ABSTRACT
Although single nucleotide polymorphism (SNP) testing for disease susceptibility is commercially available, translational studies are necessary to understand how to communicate genomic information and potential implications for public health. We explored attitudes about and initial responses to genomic testing for colon cancer risk. Following development of the educational materials, we offered testing for three colon cancer SNPs in a pilot study with primary care patients. Participants completed pre- and post-test sessions and interviews. We analyzed interview transcripts with qualitative software using thematic analysis. All 20 participants opted for SNP testing. Qualitative analysis identified several themes: Motivations for SNP Testing, Before/After: Meaning of Results, Emotional Responses to SNP Results, and Genomic Literacy/Information Delivery. Results demonstrate that individuals will pursue SNP testing in the context of pre- and post-test education. SNP results may influence health behaviors like healthy eating and exercise yet did not appear to impact colon cancer screening intentions.
KEYWORDS: Single nucleotide polymorphism, Genetic testing, Personalized medicine, Qualitative, Research, Direct-to-consumer, Health communication
INTRODUCTION
The last decade has seen a rapid proliferation in research on human genomic variation and potential disease predisposition. Genomic research has generated both excitement and expectation that the findings will translate into clinical use to improve medical outcomes, particularly for common disease [1]. Within the emerging translational genomics field, few studies have explored uptake or perceptions of genomic testing.
Since 2007, several direct-to-consumer (DTC) genetic testing companies have offered testing for susceptibility to a variety of common diseases and traits [2]. These DTC companies use data from genome-wide association studies to provide personalized risk assessments to consumers [3]. Although some DTC testing has recently expanded to exomes and whole genomes [4], most of these risk assessments are generated based upon the presence or absence of genetic variants known as single nucleotide polymorphisms, commonly referred to as SNPs. The commercial availability of SNP, exome, and whole genome testing has outpaced translational genomics research. Gaps remain in our knowledge of the communication, behavioral, and social aspects of this new entity of personal genomics [5]. These gaps fuel the debate about whether genomic information will improve overall health [6–8].
Conducting translational research to explore behavioral and psychosocial outcomes following genomic feedback is challenging in part due to the complexity involved in communicating genomic information. Challenges inherent in the return of SNP results include whether SNP data should be used to inform clinical decision making (clinical utility) and uncertainty about best practices of combining risk estimates from several SNPs [8–12]. Other questions under debate include determining the most appropriate means of communicating this genomic risk information and whether a health care professional should be involved in the process [13, 14]. Additional concerns include the possibility that consumers will interpret their genomic results as a diagnosis of disease or make medical decisions based on their results [15]. Potential harms of DTC testing cited by leading genetics organizations include risks to consumers’ privacy and confidentiality, an overstatement of clinical utility on behalf of the DTC companies, and the chance of misinterpreting results [16].
Initial research examining personal genomics has focused on early adopters of these technologies. Some studies have recruited consumers who have used (or plan to use) a commercial DTC genomic testing entity, while others have recruited individuals to be tested by a non-DTC laboratory within a research setting. Although one study of early adopters of commercial DTC testing found that half of users had concerns about the process or experience [17], another study found that users of DTC testing experienced no increase in anxiety [6]. While users expressed intention to undergo increased screening for various common diseases, they did not partake in actual increased screening nor did they change exercise or diet behaviors [6]. A qualitative study of early DTC users reported they expressed both optimism and skepticism regarding the technology and that they did little or nothing to change their health behaviors after receiving results [18]. Contrary to these studies, a recent online survey of over 1,000 DTC customers found that genomic test results spurred users to seek additional information, share their results with a healthcare provider, and make behavior changes, with 33 and 14 % of users reporting that they improved their diet and physical activity behaviors, respectively [19]. Interestingly, this study found that users’ personal context (such as presence of chronic disease, family history of disease, and a poorer self-reported health) and subjective interpretation of risk were associated with some of these health behavior changes [19].
The Coriell Personalized Medicine Collaborative (CPMC) [20] and the NIH Multiplex Initiative [21] recruited individuals to participate in research of SNP testing or genome-wide profiling. Studies of non-commercial genomic testing have identified curiosity, altruism, and interest in risk information as important motivators for testing [2, 22–24]. While a recent study from the CPMC found that participants generally understood the information conveyed, did not have negative emotional reactions, and planned to share results with medical providers [25], other data suggest that a subset of CPMC participants had misperceptions about SNP testing [23]. Recent data from the Multiplex Initiative found that most individuals did not interpret their results as deterministic, did not experience strong emotional reactions, and did not tend to share results with providers [26]. The few studies of genomic testing performed outside of the DTC setting generally suggest low rates of behavior change after testing [24, 25, 27].
Translational genomic research is in an early stage, and recent reports on early adopters yield equivocal results related to participants’ understanding, sharing, and response to SNP-based genomic risk results. In-depth qualitative research exploring participant attitudes and experiences of SNP testing can help clarify whether genomic information can be used to improve public health. We conducted a qualitative study of primary care patients to better understand how to best explain SNP testing and to evaluate participants’ initial perceptions and responses to SNP testing for colon cancer risk. Colon cancer may be a useful model in which to examine translational genomic research questions as effective screening/prevention exists, screening occurs relatively infrequently and lifestyle risk factors are modifiable. To date, over 15 SNPs have been associated with risk of colon cancer [28].
Health behavior and decision-making theories such as the informed choice model [29, 30] and self-regulation theory [31, 32] provide conceptual grounding for genomic risk communication and translational science. An informed choice is defined as one based on relevant knowledge, consistent with the decision maker’s values and behaviorally implemented [29]. Structuring SNP-testing educational materials to promote informed choice is important given the uncertainty, risks, and limitations of this type of genetic testing [33].
Recent conceptual models of uncertainty in health care apply to genomics [34]. Uncertainty surrounding predictive genomic risk information is due to its probabilistic nature, ambiguity and complexity [34]. When risk information is ambiguous—due to imprecision [34] or uncertainty in probability estimates [35]—research participants may view their risk pessimistically or may avoid making health decisions [34–36]. This concept, called “ambiguity aversion,” impacts affective, cognitive, and behavioral factors in health decisions [36, 37]. For example, perceived ambiguity about mammography recommendations was associated with diminished mammography use and increased worry [37, 38].
Response to SNP information may also be influenced by an individual’s cognitive and emotional processing of health risk information, as described by self-regulation theory [31, 32]. For example, cognitive responses (i.e., perceived risk of colon cancer) may shape subsequent health behavior [39], while emotional responses (i.e., distress/worry) may influence risk management behaviors in certain genetic testing situations [40]. Guided by the informed choice model, literature on uncertainty and self-regulation theory, we examined primary care patients’ initial cognitive, emotional, and behavioral responses to genomic risk information for colon cancer risk. We focused on primary care patients to broaden the current literature beyond DTC users by recruiting participants in a clinical research setting.
