegren P, Christensen IJ, Steiniche T. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27:3330–3337. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, Nakazawa T, Anderson P, Kiessling R. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26:1308–1313. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- Kuan CT, Wakiya K, Keir ST, Li J, Herndon JE, 2nd, Pastan I, Bigner DD. Affinity-matured anti-glycoprotein NMB recombinant immunotoxins targeting malignant gliomas and melanomas. Int J Cancer. 2011;129:111–121. doi: 10.1002/ijc.25645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]
- Makitie T, Summanen P, Tarkkanen A, Kivela T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1414–1421. [PubMed] [Google Scholar]
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Marconi C, Bianchini F, Mannini A, Mugnai G, Ruggieri S, Calorini L. Tumoral and macrophage uPAR and MMP-9 contribute to the invasiveness of B16 murine melanoma cells. Clin Exp Metastasis. 2008;25:225–231. doi: 10.1007/s10585-007-9136-0. [DOI] [PubMed] [Google Scholar]
- Metz RL, Patel PS, Hameed M, Bryan M, Rameshwar P. Role of human HGFIN/nmb in breast cancer. Breast Cancer Res. 2007;9:R58. doi: 10.1186/bcr1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, Pasquali C, Calori G, Pessi F, Sperti C, et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451–7461. [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Nikawa T, Furochi H, Kosyoji M, Hirasaka K, Suzue N, Sairyo K, Nakano S, Yamaoka T, Itakura M, et al. Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am J Physiol Cell Physiol. 2005;289:C697–C707. doi: 10.1152/ajpcell.00565.2004. [DOI] [PubMed] [Google Scholar]
- Onaga M, Ido A, Hasuike S, Uto H, Moriuchi A, Nagata K, Hori T, Hayash K, Tsubouchi H. Osteoactivin expressed during cirrhosis development in rats fed a choline-deficient, L-amino acid-defined diet, accelerates motility of hepatoma cells. J Hepatol. 2003;39:779–785. doi: 10.1016/s0168-8278(03)00361-1. [DOI] [PubMed] [Google Scholar]
- Paglia D, Oran A, Lu C, Kerbel RS, Sauder DN, Mckenzie RC. Expression of leukemia inhibitory factor and interleukin-11 by human melanoma cell lines: LIF, IL-6, and IL-11 are not coregulated. J Interferon Cytokine Res. 1995;15:455–460. doi: 10.1089/jir.1995.15.455. [DOI] [PubMed] [Google Scholar]
- Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- Porta C, Subhra Kumar B, Larghi P, Rubino L, Mancino A, Sica A. Tumor promotion by tumor-associated macrophages. Adv Exp Med Biol. 2007;604:67–86. doi: 10.1007/978-0-387-69116-9_5. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich JN, Shi Q, Hjelmeland M, Cummings TJ, Kuan CT, Bigner DD, Counter CM, Wang XF. Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model. J Biol Chem. 2003;278:15951–15957. doi: 10.1074/jbc.M211498200. [DOI] [PubMed] [Google Scholar]
- Richmond A, Yang J, Su Y. The good and the bad of chemokines/chemokine receptors in melanoma. Pigment Cell Melanoma Res. 2009;22:175–186. doi: 10.1111/j.1755-148X.2009.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA. GPMNB is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol. 2007;178:6557–6566. doi: 10.4049/jimmunol.178.10.6557. [DOI] [PubMed] [Google Scholar]
- Robinson SC, Scott KA, Balkwill FR. Chemokine stimulation of monocyte matrix metalloproteinase-9 requires endogenous TNF-alpha. Eur J Immunol. 2002;32:404–412. doi: 10.1002/1521-4141(200202)32:2<404::AID-IMMU404>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Rose AA, Grosset AA, Dong Z, Russo C, Macdonald PA, Bertos NR, St-Pierre Y, Simantov R, Hallett M, Park M, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res. 2010;16:2147–2156. doi: 10.1158/1078-0432.CCR-09-1611. [DOI] [PubMed] [Google Scholar]
- Rose AA, Siegel PM. Emerging therapeutic targets in breast cancer bone metastasis. Future Oncol. 2010;6:55–74. doi: 10.2217/fon.09.138. [DOI] [PubMed] [Google Scholar]
- Selim AA, Abdelmagid SM, Kanaan RA, Smock SL, Owen TA, Popoff SN, Safadi FF. Anti-osteoactivin antibody inhibits osteoblast differentiation and function in vitro. Crit Rev Eukaryot Gene Expr. 2003;13:265–275. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.180. [DOI] [PubMed] [Google Scholar]
- Sheng MH, Wergedal JE, Mohan S, Lau KH. Osteoactivin is a novel osteoclastic protein and plays a key role in osteoclast differentiation and activity. FEBS Lett. 2008;582:1451–1458. doi: 10.1016/j.febslet.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Sierra-Filardi E, Puig-Kroger A, Blanco FJ, Nieto C, Bragado R, Palomero MI, Bernabeu C, Vega MA, Corbi AL. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood. 2011;117:5092–5101. doi: 10.1182/blood-2010-09-306993. [DOI] [PubMed] [Google Scholar]
- Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–652. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- Somasundaram R, Jacob L, Swoboda R, Caputo L, Song H, Basak S, Monos D, Peritt D, Marincola F, Cai D, et al. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res. 2002;62:5267–5272. [PubMed] [Google Scholar]
- Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011;187:3671–3682. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- Tomihari M, Chung JS, Akiyoshi H, Cruz PD, Jr, Ariizumi K. DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells. Cancer Res. 2010;70:5778–5787. doi: 10.1158/0008-5472.CAN-09-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- Tse KF, Jeffers M, Pollack VA, Mccabe DA, Shadish ML, Khramtsov NV, Hackett CS, Shenoy SG, Kuang B, Boldog FL, et al. CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res. 2006;12:1373–1382. doi: 10.1158/1078-0432.CCR-05-2018. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S, Nathan CF. Macrophage deactivation. Altered kinetic properties of superoxide-producing enzyme after exposure to tumor cell-conditioned medium. J Exp Med. 1986;164:1319–1331. doi: 10.1084/jem.164.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–1144. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- Varney ML, Johansson SL, Singh RK. Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma: role of monocyte chemotactic protein-1 and vascular endothelial growth factor-A. Melanoma Res. 2005;15:417–425. doi: 10.1097/00008390-200510000-00010. [DOI] [PubMed] [Google Scholar]
- Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weterman MA, Ajubi N, Van Dinter IM, Degen WG, Van Muijen GN, Ruitter DJ, Bloemers HP. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- Williams MD, Esmaeli B, Soheili A, Simantov R, Gombos DS, Bedikian AY, Hwu P. GPMNB expression in uveal melanoma: a potential for targeted therapy. Melanoma Res. 2010;20:184–190. doi: 10.1097/CMR.0b013e3283364a08. [DOI] [PubMed] [Google Scholar]
- Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, Feigenbaum L, Fuchs E, Lyakh L, Young HA, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469:548–553. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.