Abstract
The increased obstetric risks of maternal obesity have been well described. These include increased risks of gestational diabetes mellitus, preeclampsia, stillbirth, and cesarean delivery. The fetal/neonatal consequences of prenatal maternal obesity have received less attention. In addition to an increased risk of stillbirth, the fetal/neonatal consequences include increased adiposity and a metabolic status that increases the lifetime risk of obesity and diabetes. This review focuses on the clinical obstetric consequences of maternal obesity and highlights recent mechanistic insights on fetal programming as well as evidence suggesting that prenatal care provides a unique opportunity to ameliorate these risks and decrease the cycle of childhood obesity.
Keywords: obesity, pregnancy, high fat diet, placenta, programming
The Obesity Epidemic
Obesity is a worldwide health epidemic and a major contributor to the increased occurrence of coronary heart disease, hypertension, and type 2 diabetes mellitus.1–3 An even more disturbing trend is the dramatic increase in metabolic disease among children including infants. According to recent studies, 12% of children are in the 97th percentile for weight, 17% are in the 95th percentile, and 32% are in the 85% percentile.4 Of greatest concern, almost 10% of infants are above the 95th percentile of body weight.4 Unfortunately, being obese in early childhood strongly predicts a lifetime of health problems in adults including cardiovascular disease and diabetes.5–8 The converse is also true. Infants born small for gestational age also increases the risk of lifelong metabolic complications including coronary artery disease, diabetes, and obesity.9–13 Because the obese gravidae is at increased risk for both small and large infants, the potential neonatal/childhood consequences in this obstetric population are profound.14,15
The increased prevalence of obesity complicating pregnancy is a direct consequence of the global obesity epidemic.16 Recent data from the Centers of Disease Control and Prevention suggest that 20% of women are obese at the start of pregnancy and that the prevalence of obesity in reproductive-age women is 30%.16 Prepregnancy maternal obesity, defined as a body mass index (BMI) of 30 kg/m2, confers an increased risk of fetal growth abnormalities, intrauterine growth restriction and macrosomia, gestational diabetes mellitus, preeclampsia, and fetal death.17–21 The mechanisms underlying these increased obstetric risks are not well understood.
Maternal obesity and diabetes, and nutritional status during pregnancy and lactation have profound long-term effects on the systems that regulate energy balance in offspring.22 The dramatic increase in juvenile obesity and diabetes is often attributed to an increase in calorically dense diets and reduced physical exercise in children. However, mounting evidence in humans and animals models indicates that early programming events also significantly contribute to this epidemic. Clinical and rodent studies have demonstrated that both pregnancy (i.e., maternal nutrition or gestational diabetes mellitus (GDM)) and early postnatal (i.e., diet and energy availability) environments significantly influence body weight and energy homeostasis in adulthood.23–28 Specifically, there is a high rate of infant morbidity and mortality associated with GDM, and infants from mothers with GDM are frequently born with macrosomia, impaired glucose tolerance, and a much greater risk of growing up to be obese and diabetic.29–32 The impaired glucose tolerance in the infants is likely due to insulin resistance in target tissues as well as defects in the pancreas. The maternal factors that cause the metabolic disorders in the offspring are unknown, but likely ones include maternal hyperinsulinemia, excess nutrients (fatty acid/triglycerides and glucose), and/or changes in placental function (blood flow and nutrient transport). Considering the prevalence of obese and overweight adult women, maternal obesity and poor nutrition may be the most common health concern experienced by the developing fetus.
