Abstract
Ependymomas are neoplasms that can occur anywhere along the craniospinal axis. They are the third most common brain tumor in children, representing 10% of pediatric intracranial tumors, 4% of adult brain tumors, and 15% of all spinal cord tumors. As the heterogeneity of ependymomas has severely limited the prognostic value of the World Health Organization grading system, numerous studies have focused on genetic alterations as a potential basis for classification and prognosis. However, this endeavor has proven difficult due to variations of findings depending on tumor location, tumor grade, and patient age. While many have evaluated chromosomal abnormalities for ependymomas as a whole group, others have concentrated their efforts on specific subsets of populations. Here, we review modern findings of chromosomal analyses, their relationships with various genes, and their prognostic implications for intracranial and spinal cord ependymomas.
Keywords: Adult, Chromosome, Ependymoma, Genetic, Pediatric, Prognosis
1. Introduction
Ependymomas are rare neoplasms that can occur anywhere along the craniospinal axis. Recent findings suggest that they are derived from radial glial cells,1,2 which give rise to ependymal cells during normal cellular development.3 Ependymomas are the third most common brain tumor in children,1 representing 8% to 10% of pediatric intracranial tumors and approximately 4% of adult brain tumors.4,5 They constitute 60% of spinal cord gliomas and 15% of all spinal cord tumors.4–6 Interestingly, they have also been reported in the sacrococcygeal region, mediastinum, and ovaries,7–9 indicating that abnormalities in cellular migration or differentiation may have a role in ependymoma development.7
The World Health Organization (WHO) established the following classification system for ependymomas in 2007: WHO grade 1 (subependymomas and myxopapillary ependymomas), WHO grade 2 (classic ependymomas), and WHO grade 3 (anaplastic ependymomas).4 WHO grade 2 ependymomas have been further subdivided into cellular, papillary, clear cell, and tanycytic variants (Supplementary Fig. 1).10 However, this WHO classification has been a subject of controversy regarding its prognostic capabilities and overall usefulness. While a meta-analysis of 2400 patients showed that WHO grading was an independent outcome predictor, 11,12 other studies have suggested that ependymoma grading, especially differentiation between grades 2 and 3, is highly dependent upon the experience of the neuropathologist11,13–18 and a poor clinical correlate. Some authors have failed to find any association between survival and grading,4,19,20 while others have reported an obvious improvement in overall and progression- free survival (PFS) for lower grade ependymomas.4,15,16,21–23 One study reported that WHO grading was the most powerful prognostic factor for ependymomas in the adult population.4,24
As the incidence of ependymomas is relatively low,4,5,25 many researchers have chosen to pool data from both pediatric and adult populations, as well as combine grade 2 and grade 3 lesions in their reports. These retrospective studies have often analyzed data collected over several decades, during which diagnostic criteria and treatment strategies were being modified. As such, providing evidence to support universally accepted prognostic factors and implementing a standardized treatment protocol have been difficult endeavors.4 Given these difficulties in grading and prognosis, potential genetic markers may serve as a more reliable risk stratification for patients with ependymomas. Here, we review the most promising chromosomal gains and losses common to ependymomas within the mixed population (adults and children), as well as unique findings in specific subgroups (for instance in the pediatric population, for tumor location, for tumor grade) and their potential for prognostic significance.
2. Intracranial ependymoma overview
Intracranial ependymomas are characteristically found in pediatric populations, and they are rare in adults.1 Overall, 90% of all pediatric ependymomas are intracranial, and are generally grade 2 or 3.7 Supratentorial tumors account for 50% to 60% of adult intracranial ependymomas,4 while only 25% to 35% of ependymomas are found in this region in pediatric patients. Supratentorial lesions generally develop in the lateral or third ventricles, but may also arise within the white matter or rarely in the cortex.1,26,27
Tumors of the infratentorial region occur in the midline along the 4th ventricle or more laterally within the cerebellopontine angles. Ependymomas have also been described with invasion of the brainstem and extension beyond the foramen magnum.4 Infratentorial ependymomas and grade 3 tumors are reported to be more prone to seeding of the cerebrospinal fluid (CSF), which occurs in 3% to 15% of intracranial lesions.4,28–31
Historically, the extent of tumor resection has been regarded as the most important prognostic factor for pediatric intracranial tumors, 1,14 with a five-year overall survival (OS) of 50% to 60% and PFS of 30% to 50%.32 Children with ependymoma have decreased survival compared to adults, as 40% to 60% of the pediatric population die of their disease.33,34 The five-year OS for adults with intracranial lesions is 62% to 84.8%, with a five-year PFS of 43% to 65.3%.4,19,21,24
Tumor location has been observed as having potential prognostic value. Infratentorial ependymomas in adults demonstrate a trend towards a better prognosis,4,19,21,24 perhaps because infiltrative grade 3 lesions are more often found in supratentorial regions. 4,22,24 These anaplastic tumors are also associated with an increased risk of recurrence.4,35
Other potential prognostic factors include age, which has been associated with a better prognosis by Reni et al. (when <40 years)4,19 and Metellus et al. (when <55 years),4,24 yet no such findings were detected by Guyotat et al.4,21 The Karnofsky Performance Scale score may also be associated with improved survival.4,24
3. Spinal cord ependymoma overview
Ependymomas affecting the spinal cord most frequently occur in adults of 20 to 40 years of age.10 They represent the most common spinal cord tumor in adults, accounting for 37% to 47% of intramedullary tumors.10 Myxopapillary ependymomas (MEPN) represent 13% of all ependymomas,20,36 and 50% of all adult spinal cord tumors. This subtype is primarily located in the cauda equina, with occasional extension into the conus medullaris.4 While pediatric spinal cord tumors account for only 5% to 10% of all ependymomas, 37 pediatric patients with MEPN develop more aggressive tumors, with a greater metastatic potential36,38–41 and a higher recurrence rate than adult MEPN.36
The remaining spinal cord ependymomas generally consist of the classic ependymoma type, which are mainly located in the cervical, or less frequently thoracic, regions. Half of all lesions extend to three or more vertebral levels, with 90% of all spinal cord ependymomas being slow growing and benign. During their expansion, these tumors have a propensity for compressing adjacent structures, rather than infiltration.4
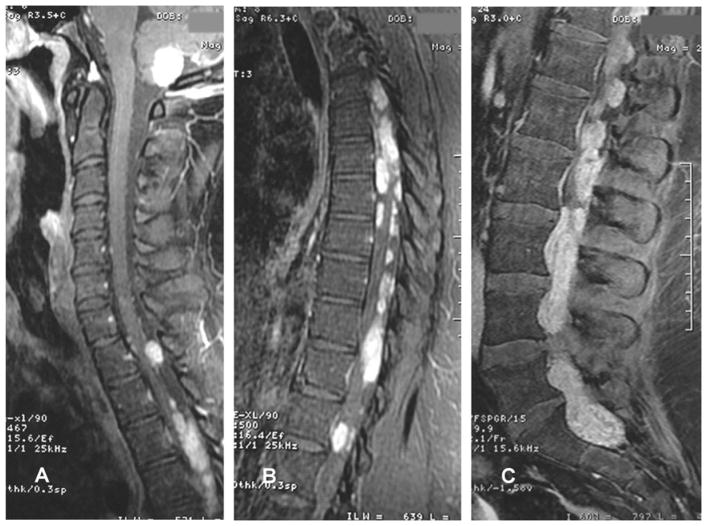
Although rare, CSF dissemination occurs in 7% of patients with spinal ependymomas,4,42 with rare instances involving the brain (Fig. 1).4,42,43 Metastasis to extra-neural structures has also been documented,4,44 and originates predominantly from clear cell ependymomas.37
Fig. 1.