MATERIALS AND METHODS
Participants and setting
We conducted a prospective study at the Lombardi Comprehensive Cancer Center at Georgetown University and the Division of General Internal Medicine at Georgetown University Hospital. Participants were recruited from the Division of General Internal Medicine. Persons eligible for this study were male and female primary care patients aged 40 and older. Exclusion criteria were (a) inability to read or understand English or (b) cognitive impairment that precluded informed consent. There were no exclusions based on prior cancer history. Procedures were approved by the Institutional Review Board at Georgetown University/MedStar Health.
Recruitment
We recruited participants through a mailed study invitation letter or in-person recruitment efforts in the Division of General Internal Medicine primary care clinic. Study invitation letters described the study and included the option to decline further contact. In-person recruitment occurred in the waiting room of the Division of General Internal Medicine primary care clinic; trained study personnel approached individuals waiting for medical appointments to describe the study and assess interest and eligibility.
Interested participants were given a copy of the consent document at the time of recruitment or immediately prior to participation. Recruitment materials explained that participants could choose whether or not to be tested for the SNP panel. The consent document emphasized the uncertain clinical utility of SNP results and the possibility that participants could experience anxiety or worry as a result of testing. The benefits of participating included free SNP testing and the potential to gain information about colon cancer risk factors and prevention.
Procedures
Overview
After obtaining written informed consent, participants completed a brief questionnaire that assessed sociodemographics and personal history of cancer and family history of colon cancer. Participants then completed a pre-test education session with a certified genetic counselor followed by a semi-structured interview; education sessions and interviews were completed in-person. If the participant opted to pursue SNP testing, we collected a mouthwash DNA sample after the semi-structured interview. When results were available (approximately 8–10 weeks), we contacted the participant to schedule an in-person results disclosure education session and second semi-structured interview. All education sessions and interviews were audio-recorded if participants agreed. Audio-recordings were transcribed in house and names were removed from the transcripts. Participants received gift cards valued at $30 for the first semi-structured interview and $25 for the second semi-structured interview.
SNP panel and genotyping
We selected three SNPs for inclusion in the research panel: rs6983267 (8q24.21) [41], rs4779584 (CRAC1) [42], and rs3802842 (11q23.1) [43] (Table 1). We chose these SNPs based upon careful review of the literature at the time of study initiation and our a priori selection criteria. The selection criteria we considered when performing literature reviews for published SNP studies included: (1) number of publications which verified the association, (2) size of the populations studied, (3) whether the demographics of the study population reflected our population, and (4) whether statistically significant results were reported. We selected only SNPs associated with increased colon cancer risk (relative to the general population risk) as we planned to assess intentions to screen for this cancer. Importantly, our three SNPs are included in the current colon cancer panels of each of the three major commercial DTC companies [44–46]. From the published studies available, we chose the odds ratios from a specific study for each of our three SNPs (see Table 1).
Table 1.
Colon cancer SNP research panel
| Odds ratio | ||||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Gene or location | Risk allele | Reference allele | Heterozygous (one risk allele and one reference allele) | Homozygous (two risk alleles) | Study | ||
| SNP A: rs6983267 | 8q24.21 | G | T | 1.22 | With family history of colon cancer, 1.27 | 1.38 | With family history of colon cancer, 1.47 | Tomlinson [41] |
| SNP B: rs4779584 | CRAC1 | T | C | 1.23 | 1.70 | Jaeger [42] | ||
| SNP C: rs3802842 | 11q23.1 | C | A | 1.18 | 1.35 | Pittman [43] | ||
Participants who elected to undergo SNP testing provided DNA using a mouthwash oral rinse solution by standard collection procedures. We processed and stored mouthwash samples as pellets at −80 °C until analysis. SNP analysis was performed using allelic discrimination techniques based on real-time PCR methods with Taqman® probes. PCR reactions were performed on the ABI 7900HT sequence detection system (Applied Biosystems, Foster City, CA, USA). All samples were interpreted independently by two reviewers; 20 % of randomly selected samples were analyzed twice for consistency. The CLIA-approved Genomics and Epigenomics Shared Resource at Lombardi Comprehensive Cancer Center performed the genotyping.
SNP risk estimation
We used a multiplicative model to generate lifetime risk estimates [45, 47]. Specifically, we multiplied the odds ratios of each genotype and then multiplied that total by the average population risk of 6 % [41–43]. In the absence of an established method for combining SNP risk estimates [33], we chose the multiplicative model because (1) results generated from alternative models are highly correlated with multiplicative results [47], (2) GWAS studies with our three SNPs have reported that increasing numbers of risk alleles are associated with greater risk [48], and (3) DTC testing companies typically employ a multiplicative model [45]. This model assumes that the individual SNPs occur and behave independently. Table 2 outlines specific examples of the multiplicative model calculation.
Table 2.
Example multiplicative model calculations
| SNP A genotype | SNP B genotype | SNP C genotype | Multiplicative calculation | Lifetime risk calculation | |
|---|---|---|---|---|---|
| Example 1 | GT | CC | AA | 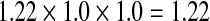 |
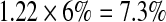 |
| Example 2 | GG | TC | CA | 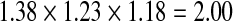 |
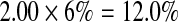 |
Development of educational materials
First, we conducted a thorough review of online materials from the DTC companies and two large SNP-related research studies [20, 21]. These sources guided material development, along with the informed choice model (value-clarification exercises, provision of factual information), literature on uncertainty, and the self-regulation theory (addressing possible cognitive and emotional responses to SNP information).