Obesity and Inflammation
The search for a unifying mechanism in the spectrum of obesity-associated diseases such as diabetes, hypertension, and hyperlipidemia links nutrient excess with abnormalities in the mediators of inflammation.33 This chronic low-grade inflammatory response to obesity has been termed meta-inflammation.34 This meta-inflammation negatively affects organs systemically including the brain, pancreas, adipose tissue, and skeletal muscle resulting in dysregulation of metabolic homeostasis, ultimately resulting in what is referred to as the metabolic syndrome.33 The initiating trigger for this meta-inflammation and its link to insulin resistance are areas of active investigation and beyond the scope of this review. In the nonpregnant state, obesity is associated with increased production of proinflammatory cytokines.35,36 Similarly, obese gravidae demonstrate increased inflammatory cytokines, insulin, and lipids when compared with lean gravidae.29,37,38 The normal physiological inflammation and insulin resistance of pregnancy exacerbates this chronic low-grade inflammation.39 Furthermore, increased postprandial cytokines in response to high-fat meals may further exacerbate the overall inflammatory state.33,40,41
Inflammation has detrimental effects on insulin secretion, insulin sensitivity, and lipid metabolism. Inflammation decreases pancreatic islet cell mass and triggers beta cell apoptosis, and it reduces insulin secretion.33,42,43 Obesity in both animals and adults is associated with expansion of both adipocytes and adipocyte proinflammatory macrophages.44 This adipose tissue inflammation is so robust that an excess 20 to 30 million macrophages are estimated to accumulate with each kilogram of excess fat in humans.33 The adipose tissue is metabolically active and secretes proinflammatory cytokines, such as tumor necrosis factor (TNF)α and interleukin (IL)-8, which further contribute to peripheral insulin resistance.34,45,46 Insulin typically stimulates storage of lipid into fat; however, the inflamed adipose tissue is less responsive to insulin resulting in elevated levels of free fatty acids (FFAs).44,47 The elevated circulating FFAs may directly stimulate the local and systemic inflammatory response by binding to innate immune receptors, such as toll-like receptor (TLR)4, which in turn activates nuclear factor-κB, a robust proinflammatory transcription factor.48–50 Skeletal muscle infiltration with activated macrophages and lipid accumulation further contributes to the inflammation and insulin resistance in obesity.51,52
In addition to changes in insulin sensitivity, lipid metabolism in the obese gravidae differs from her lean counterparts. In the first and early second trimester of pregnancy, lean women increase their lipid stores more than obese women.53 This discrepancy may be secondary to increased insulin resistance in obese gravidae resulting in decreased lipid uptake and lipogenesis. An alternative explanation is an inability of obese women to expand their hypertrophic adipocytes like their lean counterparts. On the surface, this may appear beneficial because it might limit gestational weight gain, but adipose tissue is critical for storing the excess intake of lipids. Otherwise this leads to increased lipolysis, increased systemic FFAs, and ectopic fat deposition in other organs like the liver, skeletal muscle, and the developing fetus that lead to other metabolic complications.53,54 The FFAs, in combination with elevated triglycerides and cholesterol, contribute to the increased oxidative stress seen in obesity.55 Furthermore, the hyperlipidemia may act both directly and indirectly on the vasculature contributing to the vascular dysfunction seen in obesity.37,56,57
In summary, obesity is associated with a metabolic environment characterized by multiorgan inflammation, insulin resistance, hyperlipidemia, and vascular dysfunction. All of these factors, unfortunately, pose significant challenges to the developing fetoplacental unit.
Obesity, Placental Function, and Fetal Growth
In the metabolic environment of the obese gravidae, the fetoplacental unit develops under conditions of both excess nutrients and inflammation. Because the placenta regulates nutrient flow from mother to fetus, it likely occupies a central role in mediating the adverse obstetric risks associated with pregnancy. The placenta is the primary organ for nutrient exchange during pregnancy, and abnormal placental development has been associated with virtually every adverse obstetric outcome including abnormalities in fetal growth, preeclampsia, preterm labor, and stillbirth.58–61 Diseases associated with obesity that also increase these risks include diabetes, hypertension, and preeclampsia. However, obese human gravidae demonstrate metabolic, inflammatory, and vascular abnormalities even when there are no associated medical conditions, suggesting that obesity by itself is a contributor to these adverse obstetric outcomes.35 The mechanisms underlying these increased risks are incompletely understood, but insights from both animal models and humans suggest placental inflammation and placental dysfunction as possible contributing factors.