Sagittal T1-weighted MRI with contrast demonstrating (A) an expansive, contrast-enhancing intra-axial lesion in the cerebellum with extension into the foramen magnum, and (A, B, C) multiple well-circumscribed enhancing intradural and extramedullary lesions predominantly along the thoracic and lumbar regions. From 85 Macedo LT, Rogerio F, Pereira EB, et al. Cerebrospinal tumor dissemination in a patient with myxopapillary ependymoma. J Clin Oncol 29:2011 e795–798. Reprinted with permission. © 2011 American Society of Clinical Oncology. All rights reserved.
The prognosis for spinal cord ependymomas, compared to intracranial lesions, is fairly good for five-year (83–97%), 10-year (74–97%), and 15-year (61–75%) survival rates. PFS has been reported as 70% to 75% (five-year), 50% to 62% (10-year), and 35% to 46% (15-year).4,43,45,46 Improvement in OS and PFS has been associated with younger age, tumor size, and distant spinal disease.4,43,46–48
4. Chromosomal anomalies associated with ependymoma
Given the controversy regarding WHO grading and the difficulty in achieving local tumor control for ependymomas with surgery and chemo-radiotherapy, recent advances have demonstrated a strong focus on genetic analysis to elucidate the mechanisms of tumor initiation and progression (Table 1).4,7,49,50
Table 1.
Chromosomal alterations of ependymoma tumors
| Losses | Comments | Gains | Comments | ||
|---|---|---|---|---|---|
| Mixed populations | 6q21 | ADM1 and CDK11 underexpression | 1q21.1–32.1 | Associated with recurrence | |
| 6q23 | Poor event-free survival | 1q23.3 | DUSP12 overexpression, associated with aggressive tumors | ||
| 6q24–26 | SASH1 and TCP1 underexpression | ||||
| 9p24.31 | Associated with FOXD4 expression, usually expressed in embryonic stem cells | 7q34 | Found in 38%, codes for ARHGEF5 gene | ||
| 10q23.21 | Found in 19%, codes for MINPP1 | 12q12.12 | Found in 34%, associated with HOXC4 HOX associated with spinal tumors | ||
| 10q26.12 | Found in 16%, codes for TACC2 | ||||
| 11q | Inverse relationship with 22q LOH found with mutations in the MEN1 gene at 11q13 | 1q, 7q, 9q, 12, 13q, 17p, 17q, 20q, 22q | Found in high percentage of ependymomas | ||
| 22q | Associated with NF2 mutation | ||||
| 22q12.3–22q13.3 | RAC2, G22P1, MCM5, SULT4A1, FBX7, C22orf2, CBX7, and SBF1 underexpression | ||||
| 2q, 4q, 5q, 6q, 7q, 9q, 10q, 15q, 16, 17p, 19p, and 21 | Less commonly detected | ||||
| 3q, 6q, 10q, 15, 22 | Associated with recent tumors | ||||
| Ependymoma location | Intracranial | 6q, 9, 13 | 1q | ||
| Infratentorial | 17p13.3 | 9q33–34 | |||
| Supratentorial | 9p | ||||
| Spinal cord | 6, 12, 22 | Monosomy 22 | 7 | Found in 95% of lesions | |
| Pediatric spinal | 1, 2, 10 | Whole chromosome imbalance | 7, 9, 11, 18, 20 | Whole chromosome imbalance | |
| Pediatric ependymomas | 6q | 15% of patients, also identified upon tumour relapse | 1q | 15–50% in pediatric, 8% in adults | |
| 1q21 | Highest copy numbers present in SHC1 (41%), S100A11 (31%), and JTB (28%) | ||||
| 1q21.3–23.1 1q24-q21 |
High amplification found in both primary and recurrent pediatric patients | ||||
| 1q21–32 | Anaplastic tumors | ||||
| 1q25 | Poor prognosis, abnormalities involving TPR in 38% of ependymomas | ||||
| 16 | 1q31.1–31.3 7q, 9p24.3-qter |
Found in 58% of patients < 16 years old | |||
| 17p | 50% of sporadic pediatric ependymomas | 9qter | Amplification increased upon subsequent relapse, more frequent in older children | ||
| 9q33–34 | Found to correlate with recurrence, covering oncogenes Notch1 and TNC | ||||
| Monosomy 22 | 30% of pediatric patients | 11q13 | Flanks CCND1 oncogene | ||
| 22p13 | 55% of pediatric patients | ||||
| Posterior fossa | 6, 17, 22 | Silencing of HIC–1 on 17p | |||
| Supratentorial Grade III (case study) | 1p, 14q | Found in local recurrences and upon tumor metastasis | |||
| 1p36 | Frequency of loss increased in relation to distance from the site of local recurrence | ||||
| Ependymoma subtypes | Myxopapillary | 1, 10, 22 | 7 | Polysomy found in 13/13 patients | |
| 3, 4, 7, 8, 9, 10, 11, 13, 17q, 18, 20 | Simultaneous gains on 9 and 18 | ||||
| Classic | 22 | NF2 | |||
| –Clear cell | 9 | ||||
| Anaplastic | 1p, 9 | Implicates cyclin D/CDK4 and p53 |
NF2 = neurofibromatosis type 2.