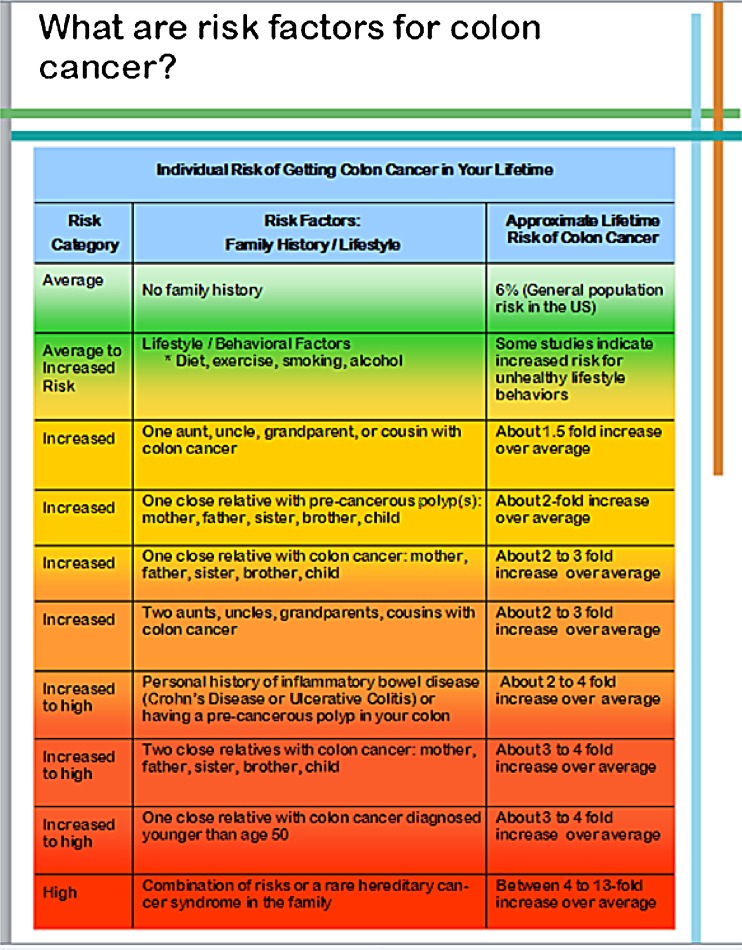
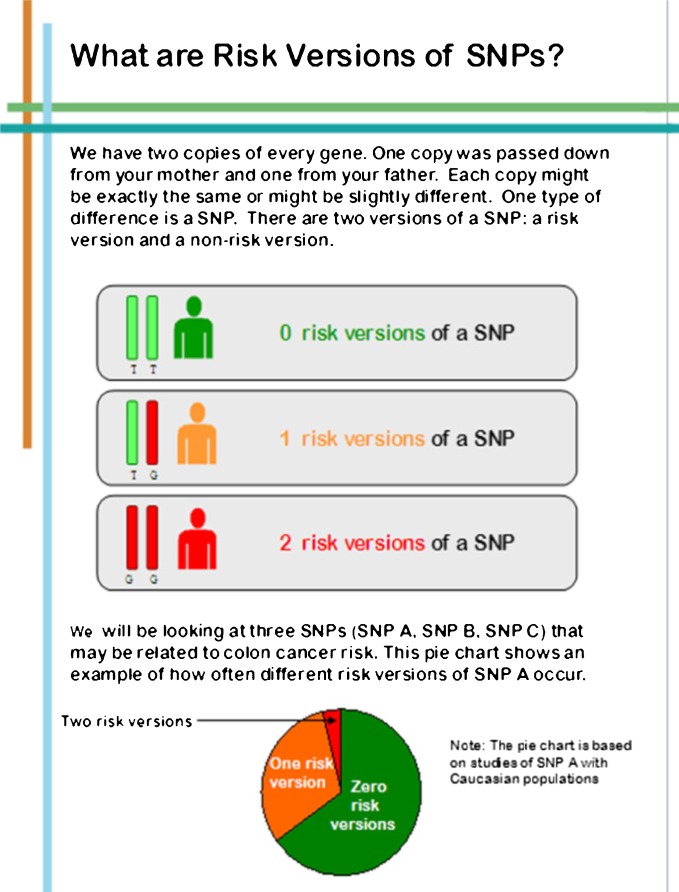
The initial content outline was reviewed by our transdisciplinary research team (behavioral scientists, genetic counselors, bioethicist, molecular biologist, medical oncologist, and internist). Sections of the pre-test education booklet were (1) facts and established risk factors for colon cancer (Fig. 1); (2) definition of SNPs and risk versions with graphical representation (Fig. 2); (3) sample SNP results with associated risk information; (4) benefits, risks and limitations of SNP testing; (5) confidentiality concerns with information regarding the Genetic Information Nondiscrimination Act (GINA) [49]; and (6) steps to reduce colon cancer risk. We emphasized the lack of current evidence for clinical utility of SNP testing for colon cancer.
Fig 1.
Screen shots: risk factors
Fig 2.
Screen shots: risk versions of SNPs
The materials also included exercises designed to elicit participants’ attitudes about SNP testing and general health values (e.g., information seeking). These value clarification exercises, derived from the informed choice model, help people think about their personal opinions and attitudes toward SNP testing—and whether testing was the right decision for them. In addition, these questions prompted individuals to think about how they might respond to SNP risk information. We further highlighted potential cognitive and emotional responses in the section on benefits, risks and limitations of SNP testing. Sample questions included “What does cancer risk mean to me?,” “What action would I think about taking, if any, based on my SNP results?,” and “How do I feel about all that is unknown about what SNP results mean?” among others.
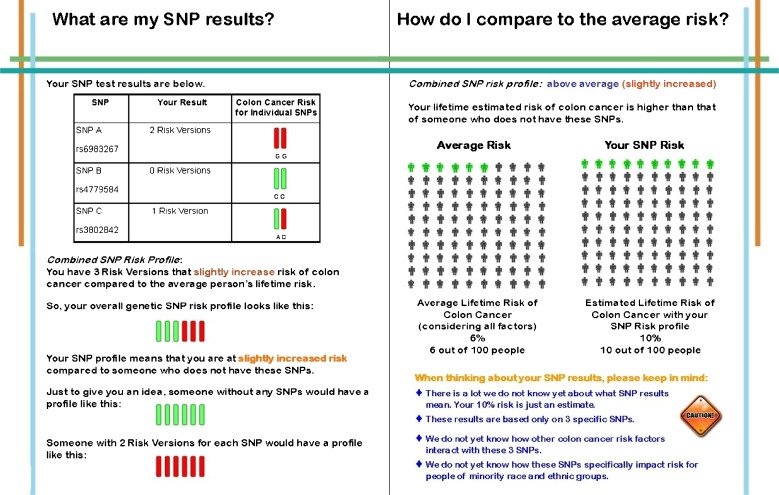
For participants who chose to have SNP testing, we provided test results via an individually tailored, printed booklet and a one-page technical report. Sections in the post test booklet were (1) personalized risk factors for colon cancer, (2) individual SNP test results and estimated lifetime risk of colon cancer (Fig. 3), (3) general colon cancer screening recommendations, (4) limitations related to clinical validity and utility, and (5) suggestions to reduce risk based on population recommendations for screening and health behaviors. The one-page technical report was issued by the laboratory as the formal documentation of the SNP results (e.g., method of SNP analysis and genotype frequencies in reference populations from HapMap) [28]. The lifetime risk estimates were determined only by SNP test results and were not modified based on family history or other lifestyle risk factors for colon cancer.
Fig 3.
Screen shots: my SNP results
To reduce the uncertainty (“ambiguity aversion”) related to genomic risk information interpretation, we included specific and actionable steps tailored for each participant about screening and health behaviors, placed their SNP test result in context compared to average risk, and included a list of cancer- and health-related information resources. The development process for the pre- and post-test materials and education sessions was iterative and included mock sessions with observation and feedback.
Pre-test education session and semi-structured interviews
Following recommendations of the American Society of Clinical Oncology [33], we offered SNP testing within the setting of in-person pre- and post-test counseling conducted by experienced genetic counselors. The study-specific educational materials (i.e., booklet) were used to structure the in-person education session. At the end of the session, participants were asked if they were interested in testing.