Rodent studies of a high-fat diet (HFD) during pregnancy have reported variable results on fetal birthweight including no effect, decreased birthweight, and increased birthweight.62–69 A recent rat study of chronic administration of a HFD both before and during pregnancy resulted in decreased fetal birthweight, which suggests some degree of placental insufficiency.66 Another recent rat study demonstrated that a maternal HFD reduced both fetal growth and growth of the placental junctional zone, again suggesting that abnormalities in placental development secondary to a HFD may contribute to aberrant fetal growth.70 Direct comparisons among these studies are limited by the varying compositions of the HFD and the duration of exposure. The rodent studies are also limited by their short gestation relative to primates and the structural differences in their placentas when compared with primate placentas.71,72 However, these rodent studies are consistent with sheep studies of excess nutrition. In a well-characterized sheep model of acute excess nutrition during pregnancy, overnourished ewes had a significant reduction of uterine blood flow at midgestation compared with controls.73 By late gestation in this sheep model, both uterine and umbilical blood flows were reduced.74 The placentas in this adolescent sheep model demonstrate reduced capillary density and decreased placental mass that may explain the reductions in uterine and umbilical blood flow.75 Although the fetuses of the overnourished ewes weighed more in midgestation, they were reduced by 20% compared with controls by late gestation, suggesting that the consequences of decreased uteroplacental perfusion may not be seen until later in gestation when the fetal nutrient demands exceed the placental capabilities.75 The sheep placenta is markedly different in structure from the primate placenta, so direct comparisons with humans is not possible.71,76
Despite these species differences, the variation in fetal weight, both large and small, in the animal studies of excess nutrition is consistent with the observed human data. Obese women have increased rates of both fetal macrosomia and growth-restricted infants.14,15 Although both these fetal conditions are associated with risks of childhood metabolic disease, neonatal fat mass and insulin resistance may be more important mediators of childhood obesity and metabolic dysfunction.
Obesity in pregnancy is associated with an increase in proinflammatory mediators and nitrosative stress in the human placenta.77,78 Although the data are limited, evidence suggests that inflammatory cytokines can alter maternal nutrient transport. IL-6 and TNF-α stimulate system A amino acid transport in cytotrophoblasts; IL-1β inhibits this same system.79,80 A murine study also demonstrated that a HFD both before and during pregnancy upregulated placental nutrient transport suggesting alterations in placental nutrient transport may contribute to fetal overgrowth.81 The mechanisms whereby the placenta senses maternal nutrient availability and the mediators of alterations in placental nutritional transport is an understudied area that merits significant investigation.
The Role of Nutrition on the Risk of Childhood Obesity and Metabolic Disease: Insights from the Nonhuman Primate
In humans, maternal obesity is often associated with consumption of a HFD, and a HFD is used to promote maternal obesity in most animal models. Therefore, a major challenge faced by the obstetric field is the ability to separate the effects of the HFD from the maternal metabolic phenotype. The nonhuman primate (NHP) shares developmental ontogeny similar to human fetuses including placental function, brain development, and the full spectrum of metabolic disease when placed on a HFD. Recent work from our NHP model of excess nutrition in pregnancy provides insight into the contributions of nutrition, independent of obesity, on placental function, pregnancy complications, and fetal metabolic disorders.
Briefly, in our model of obesity, young adult female macaques are maintained on chow or a HFD (up to 6 years).82 The HFD is high in saturated and monounsaturated fatty acids; however, polyunsaturated fatty acid levels are comparable between the two diets. The adults on the HFD segregate into either a diet sensitive (obese and insulin resistant) or diet resistant (normal adiposity and insulin sensitivity).82,83 Thus this model allows us to distinguish the relative impact of maternal diet versus the maternal metabolic phenotype.