4.1. Chromosome 22 loss (Mixed populations)
Ependymomas have a high incidence of loss of heterozygosity (LOH) on chromosome 22q, which is associated with neurofibromatosis type 2 (NF2) mutations.4,51,52 Ebert and colleagues identified six NF2 mutations in grade 2 spinal ependymomas, but no mutations were found in MEPN tumors within the study.36,51 Furthermore, deletion of NF2 does not appear to have a significant impact on pediatric intracranial lesions.7,53,54 While monosomy 22 has a higher incidence in adult patients, only 31% of pediatric ependymomas display this finding.10
LOH on chromosome 22q also has an inverse relationship with LOH on 11q. Along with the 11q LOH, mutations in the MEN1 gene (located at 11q 13) were occasionally found. MEN1 was initially found intact within WHO grade 2 ependymomas, but upon tumor recurrence with malignant transformation, MEN1 was discovered to be mutated. This finding implicates MEN1 as a potential gene involved in tumor recurrence and progression.4 In addition, recurrent tumors have also been found to have losses of chromosome 3q, 6q, 10q, 15, and 22, with no such anomalies detected during initial tumor presentation.37
Genetic underexpression of several transcripts was found in 22q12.3–22q13.3, including RAC2, G22P1, MCM5, SULT4A1, FBX7, C22orf2, CBX7, and SBF1. CBX7 is thought to regulate both the p16(INK4A)/Rb and ARF/p53 pathways involved in cellular lifespan. 7,10,55–57 SMARCB1 (hSNF5/INI1) is also located on 22q and has been implicated in the pathogenesis of various other tumors.10
While the high incidence of 22q LOH in ependymoma tumors and subsequent alteration of specific genetic expression profiles warrants further investigation, many other chromosomal abnormalities have also been detected in the adult and pediatric populations.
4.2. Other chromosomal losses (Mixed populations)
Although less common, chromosomal losses have also been detected on 2q, 4q, 5q, 6q, 7q, 9p, 10q, 15q, 16, 17p, 19p, and 21.4,10,58–60 While common in malignant gliomas, chromosome 10q LOH was uncommon in a wide variety of ependymomas.4 However, one study found this genetic alteration in 19% of patients, including 10q23.21, which codes for MINPP1 and is implicated in follicular thyroid carcinomas. In 16% of patients, TACC2, believed to be involved in mitotic spindle maintenance61, had a deletion at 10q26.12, and TACC2 mRNA showed increased expression in all samples. Loss on 6q24–26 is also frequently identified, 7,59,62 with related decreased expression of SASH1 (deleted in breast cancer) and TCP1 (involved in tubulin production). ADM1 and CDK11 at 6q21 are implicated in cellular proliferation and are also underexpressed.55,56 Loss on chromosome 6q23 was correlated with a poor event-free survival.7,63 FOXD4 (9p24.31) is a member of the forkhead box family expressed in embryonic stem cells and is also found with losses in ependymoma patients.61,64
4.3. Chromosomal gains (Mixed populations)
While there remains a great deal of variability, comparative genomic hybridization has been utilized to appreciate chromosome gains in a high percentage of ependymomas, including 1q, 7q, 9q, 12, 13q, 17p, 17q, 20q, and 22q.4,61,65–67 Gains at 1q21.1–32.1 were associated with recurrence in patients of mixed age groups.61 DUSP12 (1q23.3) was overexpressed in all samples in one study, with suspected involvement with aggressive ependymomas. DUSP12 mRNA levels were also associated with cyclin D1 levels throughout the cell cycle, implicating a potential role in the regulation of cell division and tumor development.61 The ARHGEF5 gene located at 7q34 was identified with gains in 38% of ependymomas. This gene is involved in cytoskeletal organization and progression. Gains were also detected in 34% of tumors within the HOXC4 gene at 12q12.12, which is implicated in neuronal morphogenesis. Furthermore, increased expression of this gene was found in 90% of ependymomas.61 HOX (homeobox-containing) family genes have been specifically associated with spinal ependymoma tumorigenesis.55,60,68,69
4.4. Ependymoma location (Mixed populations)
Chromosome anomalies have also been associated with specific tumor locations, implying a unique pathogenesis for ependymoma formation in the infratentorial, supratentorial, and spinal cord regions. Intracranial lesions were associated with gain of 1q10 and losses on 6q, 9 and 13.65,67,68,70 Infratentorial ependymomas demonstrated chromosomal gains of 9q33–34,1,32 and losses of 17p13.3. Supratentorial tumors were identified with losses of 9p.10 Spinal cord ependymomas included gains on chromosome 7, which were described in 95% of lesions.10 Additional findings for spinal cord tumors include monosomy 22, and loss of chromosomes 6 and 12.62,65,68,71–73 Pediatric spinal ependymomas were found to display whole chromosomal imbalances (gain of chromosome 7, 9, 11, 18, 20, or loss of 1, 2, 10).7
4.5. Pediatric ependymomas
While limited data have forced many researchers to combine their findings from mixed age groups, some have analyzed results exclusively from the pediatric population. In pediatric intracranial ependymomas, approximately 90% were found to have abnormal karyotypes. One review of 21 karyotype studies reported that while adults tend to present with gains on chromosomes 2, 5, 7, 9, 12, 18, and X, and loss on 6, 10, 13q, 14q and 22, pediatric ependymomas are associated with gains on chromosomes 1q, 7, and 9, and loss on chromosomes 1p, 3, 6, 9p, 13q, 17, and 22.7
4.6. Pediatric chromosomal loss
Loss of 17p is reported in as many as 50% of sporadic pediatric ependymomas,10 with loss of 6q in about 15% of patients.74 Loss of chromosome 6q has also been identified in children upon tumor relapse.7 Ependymomas in the posterior fossa have been associated with loss of chromosomes 6, 17, and 22, as well as silencing of the tumor suppressor gene HIC-1 on chromosome 17p.75 Monosomy 22 has been reported in 30% of pediatric patients, with loss of 22q13 in 55% of this population.57 Losses on chromosome 16 have also been noted.10,32
One report of an 18-year-old patient with a supratentorial grade 3 ependymoma revealed deletions at 1p and 14q found only in local recurrences and upon tumor metastasis. Interestingly, the frequency of loss at 1p36 increased in relation to distance from the site of local recurrence. These findings implicate a potential gene associated with tumor recurrence and metastasis. One candidate gene is AJAP1/SHREW1, located at 1p36.32. This gene is believed to inhibit adhesion and migration in oligodendrogliomas. Furthermore, immunohistochemistry staining of this patient’s metastatic lesion demonstrated a complete loss of this gene expression compared to the initial primary ependymoma.37