Immediately after the pre-test education session, participants completed in-person, semi-structured interviews conducted by a trained research assistant to elicit their experiences with and opinions about the session. Interview questions assessed participants’ thoughts about SNP testing for colon cancer risk (i.e., possible cognitive responses to SNP testing per the self-regulation theory), impressions of the education booklet and session, whether they planned to provide DNA for testing, and anticipated responses to their test results, including potential worry or distress (i.e., possible emotional responses per the self-regulation theory).
Post-test education session
Participants who provided a DNA sample received test results during an in-person session with a genetic counselor. We provided participants with the technical report and individually tailored results booklet. During the post-test session, the genetic counselor emphasized the preliminary nature of the SNP results and that it is not known how SNPs interact with other genes or the environment to influence risk. The uncertainty of the risk estimate was particularly stressed for individuals with established colon cancer risk factors (e.g., personal history of polyps, irritable bowel disease and/or family history of colon cancer).
Participants completed a second semi-structured interview immediately after the post-test disclosure session, assessing their understanding of the test results, cognitive and emotional responses to results, anticipated communication about results, and whether and how SNP results might impact health behaviors (screening for colon cancer, physical activity, diet). We elicited feedback on the education materials and participants’ satisfaction with the SNP testing process.
Analysis
We tabulated descriptive statistics from the demographic surveys to characterize the sociodemographics and cancer history variables of the sample.
Qualitative analyses
We used NVIVO 9, a qualitative research analysis program (NVIVO Software, QSR International) to analyze transcripts of the education sessions and interviews. Our analytic approach was informed by the qualitative research approaches of thematic analysis and qualitative description [50, 51]. Our analyses were guided by the informed choice model and self-regulation theory, in addition to our empirical and clinical experiences related to genetics and cancer risk. Specifically, we examined the transcripts for evidence to support or refute an informed choice about SNP testing (e.g., whether the decision to test appeared consistent with the participants’ values and was based on appropriate knowledge of the risks and limitations of testing). The self-regulation theory guided interpretation of participants’ cognitive and emotional responses to SNP testing and their colorectal cancer risk.
Prior to codebook development, we read all transcripts thoroughly to gain an in-depth understanding of the participants’ experiences. We developed and iteratively refined the codebook as analysis progressed and broad themes became more apparent [52]. The iterative process reflected an attempt to limit bias by reviewing the codebook with members of the research team throughout the analysis. Codes were derived from the transcripts to closely describe participants’ direct comments. Analysis ensued by coding pre- and post-test transcripts from the first participant, followed by pre- and post-test transcripts from the second participant, and so on. This allowed us to closely examine any significant changes in individuals’ perceptions and responses following SNP testing. A goal of the analysis was to remain close to the data with limited abstract interpretation [51]. We used a memo-ing technique to track similarities among transcripts, relationships between codes and noteworthy ideas [53]. Through this process, we consolidated and grouped codes under similar topics formed as themes.
RESULTS
Sample characteristics
Our sample includes 20 participants (see Table 3). The mean age was 61 years (SD = 11.1 years) and five participants reported a personal history of cancer (none with colon cancer). All 20 participants chose to undergo testing for the research panel of SNPs, and at least one risk version was identified in all of these individuals. Combined SNP lifetime risk estimates of colon cancer ranged from 6 % (close to average risk) to 14 % (more than double average risk). In addition to presentation of percentage lifetime risk estimates, we categorized estimates between 6 and 8 % as average to slightly above average, 9 to 11 % as above average risk, and 12 % and higher as twice average risk. We compared study participants to individuals who declined the study when approached in clinic (n = 12). Although sample sizes are small, we found no differences between participants and decliners on age, race, or gender.
Table 3.
Demographics of participants based on self-report (n = 20)
| Characteristics | Number (percent) |
|---|---|
| Age ≥50 | 17 (85) |
| Female | 12 (60) |
| Race | |
| Caucasian | 13 (65) |
| African-American | 5 (25) |
| Multi-racial | 2 (10) |
| Education | |
| <College | 3 (15) |
| ≥College | 17 (85) |
| Personal cancer history (yes) | 5 (25) |
| At least one first or second degree relative with colon cancer | 7 (35) |
| Adherent to general population screening guidelines for colorectal cancer | 18 (90) |
Themes
Through our qualitative analysis of the transcripts, we identified four broad themes: (1) “Motivations for SNP Testing,” (2) “Before and After: Meaning of Results,” (3) “Emotional Responses to SNP Results,” and (4) “Genomic Literacy and Information Delivery.” Sub-themes that emerged within these broad themes are reported below.
Motivations for SNP testing
At pre-test, the two most frequently reported reasons for pursuing SNP testing were to seek medical information (coded “Information gathering”) and to make a contribution to research (coded “Altruism”). Some participants with a personal history of cancer were interested in information for personal use, such as this female participant (SNP08, pre-test):
I feel as though it’s better to have all the information that you possibly can about your health. I have had one cancer and that makes me even more interested in knowing the potential risks for other types of cancer.
Participants without personal or familial experiences with cancer also sought testing with the intent of information gathering: “Well I don’t have any family history of colon cancer so it’s more potential health information for me” (SNP23, female, pre-test). A participant whose mother died of metastatic colon cancer describes her altruistic interest in testing:
I think I would provide a DNA sample for you even if I never got to know the results and you were just going to track me and I was some unknown number. Obviously trying to figure out cancer is important so I would just donate to help further science. Part of it is altruistic I guess… I think giving back is important (SNP17, female, pre-test).
Participants without personal or familial experiences with cancer also sought testing with the intent of information gathering: “Well I don’t have any family history of colon cancer so it’s more potential health information for me” (SNP23, female, pre-test). A participant whose mother died of metastatic colon cancer describes her altruistic interest in testing:
I think I would provide a DNA sample for you even if I never got to know the results and you were just going to track me and I was some unknown number. Obviously trying to figure out cancer is important so I would just donate to help further science. Part of it is altruistic I guess… I think giving back is important (SNP17, female, pre-test).
Other motivations for SNP testing described by the participants included “Curiosity,” “Implications for future generations,” and “Nothing to lose” (Table 4). For example, in the “Nothing to lose” sub-theme, one participant with a family history of colon cancer stated, “…since I already view myself at risk, I can’t imagine how much more at risk you’re going to convince me I’ll be, so I have nothing to lose” (SNP12, male, pre-test).
Table 4.
Motivations for SNP testing: sub-themes
| Sub-themes | Representative quotes |
|---|---|
| Positive attitudes toward testing | |
| Information gathering | It will just be more data about my health and the health of everyone who carries my genes |
| Altruism | Well I see the SNP testing as a pioneer for what’s to come later on. It can… provide very good information to hopefully find a cure for colon cancer in the future |
| Curiosity | I think it’s interesting. I’m curious about it. It’s good to be aware because I don’t feel like I have a risk for colon cancer |
| Implications for future generations | …let’s just see what’s in my body and see if it will help my kids and my grandkids |
| Nothing to lose | I haven’t given too much thought to it. I thought this would be interesting and the testing is harmless |
| Negative attitudes toward testing | |
| Limited information | I think it’s too bad that it doesn’t tell you more |
| Privacy | Quite frankly the one thing that might concern me a little bit is if in fact the SNP results became part of my medical record and then an insurance company may have access to that information at some point |
| Worry | I’d still be upset a little but I wouldn’t be distressed |
Although all participants chose to be tested and receive results, a subset of participants did express negative attitudes regarding SNP testing. These viewpoints were divided into the sub-themes of “Limited information,” “Privacy,” and “Worry” (Table 4). Importantly, of the five participants whose pre-test statements were classified into the “Worry” sub-theme, none felt that SNP results would significantly increase personal distress.