During pregnancy in HFD animals, in the maternal and fetal circulation, fasting levels of n3 fatty acids are low while there is a significant increase in the proinflammatory n6:n3 ratio.84 Fasting saturated fatty acid levels are not different; however, maternal postprandial levels are significantly elevated in HFD females.84 The excess systemic lipids may increase substrate availability of FFAs for placental transport resulting in a fetal lipid profile that mirrors the maternal lipid profile.84
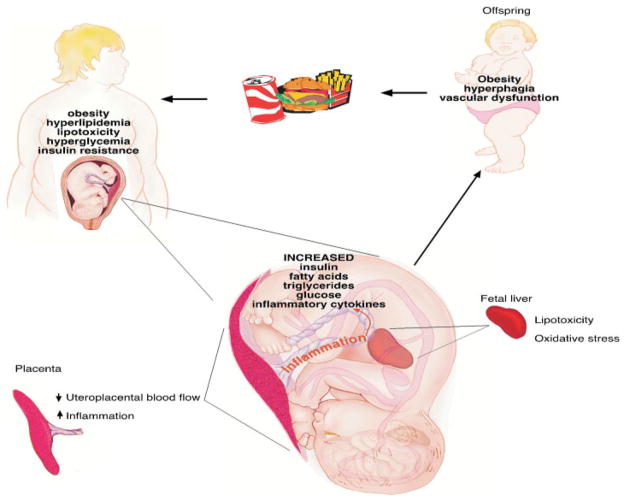
Our published studies using this model have demonstrated that consumption of a high-fat/calorie (Western-style) diet during pregnancy, in the absence of obesity or diabetes, causes damage and reprogramming in the liver, brain, pancreas, and placenta.82,83,85,86 We have demonstrated that maternal HFD consumption results in increased liver triglycerides and oxidative damage in the fetal offspring and that this effect persists into the postnatal period.82 These same fetal offspring have increased cytokine expression in the hypothalamus along with abnormal development of the melanocortin and serotonin systems, which are important for the regulation of food intake and glucose homeostasis.85,86 Furthermore, our studies suggest that a HFD diminishes uterine blood flow and that obesity with a HFD causes placental ischemia and decreases placental blood flow resulting in an increased risk of stillbirth.83 The inflammation in the fetal brain, liver, and placenta is secondary to a HFD and independent of maternal obesity. During the postnatal period the HFD offspring, independent of maternal obesity, have accelerated weight gain and increased adiposity and glucose intolerance; again suggesting that diet alone may cause lipotoxicity in the developing fetus and may be a major contributor to adverse obstetric and childhood outcomes.82 Finally, juvenile offspring of HFD mothers, both lean and obese, display increased intimal thickening in the vasculature and demonstrate impaired endothelial function, manifesting as depressed endothelium-dependent vasorelaxation (Grove, unpublished data). In summary, maternal HFD consumption, independent of obesity, impacts both placental function and the developing fetus, resulting in offspring that are predisposed to abnormalities in metabolic homeostasis and cardiovascular dysfunction destined to repeat the cycle (Fig. 1).
Figure 1.
Maternal obesity and high-fat diet (HFD) consumption have an impact on the developing placenta and fetus. The fetus and placenta from a HFD-consuming mother experiences an environment characterized by elevated levels of glucose, insulin, fatty acids, triglycerides, and inflammatory cytokines. This environment leads to changes in placenta function/transport that result in systemic fetal inflammation, hyperinsulinemia, hyperlipidemia, and lipotoxicity, resulting in offspring at increased risk for obesity, diabetes, and vascular dysfunction.
Nutrition and the Transgenerational Problem: How Do We Break the Cycle?
One of the goals of prenatal care is to optimize maternal health so that we can ensure a healthy pregnancy and newborn. Because approximately two thirds of pregnant women in the United States are currently overweight or obese at the time of conception, how can we modify the adverse obstetric outcomes associated with obesity?16 As a result of the prevalence of obesity in reproductive-age women, obesity may be a greater contributor to perpetuating the obesity epidemic than diabetes.44,87 How can we break the cycle?
Recent studies on nutrition converge on a powerful solution: better prenatal nutrition. A murine study recently demonstrated that maternal diet-induced obesity increased mitochondrial oxidative stress in both mouse oocytes and zygotes suggesting prenatal mitochondrial injury.88 Our own NHP studies provide evidence of multiple metabolic perturbations to the placenta, fetus, and neonate directly related to a high-fat diet, independent of obesity.82–86 A recent rat study implicated chronic high-fat diet in fathers with pancreatic β-cell dysfunction of female offspring.89 While we await determination of the optimal nutritional strategy to ensure fetal health, a worthy interim solution is to actively promote lifestyle changes prior to and during pregnancy that include a nutrient-dense diet low in saturated fats. If the pregnancy is used as a commitment device to alter diet, perhaps we can impact not only fetal but childhood health. Fostering better nutrition during pregnancy may modify postnatal nutritional choices. Ongoing experiments in our laboratory and others will seek to determine whether additional nutritional supplements can mitigate some of the deleterious effects of a HFD on the placenta and developing fetus.
Future areas of investigation require research innovation in both the basic and clinical sciences. First, improved methods to stratify obese patients at risk for adverse obstetric and neonatal/childhood complications need to be developed.