4.7. Pediatric chromosomal gain
Chromosomal gains at 1q were particularly more common in pediatric (15–50% compared to 8% in adults)7,57 and anaplastic ependymomas (gain at 1q21–32).4,7,61,67,70 Kilday et al.7 reported that gain at 1q was the most common imbalance (with high amplification at 1q24–q31) and this gain was found in both primary and recurrent pediatric tumors. In addition, this genomic aberration has been associated with a poor prognosis in various other tumors, further validating its importance.7,57,76–78 Specifically, gains of 1q25 have been associated with a poor prognosis in pediatric patients, with genomic abnormalities involving the translocated promoter region (TPR) identified in 38% of ependymomas. Both TPR amplification and RAC2 loss were associated with decreased survival, while RAC2 loss was also correlated with increased recurrence in patients younger than two years of age.49,57,61 In conjunction with gain on 1q, losses on 6q and 22 have also been associated with recurrence.7 Furthermore, numerous other potential candidate oncogenes on 1q have been suggested, including HSPA6 (1q23), laminin (1q31), PRELP (1q32), GAC1 (1q32), and CHI3L1 (1q32.1).7,56,79–81
Karakoula et al.57 investigated pediatric ependymomas and found increased expression of various genes located in the “epidermal differentiation region” that are implicated in tumorigenesis and are frequently abnormal in several other tumor types. Genetic gains were identified in 61% of patients and were most commonly found in 1q21 and 1q25. Chromosome 1q21 had the highest copy numbers present in SHC1 (41%), S100A11 (31%), and JTB (28%). Additional gains at 1q21.3–23.1 and 1q31.1–31.3 have also been identified.57
One study found that 58% of patients younger than sixteen years old had gains on chromosomes 7q and 9p24.3-qter.32 In patients less than three years old, gain in 9q33–34 (covering oncogenes Notch1 and TNC) was found to correlate with recurrence. Interestingly, 9qter amplification increased upon subsequent relapses, consistent with the fact that 9qter was more frequent in older children.32 In addition, this young population is also reported with gain on 11q13 flanking the CCND1 oncogene.10,32
4.8. Ependymoma subtypes
Regarding genetic abnormalities associated with specific ependymoma subtypes, MEPN tumors displayed frequent gains simultaneously on chromosomes 9 and 18, with others detected on chromosomes 3, 4, 7, 8, 11, 13, 17q, and 20. Losses were noted on chromosomes 1, 10, and 22.10 Santi and colleagues36,82 found all 13 MEPN within their study to display polysomy of chromosome 7, whereas no such abnormality was present in classic ependymomas. Anaplastic ependymomas displayed losses on chromosome 9 and 1p, which implicated several potential pathways, including the cyclin D/CDK4 pathway (INK4A on 9p) and p53 pathway (ARF on 9p). ARF is involved in the stabilization of p53, but its role in anaplastic ependymomas is uncertain. While decreased p14ARF and p27 expression have been associated with increased aggressive tumor behavior, one report failed to detect deletions or hypermethylation of ARF. However, positive staining in grade 3 ependymomas for p53, tenascin, vascular endothelial growth factor (VEGF), and epidermal growth factor receptor (EGFR) have been associated with decreased PFS.10,37 Loss of chromosome 9 has also been implicated in the development of clear cell ependymomas.37
Classic ependymomas were found to contain high expression levels of dynein genes, which are involved in the development of cytoskeleton and mitotic spindles.68,83 As grade 2 tumors are found with more gross chromosomal abnormalities than grade 3 lesions, it has been postulated that dynein dysregulation may be the cause of these anomalies.68
5. Prognosis
To create a more consistent and reliable risk stratification, Korshunov et al.11 proposed a system based upon genetic analysis for intracranial ependymomas consisting of three prognostic subgroups:
Group 1 (34% of their study, five-year OS of 100%) – tumors with gains of chromosome 9, 15q, or 18, or loss of chromosome 6, without 1q gain or CDKN2A deletion
Group 2 (42% of their study, five-year OS of 78%) – tumors balanced for chromosome 1q, 6, 9, 15q, and 18, without a homozygous deletion of CDKN2A
Group 3 (25% of their study, five-year OS of 32%) – tumors with 1q gain or homozygous deletion of CDKN2A.11
However, they concluded that a gain in chromosome 1q is the most reliable genetic prognostic marker overall.11,61,67,70,81 Current therapy standards demonstrate an excellent prognosis for ependymomas possessing chromosomal changes indicative of group 1. Conversely, chromosomal alterations of group 3 are associated with anaplastic ependymomas and a poor prognosis.11,61,65–67,69,70 Tumors of this group manifest aggressive clinical behavior and demonstrate a propensity for metastasis.11,37,61,84 In accordance with Korshunov’s group 3, intracranial ependymomas with gains at 1q21.1–32.1 are also associated with increased recurrence.10
Yet contrary findings for these prognostic markers have also been reported. Loss of 6q23 (group 1) has been associated with a decreased PFS,11,63 while anaplastic ependymomas with loss of 6q25.3 demonstrate an improved OS. In pediatric patients, gain of 9q (group 1) have also been correlated with frequent recurrence.11,12
Kilday et al.7 reported that adult ependymomas have more chromosomal abnormalities (7.5 per tumor) than their pediatric counterpart (3.8 per tumor), which is further supported by the evidence that a balanced genomic profile was observed in 36% to 58% of pediatric patients compared to less than 10% of adult patients. Adult and spinal imbalances tended to incorporate whole chromosomal rearrangements, rather than the partial and complex imbalances observed in pediatric and aggressive adult ependymomas. These observations seem to support Korshunov’s classification system, as a higher number of genomic aberrations was associated with a better prognosis. Consistent with this concept, Dyer et al. found that almost all recurrent tumors possessed a genomic signature demonstrating infrequent and often partial imbalances.7,70
Although the application of current chromosomal findings for the purposes of risk stratification still necessitates further validation, the use of genetic biomarkers as prognostic indicators may allow for more individualized therapeutic strategies. Patients requiring more aggressive treatment may be identified to prevent progression and recurrence, whereas low-risk groups may benefit from reduced toxicity of unnecessarily intense adjuvant therapy.