-
Theme 2:
Before and after: meaning of results
We compared concepts that emerged from the pre- and post-test educations sessions. This broad theme of “Before and After: Meaning of Results” was divided into the sub-themes of “Cognitive Responses: Perceived Importance for Health,” “Health Behavior Change,” and “Sharing Results.”
COGNITIVE RESPONSES: PERCEIVED IMPORTANCE FOR HEALTH
Prior to testing, most participants understood the uncertain clinical utility of the test, but in spite of this, wished to proceed. For example, a male participant (SNP06) said at pre-test:
I think it’s potentially good information and I think, well I know it’s in its early stages and the scientists don’t know how to completely relate it to…well they know it has some relationship but the actual quantitative value is still somewhat questionable. But I believe more data is better so I kind of like what everybody is trying to do.
SNP results did not appear to have a substantial impact on the way participants viewed their risk of colon cancer. Most participants perceived their SNP risk profile as indicating slightly increased risk for colon cancer relative to their risk perception prior to the study. This understanding is consistent with the messages delivered by the genetic counselors. Several participants expressed sentiments similar to this female participant’s (SNP14, average risk) comments at post-test: “I am at a very slightly increased risk compared to the average population. It’s very small. I feel OK about them [SNP results].” Two of the 20 participants, one of whom had a personal history of a non-colon cancer, explicitly stated that they perceived their combined risk profiles as high:
It is what it is. It’s a bit high. (SNP07, female, twice average risk, post-test)
So, it’s 9 out of 100 people. …that’s kind of high. (SNP08, female, slightly above average risk, post-test)
Some participants viewed the results as irrelevant. Participants with a personal or family history of cancer placed greater weight on these established risk factors than on genomic information. Consistent with self-regulation theory, when genetic risk information does not fit into a person’s cognitive or emotional views of a health threat, then the information is deemed less relevant [32]. For example, one male participant with a family history of colon cancer said this prior to being tested (SNP21, pre-test):
I was 21 when my father died so I’ve been thinking about colon cancer for a long time. If I had all six risk versions it wouldn’t knock me out of my chair. I’ll tell you that. It won’t substantially alter the way I look at this. I would keep getting screened every three years. No matter what my results are I would do that.
Upon receiving his SNP results, this participant stated (SNP21, average risk, post-test):
It’s a reminder in terms of lifestyle and diet and exercise and screening which I don’t really need much of a reminder but why not be safe? The results themselves were not surprising but at the same time I thought that I would show up higher but it doesn’t mean too much in terms of what I’m already doing in my life as it applies to colon cancer.
HEALTH BEHAVIOR CHANGE
We prompted participants at both pre- and post-test to discuss the ways in which their SNP results might impact their health behaviors, a sub-theme coded as “Health Behavior Change.” At least half of the sample discussed making dietary and exercise changes at both pre- and post-test sessions, particularly decreased red meat consumption and alcohol intake and plans for increased exercise. Several participants stated that the SNP results would not lead them to make behavior changes because they were already in good health and adherent to colon screening. For example, one woman (SNP11) stated at pre-test, “I wouldn’t change anything [based on SNP results]” and confirmed this at post-test with: “I’m doing a great job right now so I don’t think I’d change much of what I’m doing.”
Some participants appeared motivated by the discussion of modifiable risk factors in the pre-test session and made behavior changes prior to receiving results. For example, one male participant (SNP22) stated at his pre-test session, “I’m thinking that even if it came back with no risk factors, I should exercise more and not eat as much red meat.” When SNP22 returned for his results he stated: “I told her [genetic counselor] I was going to cut back and now I only have red meat once a week.” In contrast, another participant was motivated by her SNP results:
I do need to exercise and eat better so I’ve been thinking about that a lot. These results aren’t the reason why I’d start incorporating those new things, but they’d be a motivator for me perhaps. (SNP23, female, average risk, post-test)
SNP results did not appear to alter participants’ plans for colon cancer screening, with two exceptions. One participant, who had not had a colonoscopy in over 10 years, mentioned she would probably “go get another colonoscopy soon” (SNP03, average risk, post-test) and another participant with a personal history of colon polyps and cancer other than colon cancer stated, “I’m doing the colonoscopies and I will talk to my doctor to let her know about these results and maybe get it more frequently than the five or seven years that it’s at now” (SNP22, male, slightly above average risk, post-test). Overall, only two participants were not up-to-date with general population screening recommendations for colon cancer.
SHARING RESULTS
With family
In both pre- and post-test interviews, participants frequently discussed plans to share SNP results with family members, including spouses, children and siblings. A small subset of participants did not plan on discussing results with any family members as results were not perceived as “a big deal” (SNP13, female, average risk, post-test).
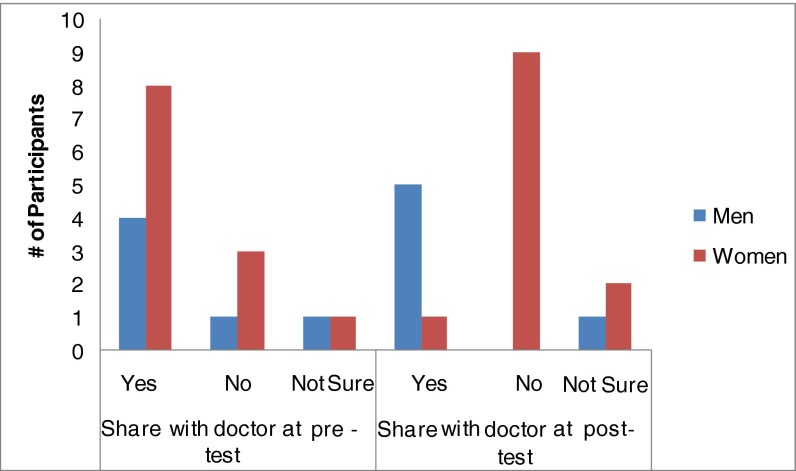
With doctors
We identified different responses at pre- and post-test within the “Sharing Results: with doctors” Category. At the post-test visit, five men (of eight) and one woman (of 12) reported that they would discuss SNP results with their doctors. Interestingly, six women who had stated at the pre-test visit that they planned to discuss results with their doctors changed their minds and said they would not discuss with their doctors after their SNP results were disclosed (Fig. 4). One of these women stated at her pre-test session that she would mention the results to her doctor. She said, “I know it’s new but maybe it would be nice to fill him in because it has to do with my health” (SNP24, pre-test). However, after she received her results she changed her mind, stating:
I don’t think I’m going to share them with my doctor. Since this risk is just an estimate, nothing has been confirmed. This is just preliminary research and I think we have to go a long before these SNPs affect us in a bigger way. (SNP24, average risk, post-test)
Fig 4.