Such a strategy will require significant improvement in diagnostic methods including both physiological biomarkers of disease as well as improved imaging modalities of placental function. A lack of data is present in terms of assessing placental function in the obese gravidae. The improved diagnostic tools will require implementation of better strategies than the deficient BMI to define maternal body composition. Second, investigation of placental lipid transport and how both uteroplacental perfusion and the obesogenic maternal environment modify it is necessary. Again, such a strategy will require the development of improved imaging methods to connect in vivo function with transport. Third, resource development is needed to identify strategies and provide infrastructure so that prenatal nutrition education is available to all pregnant women as well as access to better food choices.
Acknowledgments
This work was funded by National Institutes of Health grants R24 DK0909640–01, R01 DK-79194, P51 RR00163, P01 HD34430, and WRHR K12 HD001243–10.
References
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404 (6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282(16):1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 5.Caprio S. Relationship between abdominal visceral fat and metabolic risk factors in obese adolescents. Am J Hum Biol. 1999;11(2):259–266. doi: 10.1002/(SICI)1520-6300(1999)11:2<259::AID-AJHB13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Dietz WH, Bellizzi MC. Introduction: the use of body mass index to assess obesity in children. Am J Clin Nutr. 1999;70(1):123S–125S. doi: 10.1093/ajcn/70.1.123s. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics. 2005;115(6):1623–1630. doi: 10.1542/peds.2004-2588. [DOI] [PubMed] [Google Scholar]
- 8.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1 (8489):1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 11.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 12.Barker DJ, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11 (4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 14.Perlow JH, Morgan MA, Montgomery D, Towers CV, Porto M. Perinatal outcome in pregnancy complicated by massive obesity. Am J Obstet Gynecol. 1992;167(4 Pt 1):958–962. doi: 10.1016/s0002-9378(12)80019-6. [DOI] [PubMed] [Google Scholar]
- 15.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964–968. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 16.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 2007;15(4):986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 17.Nohr EA, Vaeth M, Bech BH, Henriksen TB, Cnattingius S, Olsen J. Maternal obesity and neonatal mortality according to subtypes of preterm birth. Obstet Gynecol. 2007;110(5):1083–1090. doi: 10.1097/01.AOG.0000286760.46679.f8. [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189(6):1698–1704. doi: 10.1016/s0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- 19.Cnattingius S, Bergström R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338(3):147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. 2005;112(4):403–408. doi: 10.1111/j.1471-0528.2005.00437.x. [DOI] [PubMed] [Google Scholar]
- 21.Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, Olsen J. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet Gynecol. 2005;106(2):250–259. doi: 10.1097/01.AOG.0000172422.81496.57. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan EL, Grove KL. Metabolic imprinting in obesity. Forum Nutr. 2010;63:186–194. doi: 10.1159/000264406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strauss RS, Dietz WH. Effects of intrauterine growth retardation in premature infants on early childhood growth. J Pediatr. 1997;130(1):95–102. doi: 10.1016/s0022-3476(97)70316-0. [DOI] [PubMed] [Google Scholar]
- 24.Ozanne SE, Hales CN. The long-term consequences of intra-uterine protein malnutrition for glucose metabolism. Proc Nutr Soc. 1999;58(3):615–619. doi: 10.1017/s0029665199000804. [DOI] [PubMed] [Google Scholar]
- 25.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000;72(5):1101–1106. doi: 10.1093/ajcn/72.5.1101. [DOI] [PubMed] [Google Scholar]
- 26.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50.y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70(5):811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 27.Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351 (9097):173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 28.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 29.Silverman BL, Landsberg L, Metzger BE. Fetal hyperinsulinism in offspring of diabetic mothers. Association with the subsequent development of childhood obesity. Ann N Y Acad Sci. 1993;699:36–45. doi: 10.1111/j.1749-6632.1993.tb18835.x. [DOI] [PubMed] [Google Scholar]