6. Conclusion
Recent findings suggest that the histologic diagnosis of ependymomas may be insufficient to assign an appropriate risk stratification strategy. Furthermore, conventional therapies may fail to effectively control tumor growth and progression due to the inherent heterogeneity of ependymoma abnormalities, as demonstrated by analysis of genetic and molecular anomalies. A more detailed understanding of these various mechanisms may facilitate the identification of more specific prognostic markers, as well as the development of novel agents for individually tailored therapy.
Supplementary Material
Acknowledgments
Isaac Yang (first author) was partially supported by an Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research UCLA Scholars in Translational Medicine Program Award, Visionary Fund Grant, and the Stein Oppenheimer Endowment Award. Daniel Nagasawa (second author) was partially supported by an American Brain Tumor Association Medical Student Summer Fellowship in Honor of Connie Finc. Marko Spasic (fourth author) was partially supported by an American Association of Neurological Surgeons Fellowship grant.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jocn.2011.11.004.
References
- 1.Andreiuolo F, Puget S, Peyre M, et al. Neuronal differentiation distinguishes supratentorial and infratentorial childhood ependymomas. Neuro Oncol. 2010;12:1126–34. doi: 10.1093/neuonc/noq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadjipanayis CG, Van Meir EG. Brain cancer propagating cells: biology, genetics and targeted therapies. Trends Mol Med. 2009;15:519–30. doi: 10.1016/j.molmed.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spassky N, Merkle FT, Flames N, et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–8. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruda R, Gilbert M, Soffietti R. Ependymomas of the adult: molecular biology and treatment. Curr Opin Neurol. 2008;21:754–61. doi: 10.1097/WCO.0b013e328317efe8. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain MC. Ependymomas. Curr Neurol Neurosci Rep. 2003;3:193–9. doi: 10.1007/s11910-003-0078-x. [DOI] [PubMed] [Google Scholar]
- 6.Tseng JH, Tseng MY. Survival analysis of 459 adult patients with primary spinal cancer in England and Wales: a population-based study. Surg Neurol. 2007;67:53–8. doi: 10.1016/j.surneu.2006.04.011. [discussion 8] [DOI] [PubMed] [Google Scholar]
- 7.Kilday JP, Rahman R, Dyer S, et al. Pediatric ependymoma: biological perspectives. Mol Cancer Res. 2009;7:765–86. doi: 10.1158/1541-7786.MCR-08-0584. [DOI] [PubMed] [Google Scholar]
- 8.Aktug T, Hakguder G, Sanoglu S, et al. Sacrococcygeal extraspinal ependymomas: the role of coccygectomy. J Pediatr Surg. 2000;35:515–8. doi: 10.1016/s0022-3468(00)90228-8. [DOI] [PubMed] [Google Scholar]
- 9.Hirahara F, Yamanaka M, Miyagia E, et al. Pure ovarian ependymoma: report of a case treated with surgery, chemotherapy, irradiation and hyperthermotherapy. Eur J Obstet Gynecol Reprod Biol. 1997;75:221–3. doi: 10.1016/s0301-2115(97)00134-6. [DOI] [PubMed] [Google Scholar]
- 10.Hasselblatt M. Ependymal tumors. Recent Results Cancer Res. 2009;171:51–66. doi: 10.1007/978-3-540-31206-2_3. [DOI] [PubMed] [Google Scholar]
- 11.Korshunov A, Witt H, Hielscher T, et al. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol. 2010;28:3182–90. doi: 10.1200/JCO.2009.27.3359. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez D, Cheung MC, Housri N, et al. Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973–2005) J Surg Res. 2009;156:340–51. doi: 10.1016/j.jss.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messahel B, Ashley S, Saran F, et al. Relapsed intracranial ependymoma in children in the UK: patterns of relapse, survival and therapeutic outcome. Eur J Cancer. 2009;45:1815–23. doi: 10.1016/j.ejca.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Figarella-Branger D, Civatte M, Bouvier-Labit C, et al. Prognostic factors in intracranial ependymomas in children. J Neurosurg. 2000;93:605–13. doi: 10.3171/jns.2000.93.4.0605. [DOI] [PubMed] [Google Scholar]
- 15.Korshunov A, Golanov A, Sycheva R, et al. The histologic grade is a main prognostic factor for patients with intracranial ependymomas treated in the microneurosurgical era: an analysis of 258 patients. Cancer. 2004;100:1230–7. doi: 10.1002/cncr.20075. [DOI] [PubMed] [Google Scholar]
- 16.Kurt E, Zheng PP, Hop WC, et al. Identification of relevant prognostic histopathologic features in 69 intracranial ependymomas, excluding myxopapillary ependymomas and subependymomas. Cancer. 2006;106:388–95. doi: 10.1002/cncr.21608. [DOI] [PubMed] [Google Scholar]
- 17.Merchant TE, Jenkins JJ, Burger PC, et al. Influence of tumor grade on time to progression after irradiation for localized ependymoma in children. Int J Radiat Oncol Biol Phys. 2002;53:52–7. doi: 10.1016/s0360-3016(01)02801-2. [DOI] [PubMed] [Google Scholar]
- 18.Tihan T, Zhou T, Holmes E, et al. The prognostic value of histological grading of posterior fossa ependymomas in children: a Children’s Oncology Group study and a review of prognostic factors. Mod Pathol. 2008;21:165–77. doi: 10.1038/modpathol.3800999. [DOI] [PubMed] [Google Scholar]