Share results with doctors: pre versus post-test. Two participants were removed from this chart due to missing data
This sentiment about the preliminary and inconclusive nature of the SNP results was echoed by several other women who changed their minds about sharing results with their physicians. Some participants indicated they would share with their doctors if their SNP risk profile had been more significantly elevated: “If I had a higher risk than this then I would want him to know but this isn’t anything to be terribly excited about” (SNP23, female, average risk, post-test). Another factor that emerged in this discussion at both pre- and post-test time points was concern about SNP results being entered into medical records. Despite discussion and description of GINA in the education materials, participants remained concerned about the potential for discrimination. A female participant (SNP07, pre-test) stated:
Now with the new healthcare reform and there is all this that went on before healthcare reform; pre-existing conditions. Do I want to be classified [with] a SNP test as a preexisting condition for denial of X, Y, Z? No, not at this point.
-
Theme 3:
Emotional responses to SNP results
As a distinction is made between cognitive and emotional responses in self-regulation theory [32], we analyzed the transcripts for insight into the emotional process following receipt of SNP results. None of the participants expressed anxiety or distress during the post-test session or interview. Several participants stated that they were not surprised by their results, such as SNP03 (female, average risk, post-test): “I feel comfortable about them. It’s about average. You might get colon cancer but you might not so these risk versions don’t make me too nervous.” Fewer participants expressed some relief upon receipt of their SNP results. One woman (SNP15, average risk, post-test) was “relieved to know I don’t have any higher risk genetically. Otherwise I’m business as usual.” Finally, only one participant with a family history of colon cancer expressed disappointment; he stated he was “hoping for much better results” (SNP06, above average risk, post-test). This participant perceived his SNP risk estimate as high—despite this disappointment; he did not report being distressed by his results.
-
Theme 4:
Genomic literacy and information delivery
An important aspect of the informed choice model is that factual information is conveyed and the problem is defined. Analysis of the transcripts to elicit participant understanding found that although participants had difficulty grasping genomics concepts (Table 5), the in-person format allowed genetic counselors to readily answer questions:
SNP23, female, pre-test: I do have a question. What would this test find that a colonoscopy wouldn’t or vice versa?
Genetic Counselor: I think that’s a great question. With the SNPs, we’re talking about risk and predispositions in your genes. That’s something you were born with… Colonoscopies are looking at actual changes that indicate that you either have colon cancer or have polyps that increase the risk because they go on to become colon cancer. So it’s a screening test. Is there colon cancer present? With this [SNP test] it’s a genetic predisposition.
Table 5.
Difficult genomics concepts
| Somatic versus germline mutations |
| Graphic depiction of a SNP |
| Mendel’s law of segregation |
| Distinctions between SNPs A, B, and C |
| Gene–gene interactions; gene–environment interactions |
| Odds ratios |
| Genetic testing for SNPs versus screening for colon cancer |
Despite some difficulty with certain genomic concepts, most participants felt that they understood the gist of the information, as affirmed by the comments below.
Some of it was not something that I was able to have a deep understanding of. In other words, a lot of the stuff referring to SNP A and risk, I kind of glazed over that. I understood what she was trying to say overall but I didn’t spend an awful lot of time trying to drill down to the molecular biology of it. I just sort of understood it in a general way what it was trying to say and I think that’s all I need to know. (SNP21, male, pre-test)
I think the SNPs are very difficult to understand for anyone who doesn’t have a science background. I’m trying to understand but it’s quite difficult to imagine in your head. But I heard what you’re saying and get what you’re talking about in a global sense. (SNP27, female, pre-test)
INFORMATION DELIVERY
Participants viewed the education sessions as a useful adjunct to the printed materials. Many participants mentioned that the genetic counselor helped them understand the information. However, there were contrasting opinions of whether it was necessary that a genetic counselor deliver the information. For example, SNP12 (male, pre-test) stated, “[The genetic counselor] was very articulate in describing the stuff but again this is not rocket science. This is the easy portion of 9th grade biology” while SNP15 (female, post-test) stated: “But the interpretation, I don’t know if I could look at this on my own. I’m reasonably bright, I could figure it out, but I think it’s better to have these results and have someone walk you through them.”
DISCUSSION
Although motivations for undergoing SNP testing differed, participants expressed more positive than negative attitudes about testing and participant responses indicated an understanding of the benefits and limitations of SNP testing. Positive opinions about testing and the 100 % rate of test uptake in the present sample are consistent with the informed choice model [29] and other conceptual models of SNP testing uptake [54]. Primary care patients’ motivations for undergoing SNP testing related to colon cancer risk (e.g., curiosity and interest in risk information) aligned closely with motivations identified in other reports of individuals participating in both SNP testing and whole-genome sequencing studies [22–24].
Several participants described non-medical reasons as motivators for their pursuing SNP testing. They were motivated by what they perceived as the “personal utility” of genetic testing [55, 56]. These reasons may include perceived value of the genomic information in and of itself, desire for reassurance, or curiosity regarding one’s genes [55].
Pre-existing beliefs about colon cancer risk factors (e.g., family history) influenced perceived risk following receipt of SNP results, consistent with the self-regulation theory [31, 32]. Specifically, new risk information (SNP results) interacts with causal beliefs and identity to influence the threat representation (risk of colon cancer) [32]. Many participants, especially those with a personal or family history of cancer, placed equal or less importance on genomic information than on established risk factors. Similarly, Kaphingst et al. [26] found that genomic test results did not lead to deterministic views. Future research can explore differences in risk perception based on genomic risk, traditional risk factors, and personal/family history, as personal illness experiences play a significant role in perceived risk [57, 58].
Participants valued information regarding health behavior change to reduce risk of colon cancer, especially recommendations regarding dietary changes. The responses related to health habits at both pre- and post-test suggest that participants may have attached more importance to information about behavioral changes than information about genomics. Other studies have indicated that education alone may provide motivation for health behavior change [24, 59]; however, some individuals in the present study indicated that SNP results provided added motivation to change behaviors. McGowan et al. [18] also found that genomic results acted as a motivator for diet and exercise change in a subset of participants who underwent testing with a DTC company. Interestingly, recent reports have found that individuals at greater behavioral risk were more inclined to favor genetic explanations of disease than the role of behavior change in reducing risk [59]. While genomic information may provide incentive for some people, the existing literature suggests there is limited evidence for long-term behavior change [60]. As genomic risk data may impact motivation for behavior change, an effective approach might be to combine genomic information in the context of established behavior change interventions.