- 30.Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord. 1997;21(6):451–456. doi: 10.1038/sj.ijo.0800429. [DOI] [PubMed] [Google Scholar]
- 31.Garcia Carrapato MR. The offspring of gestational diabetes. J Perinat Med. 2003;31(1):5–11. doi: 10.1515/JPM.2003.001. [DOI] [PubMed] [Google Scholar]
- 32.Wilkin TJ, Metcalf BS, Murphy MJ, Kirkby J, Jeffery AN, Voss LD. The relative contributions of birth weight, weight change, and current weight to insulin resistance in contemporary 5-year-olds: the EarlyBird Study. Diabetes. 2002;51(12):3468–3472. doi: 10.2337/diabetes.51.12.3468. [DOI] [PubMed] [Google Scholar]
- 33.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 35.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87(9):4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. 2007;92(3):969–975. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- 38.Madan JC, Davis JM, Craig WY, et al. Maternal obesity and markers of inflammation in pregnancy. Cytokine. 2009;47(1):61–64. doi: 10.1016/j.cyto.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Okereke NC, Huston-Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2004;287(3):E472–E479. doi: 10.1152/ajpendo.00589.2003. [DOI] [PubMed] [Google Scholar]
- 40.Blackburn P, Després JP, Lamarche B, et al. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring) 2006;14(10):1747–1754. doi: 10.1038/oby.2006.201. [DOI] [PubMed] [Google Scholar]
- 41.Alipour A, Elte JW, van Zaanen HC, Rietveld AP, Cabezas MC. Postprandial inflammation and endothelial dysfunction. Biochem Soc Trans. 2007;35(Pt 3):466–469. doi: 10.1042/BST0350466. [DOI] [PubMed] [Google Scholar]
- 42.Donath MY, Böni-Schnetzler M, Ellingsgaard H, Halban PA, Ehses JA. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol Metab. 2010;21(5):261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Ehses JA, Böni-Schnetzler M, Faulenbach M, Donath MY. Macrophages, cytokines and beta-cell death in Type 2 diabetes. Biochem Soc Trans. 2008;36(Pt 3):340–342. doi: 10.1042/BST0360340. [DOI] [PubMed] [Google Scholar]
- 44.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R711–R722. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 46.Straczkowski M, Dzienis-Straczkowska S, Stêpieñ A, Kowalska I, Szelachowska M, Kinalska I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J Clin Endocrinol Metab. 2002;87 (10):4602–4606. doi: 10.1210/jc.2002-020135. [DOI] [PubMed] [Google Scholar]
- 47.Chen YD, Golay A, Swislocki AL, Reaven GM. Resistance to insulin suppression of plasma free fatty acid concentrations and insulin stimulation of glucose uptake in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64(1):17–21. doi: 10.1210/jcem-64-1-17. [DOI] [PubMed] [Google Scholar]
- 48.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276 (20):16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 50.Laine PS, Schwartz EA, Wang Y, et al. Palmitic acid induces IP-10 expression in human macrophages via NF-kappaB activation. Biochem Biophys Res Commun. 2007;358(1):150–155. doi: 10.1016/j.bbrc.2007.04.092. [DOI] [PubMed] [Google Scholar]
- 51.Hong EG, Ko HJ, Cho YR, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58(11):2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97(4):1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehrenberg HM, Huston-Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol. 2003;189(4):944–948. doi: 10.1067/s0002-9378(03)00761-0. [DOI] [PubMed] [Google Scholar]
- 54.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. 2003;189(5):1423–1432. doi: 10.1067/s0002-9378(03)00596-9. [DOI] [PubMed] [Google Scholar]
- 55.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 56.Williams IL, Wheatcroft SB, Shah AM, Kearney MT. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord. 2002;26(6):754–764. doi: 10.1038/sj.ijo.0801995. [DOI] [PubMed] [Google Scholar]
- 57.Silver AE, Beske SD, Christou DD, et al. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115(5):627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- 58.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61(12):1254–1260. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 59.Salafia CM, Vogel CA, Bantham KF, Vintzileos AM, Pezzullo J, Silberman L. Preterm delivery: correlations of fetal growth and placental pathology. Am J Perinatol. 1992;9(3):190–193. doi: 10.1055/s-2007-999318. [DOI] [PubMed] [Google Scholar]
- 60.Kidron D, Bernheim J, Aviram R. Placental findings contributing to fetal death, a study of 120 stillbirths between 23 and 40 weeks gestation. Placenta. 2009;30(8):700–704. doi: 10.1016/j.placenta.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Amir H, Weintraub A, Aricha-Tamir B, Apel-Sarid L, Holcberg G, Sheiner E. A piece in the puzzle of intrauterine fetal death: pathological findings in placentas from term and preterm intra-uterine fetal death pregnancies. J Matern Fetal Neonatal Med. 2009;22(9):759–764. doi: 10.3109/14767050902929396. [DOI] [PubMed] [Google Scholar]