- 19.Reni M, Brandes AA, Vavassori V, et al. A multicenter study of the prognosis and treatment of adult brain ependymal tumors. Cancer. 2004;100:1221–9. doi: 10.1002/cncr.20074. [DOI] [PubMed] [Google Scholar]
- 20.Schiffer D, Chio A, Cravioto H, et al. Ependymoma: internal correlations among pathological signs: the anaplastic variant. Neurosurgery. 1991;29:206–10. [PubMed] [Google Scholar]
- 21.Guyotat J, Signorelli F, Desme S, et al. Intracranial ependymomas in adult patients: analyses of prognostic factors. J Neurooncol. 2002;60:255–68. doi: 10.1023/a:1021136029072. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin MP, Marcus RB, Jr, Buatti JM, et al. Ependymoma: results, prognostic factors and treatment recommendations. Int J Radiat Oncol Biol Phys. 1998;40:845–50. doi: 10.1016/s0360-3016(97)00893-6. [DOI] [PubMed] [Google Scholar]
- 23.Wolfsberger S, Fischer I, Hoftberger R, et al. Ki-67 immunolabeling index is an accurate predictor of outcome in patients with intracranial ependymoma. Am J Surg Pathol. 2004;28:914–20. doi: 10.1097/00000478-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Metellus P, Barrie M, Figarella-Branger D, et al. Multicentric French study on adult intracranial ependymomas: prognostic factors analysis and therapeutic considerations from a cohort of 152 patients. Brain. 2007;130:1338–49. doi: 10.1093/brain/awm046. [DOI] [PubMed] [Google Scholar]
- 25.Reni M, Gatta G, Mazza E, et al. Ependymoma. Crit Rev Oncol Hematol. 2007;63:81–9. doi: 10.1016/j.critrevonc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Lehman NL. Central nervous system tumors with ependymal features: a broadened spectrum of primarily ependymal differentiation? J Neuropathol Exp Neurol. 2008;67:177–88. doi: 10.1097/NEN.0b013e31816543a6. [DOI] [PubMed] [Google Scholar]
- 27.Roncaroli F, Consales A, Fioravanti A, et al. Supratentorial cortical ependymoma: report of three cases. Neurosurgery. 2005;57:E192. doi: 10.1227/01.neu.0000164171.29292.d6. [discussion E] [DOI] [PubMed] [Google Scholar]
- 28.Watt PM, Hoffmann K, Greene WK, et al. Specific alternative HOX11 transcripts are expressed in paediatric neural tumours and T-cell acute lymphoblastic leukaemia. Gene. 2003;323:89–99. doi: 10.1016/j.gene.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Qu Q, Sun G, Li W, et al. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12:31–9. doi: 10.1038/ncb2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma MK, Mansur DB, Reifenberger G, et al. Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res. 2007;67:890–900. doi: 10.1158/0008-5472.CAN-06-0973. [DOI] [PubMed] [Google Scholar]
- 31.Hebert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9:678–85. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puget S, Grill J, Valent A, et al. Candidate genes on chromosome 9q33–34 involved in the progression of childhood ependymomas. J Clin Oncol. 2009;27:1884–92. doi: 10.1200/JCO.2007.15.4195. [DOI] [PubMed] [Google Scholar]
- 33.Wright KD, Gajjar A. New chemotherapy strategies and biological agents in the treatment of childhood ependymoma. Childs Nerv Syst. 2009;25:1275–82. doi: 10.1007/s00381-009-0809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson PL, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: a report of the Children’s Cancer Group. J Neurosurg. 1998;88:695–703. doi: 10.3171/jns.1998.88.4.0695. [DOI] [PubMed] [Google Scholar]
- 35.Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer. 2003;98:603–9. doi: 10.1002/cncr.11534. [DOI] [PubMed] [Google Scholar]
- 36.Barton VN, Donson AM, Kleinschmidt-DeMasters BK, et al. Unique molecular characteristics of pediatric myxopapillary ependymoma. Brain Pathol. 2010;20:560–70. doi: 10.1111/j.1750-3639.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milde T, Pfister S, Korshunov A, et al. Stepwise accumulation of distinct genomic aberrations in a patient with progressively metastasizing ependymoma. Genes Chromosomes Cancer. 2009;48:229–38. doi: 10.1002/gcc.20635. [DOI] [PubMed] [Google Scholar]
- 38.Chinn DM, Donaldson SS, Dahl GV, et al. Management of children with metastatic spinal myxopapillary ependymoma using craniospinal irradiation. Med Pediatr Oncol. 2000;35:443–5. doi: 10.1002/1096-911x(20001001)35:4<443::aid-mpo13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Fassett DR, Pingree J, Kestle JR. The high incidence of tumor dissemination in myxopapillary ependymoma in pediatric patients. Report of five cases and review of the literature. J Neurosurg. 2005;102:59–64. doi: 10.3171/ped.2005.102.1.0059. [DOI] [PubMed] [Google Scholar]
- 40.Gagliardi FM, Cervoni L, Domenicucci M, et al. Ependymomas of the filum terminale in childhood: report of four cases and review of the literature. Childs Nerv Syst. 1993;9:3–6. doi: 10.1007/BF00301925. [DOI] [PubMed] [Google Scholar]
- 41.Kawagoe H, Humphries RK, Blair A, et al. Expression of HOX genes, HOX cofactors, and MLL in phenotypically and functionally defined subpopulations of leukemic and normal human hematopoietic cells. Leukemia. 1999;13:687–98. doi: 10.1038/sj.leu.2401410. [DOI] [PubMed] [Google Scholar]