Consistent with previous findings [27], most participants reported that they planned to share results with family members, a response that remained consistent both pre- and post-test. Interestingly, after undergoing SNP testing, a number of participants—mostly women—changed their mind about sharing results with a physician, indicating they no longer planned to share results with their doctor. Although some studies reported that more than half of the participants have shared or intended to share genomic test results with their doctors [23], other studies found that only 1–29 % of individuals actually shared results with providers [6, 61, 62].
Despite concerns of increased distress and anxiety following genomic testing [63], our initial data coincide with the existing literature which suggests a low risk of distress and anxiety after genomic testing for susceptibility alleles [6, 64, 65]. The lack of distress in our study may be related to our emphasis on the clinical uncertainty of SNP results. In addition, participants appeared to have a good understanding of the overall message relayed, despite difficulty with some of the genetic concepts presented. Appreciating the “gist” of SNP testing suggests that participants did indeed make informed choices about testing. The identified knowledge gaps highlight the potential importance of a genetic counselor or other health educator for relaying complex genomic information. Our data indicate that a genetic counseling model is useful for SNP testing, as it allows participants to ask questions in real time and get immediate feedback.
Our data also suggest that the specific genomic concepts behind SNP testing are less important for people than the overall message. Perhaps certain individuals need more support and expert guidance while undertaking genomic testing. One recent report suggests that the general public has the potential to misinterpret SNP results from DTC companies [13]. Most DTC companies offer genomic testing through an Internet site without requiring input from a healthcare professional [3], although at least one company, Navigenics, advertises the availability of genetic counselors to help consumers understand results [46]. Future studies can explore best practices for delivery of genomic test results to determine who might benefit from a genetic counseling approach. As the traditional in-person model is not feasible on a population-wide scale, future work can examine the streamlined DTC approach or investigate alternative delivery modes (e.g., phone, Internet). A recent study suggests that individuals confused by genomic test results could benefit from a post-test consultation with a health-care provider, offering another approach [26].
These results should be interpreted in light of the study limitations, which include a lack of generalizability of results given the self-selection of participants and homogeneity of the sample in terms of education level (85 % had college degrees or higher). In addition, SNP testing was offered free of charge which may have contributed to the high rate of test uptake. We did not inquire as to whether participants had undergone prior genetic testing; experience with prior genetic testing could have influenced a participant’s decision to be tested. We did not include SNPs that conferred a decreased risk of colon cancer, nor were we able to calculate lower than average risk based on other factors (e.g., no family history, following all recommended healthy lifestyle recommendations). Exploration of participants’ responses across a wider range of lifetime risk estimates derived from genomic and lifestyle information will be important in future translational genomic research. Another limitation was our use of participant self-report for behavior change and result sharing. Future work can explore best practices for conveying genomics information for individuals of lower literacy and examine the impact of genomic risk information on objectively measured behavior changes.
Our model of approaching all-comers to a primary care clinic for recruitment expands existing research on early adopters of genomic technology. Our results indicate individuals will engage in SNP testing and that their testing decisions are consistent with their attitudes toward SNP testing. We found no evidence of adverse effects of SNP testing. Though questions remain regarding best practices for SNP test education and disclosure of results [66], we provide initial data to examine this complex issue. As genomic testing for complex disease becomes more commonplace, additional translational genomic research studies examining the clinical and personal utility of this information are critical for genomics to eventually impact public health.
Acknowledgments
Acknowledgments
This project was supported by funding through NCI K07CA131172 and NCIK07CA131172-S2 (KG), the Genomics and Epigenomics Shared Resource at the Lombardi Comprehensive Cancer Center, Georgetown University, which is partially supported by NIH/NCI grant P30-CA051008 and from the Fisher Center for Familial Cancer Research.
Footnotes
Implications
Practice: In-person pre- and post-test genomic education results in informed uptake of SNP testing for cancer risk and may have implications for improved motivation for and uptake of certain health behaviors.
Policy: Resources for public health genomic education and continued oversight and regulation of commercially available genomic testing are warranted given the complexities of communicating and understanding genomic information.
Research: Next steps for translational genomic medicine research include larger-scale efforts to examine the clinical utility of genomic results on health behavior outcomes.
References
- 1.Freedman ML, Monteiro AN, Gayther SA, et al. Principles for the post-GWAS functional characterization of cancer risk loci. Nat Genet. 2011;43:513–518. doi: 10.1038/ng.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBride CM, Wade CH, Kaphingst KA. Consumers' views of direct-to-consumer genetic information. Annu Rev Genomics Hum Genet. 2010;11:427–446. doi: 10.1146/annurev-genom-082509-141604. [DOI] [PubMed] [Google Scholar]
- 3.Borry P, Cornel MC, Howard HC. Where are you going, where have you been: a recent history of the direct-to-consumer genetic testing market. J Community Genet. 2010;1:101–106. doi: 10.1007/s12687-010-0023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans JP, Berg JS. Next-generation DNA sequencing, regulation, and the limits of paternalism: the next challenge. JAMA. 2011;306:2376–2377. doi: 10.1001/jama.2011.1788. [DOI] [PubMed] [Google Scholar]
- 5.McBride CM, Bowen D, Brody LC, et al. Future health applications of genomics: priorities for communication, behavioral, and social sciences research. Am J Prev Med. 2010;38:556–565. doi: 10.1016/j.amepre.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364:524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med. 2007;9:665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 8.McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Putting science over supposition in the arena of personalized genomics. Nat Genet. 2008;40:939–942. doi: 10.1038/ng0808-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grosse SD, Khoury MJ. What is the clinical utility of genetic testing? Genet Med. 2006;8:448–450. doi: 10.1097/01.gim.0000227935.26763.c6. [DOI] [PubMed] [Google Scholar]
- 10.Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle—will we get our wish? N Engl J Med. 2008;358:105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- 11.Janssens AC, Gwinn M, Bradley LA, Oostra BA, van Duijn CM, Khoury MJ. A critical appraisal of the scientific basis of commercial genomic profiles used to assess health risks and personalize health interventions. Am J Hum Genet. 2008;82:593–599. doi: 10.1016/j.ajhg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swan M. Multigenic condition risk assessment in direct-to-consumer genomic services. Genet Med. 2010;12:279–288. doi: 10.1097/GIM.0b013e3181d5f73b. [DOI] [PubMed] [Google Scholar]
- 13.Leighton JW, Valverde K, Bernhardt BA. The general public's understanding and perception of direct-to-consumer genetic test results. Public Health Genomics. 2012;15:11–21. doi: 10.1159/000327159. [DOI] [PubMed] [Google Scholar]
- 14.Stack CB, Gharani N, Gordon ES, Schmidlen T, Christman MF, Keller MA. Genetic risk estimation in the Coriell Personalized Medicine Collaborative. Genet Med. 2011;13:131–139. doi: 10.1097/GIM.0b013e318201164c. [DOI] [PubMed] [Google Scholar]
- 15.McGuire AL, Diaz CM, Wang T, Hilsenbeck SG. Social networkers' attitudes toward direct-to-consumer personal genome testing. Am J Bioeth. 2009;9:3–10. doi: 10.1080/15265160902928209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldsmith L, Jackson L, O'Connor A, Skirton H. Direct-to-consumer genomic testing: systematic review of the literature on user perspectives. Eur J Hum Genet. 2012. doi:10.1038/ejhg.2012.18. [DOI] [PMC free article] [PubMed]
- 17.Bloss CS, Ornowski L, Silver E, et al. Consumer perceptions of direct-to-consumer personalized genomic risk assessments. Genet Med. 2010;12:556–566. doi: 10.1097/GIM.0b013e3181eb51c6. [DOI] [PubMed] [Google Scholar]
- 18.McGowan ML, Fishman JR, Lambrix MA. Personal genomics and individual identities: motivations and moral imperatives of early users. New Genet Soc. 2010;29:261–290. doi: 10.1080/14636778.2010.507485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman DJ, Bollinger JM, Dvoskin RL, Scott JA. Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. J Genet Couns. 2012. doi:10.1007/s10897-012-9483-0. [DOI] [PubMed]
- 20.Coriell Personalized Medicine Collaborative. Available at http://cpmc.coriell.org/. Accessed 26 Jan 2012.