- 62.Khan IY, Taylor PD, Dekou V, et al. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41(1):168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- 63.Khan IY, Dekou V, Douglas G, et al. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R127–R133. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- 64.Caluwaerts S, Lambin S, van Bree R, Peeters H, Vergote I, Verhaeghe J. Diet-induced obesity in gravid rats engenders early hyperadiposity in the offspring. Metabolism. 2007;56(10):1431–1438. doi: 10.1016/j.metabol.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 65.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R528–R538. doi: 10.1152/ajpregu.00316.2007. [DOI] [PubMed] [Google Scholar]
- 66.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587(Pt 4):905–915. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor PD, Khan IY, Lakasing L, et al. Uterine artery function in pregnant rats fed a diet supplemented with animal lard. Exp Physiol. 2003;88(3):389–398. doi: 10.1113/eph8802495. [DOI] [PubMed] [Google Scholar]
- 68.Hartil K, Vuguin PM, Kruse M, et al. Maternal substrate utilization programs the development of the metabolic syndrome in male mice exposed to high fat in utero. Pediatr Res. 2009;66(4):368–373. doi: 10.1203/PDR.0b013e3181b33375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samuelsson AM, Matthews PA, Argenton M, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51(2):383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 70.Mark PJ, Sisala C, Connor K, et al. A maternal high-fat diet in rat pregnancy reduces growth of the fetus and placental junctional zone, but not placental labyrinth zone growth. J Dev Origins Health Dis. 2011;2(1):63–70. [Google Scholar]
- 71.Carter AM. Animal models of human placentation—a review. Placenta. 2007;28(Suppl A):S41–S47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2(7):538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 73.Wallace JM, Milne JS, Matsuzaki M, Aitken RP. Serial measurement of uterine blood flow from mid to late gestation in growth restricted pregnancies induced by overnourishing adolescent sheep dams. Placenta. 2008;29(8):718–724. doi: 10.1016/j.placenta.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 74.Wallace JM, Bourke DA, Aitken RP, Leitch N, Hay WW., Jr Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am J Physiol Regul Integr Comp Physiol. 2002;282(4):R1027–R1036. doi: 10.1152/ajpregu.00465.2001. [DOI] [PubMed] [Google Scholar]
- 75.Redmer DA, Luther JS, Milne JS, et al. Fetoplacental growth and vascular development in overnourished adolescent sheep at day 50, 90 and 130 of gestation. Reproduction. 2009;137(4):749–757. doi: 10.1530/REP-08-0516. [DOI] [PubMed] [Google Scholar]
- 76.Reynolds LP, Borowicz PP, Vonnahme KA, et al. Animal models of placental angiogenesis. Placenta. 2005;26(10):689–708. doi: 10.1016/j.placenta.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 77.Challier JC, Basu S, Bintein T, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009;30(2):169–175. doi: 10.1016/j.placenta.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol. 2009;297 (5):C1228–C1235. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 80.Thongsong B, Subramanian RK, Ganapathy V, Prasad PD. Inhibition of amino acid transport system a by interleukin-1beta in trophoblasts. J Soc Gynecol Investig. 2005;12(7):495–503. doi: 10.1016/j.jsgi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 81.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23(1):271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119(2):323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frias AE, Morgan TK, Evans AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152(6):2456–2464. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grant WF, Gillingham MB, Batra AK, et al. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS ONE. 2011;6(2):e17261. doi: 10.1371/journal.pone.0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010;151(4):1622–1632. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sullivan EL, Grayson B, Takahashi D, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30(10):3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ismail-Beigi F, Catalano PM, Hanson RW. Metabolic programming: fetal origins of obesity and metabolic syndrome in the adult. Am J Physiol Endocrinol Metab. 2006;291(3):E439–E440. doi: 10.1152/ajpendo.00105.2006. [DOI] [PubMed] [Google Scholar]
- 88.Igosheva N, Abramov AY, Poston L, et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE. 2010;5(4):e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467(7318):963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]