- 42.Peschel RE, Kapp DS, Cardinale F, et al. Ependymomas of the spinal cord. Int J Radiat Oncol Biol Phys. 1983;9:1093–6. doi: 10.1016/0360-3016(83)90402-9. [DOI] [PubMed] [Google Scholar]
- 43.Akyurek S, Chang EL, Yu TK, et al. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol. 2006;80:177–83. doi: 10.1007/s11060-006-9169-2. [DOI] [PubMed] [Google Scholar]
- 44.Graf M, Blaeker H, Otto HF. Extraneural metastasizing ependymoma of the spinal cord. Pathol Oncol Res. 1999;5:56–60. doi: 10.1053/paor.1999.0056. [DOI] [PubMed] [Google Scholar]
- 45.Gomez DR, Missett BT, Wara WM, et al. High failure rate in spinal ependymomas with long-term follow-up. Neuro Oncol. 2005;7:254–9. doi: 10.1215/S1152851704001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdel-Wahab M, Etuk B, Palermo J, et al. Spinal cord gliomas: A multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys. 2006;64:1060–71. doi: 10.1016/j.ijrobp.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 47.Marks JE, Adler SJ. A comparative study of ependymomas by site of origin. Int J Radiat Oncol Biol Phys. 1982;8:37–43. doi: 10.1016/0360-3016(82)90382-0. [DOI] [PubMed] [Google Scholar]
- 48.Wen BC, Hussey DH, Hitchon PW, et al. The role of radiation therapy in the management of ependymomas of the spinal cord. Int J Radiat Oncol Biol Phys. 1991;20:781–6. doi: 10.1016/0360-3016(91)90023-w. [DOI] [PubMed] [Google Scholar]
- 49.Tamburrini G, D’Ercole M, Pettorini BL, et al. Survival following treatment for intracranial ependymoma: a review. Childs Nerv Syst. 2009;25:1303–12. doi: 10.1007/s00381-009-0874-y. [DOI] [PubMed] [Google Scholar]
- 50.Gaspar N, Grill J, Geoerger B, et al. P53 Pathway dysfunction in primary childhood ependymomas. Pediatr Blood Cancer. 2006;46:604–13. doi: 10.1002/pbc.20532. [DOI] [PubMed] [Google Scholar]
- 51.Ebert C, von Haken M, Meyer-Puttlitz B, et al. Molecular genetic analysis of ependymal tumors. NF2 mutations and chromosome 22q loss occur preferentially in intramedullary spinal ependymomas. Am J Pathol. 1999;155:627–32. doi: 10.1016/S0002-9440(10)65158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamszus K, Lachenmayer L, Heinemann U, et al. Molecular genetic alterations on chromosomes 11 and 22 in ependymomas. Int J Cancer. 2001;91:803–8. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1134>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 53.Hulsebos TJ, Oskam NT, Bijleveld EH, et al. Evidence for an ependymoma tumour suppressor gene in chromosome region 22pter-22q11. 2. Br J Cancer. 1999;81:1150–4. doi: 10.1038/sj.bjc.6690822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokota T, Tachizawa T, Fukino K, et al. A family with spinal anaplastic ependymoma: evidence of loss of chromosome 22q in tumor. J Hum Genet. 2003;48:598–602. doi: 10.1007/s10038-003-0078-3. [DOI] [PubMed] [Google Scholar]
- 55.Korshunov A, Neben K, Wrobel G, et al. Gene expression patterns in ependymomas correlate with tumor location, grade, and patient age. Am J Pathol. 2003;163:1721–7. doi: 10.1016/S0002-9440(10)63530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suarez-Merino B, Hubank M, Revesz T, et al. Microarray analysis of pediatric ependymoma identifies a cluster of 112 candidate genes including four transcripts at 22q12.1–q13. 3. Neuro Oncol. 2005;7:20–31. doi: 10.1215/S1152851704000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karakoula K, Suarez-Merino B, Ward S, et al. Real-time quantitative PCR analysis of pediatric ependymomas identifies novel candidate genes including TPR at 1q25 and CHIBBY at 22q12–q13. Genes Chromosomes Cancer. 2008;47:1005–22. doi: 10.1002/gcc.20607. [DOI] [PubMed] [Google Scholar]
- 58.Scheil S, Bruderlein S, Eicker M, et al. Low frequency of chromosomal imbalances in anaplastic ependymomas as detected by comparative genomic hybridization. Brain Pathol. 2001;11:133–43. doi: 10.1111/j.1750-3639.2001.tb00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeuken JW, Sprenger SH, Gilhuis J, et al. Correlation between localization, age, and chromosomal imbalances in ependymal tumours as detected by CGH. J Pathol. 2002;197:238–44. doi: 10.1002/path.1086. [DOI] [PubMed] [Google Scholar]
- 60.Modena P, Lualdi E, Facchinetti F. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24:5223–33. doi: 10.1200/JCO.2006.06.3701. [DOI] [PubMed] [Google Scholar]
- 61.Mendrzyk F, Korshunov A, Benner A, et al. Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res. 2006;12:2070–9. doi: 10.1158/1078-0432.CCR-05-2363. [DOI] [PubMed] [Google Scholar]
- 62.Huang B, Starostik P, Schraut H, et al. Human ependymomas reveal frequent deletions on chromosomes 6 and 9. Acta Neuropathol. 2003;106:357–62. doi: 10.1007/s00401-003-0739-5. [DOI] [PubMed] [Google Scholar]
- 63.Rajaram V, Gutmann DH, Prasad SK, et al. Alterations of protein 4. 1 family members in ependymomas: a study of 84 cases. Mod Pathol. 2005;18:991–7. doi: 10.1038/modpathol.3800390. [DOI] [PubMed] [Google Scholar]
- 64.Katoh M, Katoh M. Human FOX gene family (Review) Int J Oncol. 2004;25:1495–500. [PubMed] [Google Scholar]
- 65.Hirose Y, Aldape K, Bollen A, et al. Chromosomal abnormalities subdivide ependymal tumors into clinically relevant groups. Am J Pathol. 2001;158:1137–43. doi: 10.1016/S0002-9440(10)64061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ward S, Harding B, Wilkins P, et al. Gain of 1q and loss of 22 are the most common changes detected by comparative genomic hybridisation in paediatric ependymoma. Genes Chromosomes Cancer. 2001;32:59–66. doi: 10.1002/gcc.1167. [DOI] [PubMed] [Google Scholar]
- 67.Carter M, Nicholson J, Ross F, et al. Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br J Cancer. 2002;86:929–39. doi: 10.1038/sj.bjc.6600180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palm T, Figarella-Branger D, Chapon F, et al. Expression profiling of ependymomas unravels localization and tumor grade-specific tumorigenesis. Cancer. 2009;115:3955–68. doi: 10.1002/cncr.24476. [DOI] [PubMed] [Google Scholar]
- 69.Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–35. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Dyer S, Prebble E, Davison V, et al. Genomic imbalances in pediatric intracranial ependymomas define clinically relevant groups. Am J Pathol. 2002;161:2133–41. doi: 10.1016/S0002-9440(10)64491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazewski C, Soukup S, Ballard E, et al. Karyotype studies in 18 ependymomas with literature review of 107 cases. Cancer Genet Cytogenet. 1999;113:1–8. doi: 10.1016/s0165-4608(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 72.Vagner-Capodano AM, Zattara-Cannoni H, Gambarelli D, et al. Cytogenetic study of 33 ependymomas. Cancer Genet Cytogenet. 1999;115:96–9. doi: 10.1016/s0165-4608(99)00080-1. [DOI] [PubMed] [Google Scholar]
- 73.Zheng PP, Pang JC, Hui AB, et al. Comparative genomic hybridization detects losses of chromosomes 22 and 16 as the most common recurrent genetic alterations in primary ependymomas. Cancer Genet Cytogenet. 2000;122:18–25. doi: 10.1016/s0165-4608(00)00265-x. [DOI] [PubMed] [Google Scholar]
- 74.Michalowski MB, de Fraipont F, Michelland S, et al. Methylation of RASSF1A and TRAIL pathway-related genes is frequent in childhood intracranial ependymomas and benign choroid plexus papilloma. Cancer Genet Cytogenet. 2006;166:74–81. doi: 10.1016/j.cancergencyto.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Waha A, Koch A, Hartmann W, et al. Analysis of HIC-1 methylation and transcription in human ependymomas. Int J Cancer. 2004;110:542–9. doi: 10.1002/ijc.20165. [DOI] [PubMed] [Google Scholar]
- 76.Hing S, Lu YJ, Summersgill B, et al. Gain of 1q is associated with adverse outcome in favorable histology Wilms’ tumors. Am J Pathol. 2001;158:393–8. doi: 10.1016/S0002-9440(10)63982-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirai M, Yoshida S, Kashiwagi H, et al. 1q23 gain is associated with progressive neuroblastoma resistant to aggressive treatment. Genes Chromosomes Cancer. 1999;25:261–9. doi: 10.1002/(sici)1098-2264(199907)25:3<261::aid-gcc8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 78.Ozaki T, Paulussen M, Poremba C, et al. Genetic imbalances revealed by comparative genomic hybridization in Ewing tumors. Genes Chromosomes Cancer. 2001;32:164–71. doi: 10.1002/gcc.1178. [DOI] [PubMed] [Google Scholar]
- 79.Korshunov A, Golanov A, Timirgaz V. P14ARF protein (FL-132) immunoreactivity in intracranial ependymomas and its prognostic significance. an analysis of 103 cases. Acta Neuropathol. 2001;102:271–7. doi: 10.1007/s004010100379. [DOI] [PubMed] [Google Scholar]
- 80.Almeida A, Zhu XX, Vogt N, et al. GAC1, a new member of the leucine-rich repeat superfamily on chromosome band 1q32. 1, is amplified and overexpressed in malignant gliomas. Oncogene. 1998;16:2997–3002. doi: 10.1038/sj.onc.1201828. [DOI] [PubMed] [Google Scholar]
- 81.Rand V, Prebble E, Ridley L, et al. Investigation of chromosome 1q reveals differential expression of members of the S100 family in clinical subgroups of intracranial paediatric ependymoma. Br J Cancer. 2008;99:1136–43. doi: 10.1038/sj.bjc.6604651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santi M, Quezado M, Ronchetti R, et al. Analysis of chromosome 7 in adult and pediatric ependymomas using chromogenic in situ hybridization. J Neurooncol. 2005;72:25–8. doi: 10.1007/s11060-004-3117-9. [DOI] [PubMed] [Google Scholar]
- 83.Cassimeris L. Cell division: eg’ing on microtubule flux. Curr Biol. 2004;14:R1000–2. doi: 10.1016/j.cub.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 84.Lopez-Gines C, Gil-Benso R, Faus C, et al. Metastasizing anaplastic ependymoma in an adult. Chromosomal imbalances, metabolic and gene expression profiles. Histopathology. 2009;54:500–4. doi: 10.1111/j.1365-2559.2009.03235.x. [DOI] [PubMed] [Google Scholar]
- 85.Macedo LT, Rogerio F, Pereira EB. Cerebrospinal tumor dissemination in a patient with myxopapillary ependymoma. J Clin Oncol. 2011;29:e795–8. doi: 10.1200/JCO.2011.36.6625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.