- 21.The Multiplex Initiative. Available at https://www.multiplex.nih.gov/. Accessed 26 Jan 2012.
- 22.Facio FM, Brooks S, Loewenstein J, Green S, Biesecker LG, Biesecker BB. Motivators for participation in a whole-genome sequencing study: implications for translational genomics research. Eur J Hum Genet. 2011;19:1213–1217. doi: 10.1038/ejhg.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gollust SE, Gordon ES, Zayac C, et al. Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics. 2012;15:22–30. doi: 10.1159/000327296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Daniel JM, Haga SB, Willard HF. Considerations for the impact of personal genome information: a study of genomic profiling among genetics and genomics professionals. J Genet Couns. 2010;19:387–401. doi: 10.1007/s10897-010-9297-x. [DOI] [PubMed] [Google Scholar]
- 25.Gordon ES, Griffin G, Wawak L, Pang H, Gollust SE, Bernhardt BA. “It's not like Judgment Day”: public understanding of and reactions to personalized genomic risk information. J Genet Couns. 2011. doi:10.1007/s10897-011-9476-4. [DOI] [PMC free article] [PubMed]
- 26.Kaphingst KA, McBride CM, Wade C, et al. Patients' understanding of and responses to multiplex genetic susceptibility test results. Genet Med. 2012. doi:10.1038/gim.2012.22. [DOI] [PMC free article] [PubMed]
- 27.Marteau TM, French DP, Griffin SJ, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev. 2010; CD007275. [DOI] [PubMed]
- 28.National Cancer Institute. Variant GPS. SNPT500Cancer. Available at http://variantgps.nci.nih.gov/cgfseq/pages/snp500.do. Accessed 1 Dec 2011.
- 29.Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect. 2001;4:99–108. doi: 10.1046/j.1369-6513.2001.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michie S, Dormandy E, Marteau TM. Informed choice: understanding knowledge in the context of screening uptake. Patient Educ Couns. 2003;50:247–253. doi: 10.1016/S0738-3991(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 31.Cameron L, Leventhal H, editors. The self-regulation of health and illness behaviour. London: Routledge; 2003. [Google Scholar]
- 32.Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Soc Sci Med. 2006;62:1360–1368. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K. American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 34.Han PK, Klein WM, Arora NK. Varieties of uncertainty in health care: a conceptual taxonomy. Medical Decis Making. 2011;31:828–838. doi: 10.1177/0272989X11393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camerer C, Weber M. Recent developments in modeling preferences: uncertainty and ambiguity. J Risk Uncertain. 1992;5:325–370. doi: 10.1007/BF00122575. [DOI] [Google Scholar]
- 36.Politi MC, Han PK, Col NF. Communicating the uncertainty of harms and benefits of medical interventions. Medical Decis Making. 2007;27:681–695. doi: 10.1177/0272989X07307270. [DOI] [PubMed] [Google Scholar]
- 37.Han PK, Moser RP, Klein WM. Perceived ambiguity about cancer prevention recommendations: associations with cancer-related perceptions and behaviours in a US population survey. Health Expect. 2007;10:321–336. doi: 10.1111/j.1369-7625.2007.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han PK, Kobrin SC, Klein WM, Davis WW, Stefanek M, Taplin SH. Perceived ambiguity about screening mammography recommendations: association with future mammography uptake and perceptions. Cancer Epidemiol Biomarkers Prev. 2007;16:458–466. doi: 10.1158/1055-9965.EPI-06-0533. [DOI] [PubMed] [Google Scholar]
- 39.Ferrer RA, Hall KL, Portnoy DB, Ling BS, Han PK, Klein WM. Relationships among health perceptions vary depending on stage of readiness for colorectal cancer screening. Health Psychol. 2011;30:525–535. doi: 10.1037/a0023583. [DOI] [PubMed] [Google Scholar]
- 40.Cameron LD, Diefenbach MA. Responses to information about psychosocial consequences of genetic testing for breast cancer susceptibility: influences of cancer worry and risk perceptions. J Health Psychol. 2001;6:47–59. doi: 10.1177/135910530100600104. [DOI] [PubMed] [Google Scholar]
- 41.Tomlinson I, Webb E, Carvajal-Carmona L, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet. 2007;39:984–988. doi: 10.1038/ng2085. [DOI] [PubMed] [Google Scholar]
- 42.Jaeger E, Webb E, Howarth K, et al. Common genetic variants at the CRAC1 (HMPS) locus on chromosome 15q13.3 influence colorectal cancer risk. Nat Genet. 2008;40:26–28. doi: 10.1038/ng.2007.41. [DOI] [PubMed] [Google Scholar]
- 43.Pittman AM, Webb E, Carvajal-Carmona L, et al. Refinement of the basis and impact of common 11q23.1 variation to the risk of developing colorectal cancer. Hum Mol Genet. 2008;17:3720–3727. doi: 10.1093/hmg/ddn267. [DOI] [PubMed] [Google Scholar]
- 44.23andMe. Available at https://www.23andme.com/health/Colorectal-Cancer/. Accessed 1 Dec 2011.
- 45.deCODE genetics. Risk calculations. Available at http://www.decodeme.com/health-watch-information/risk-calculation. Accessed 1 Dec 2011.
- 46.Navigenics. Available at http://www.navigenics.com/visitor/what_we_offer/conditions_we_cover/colon_cancer/. Accessed 1 Dec 2011.
- 47.Weedon MN, McCarthy MI, Hitman G, et al. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med. 2006;3:e374. doi: 10.1371/journal.pmed.0030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kupfer SS, Anderson JR, Hooker S, et al. Genetic heterogeneity in colorectal cancer associations between African and European Americans. Gastroenterology. 2010;139:1677–1685. doi: 10.1053/j.gastro.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hudson KL. Genomics, health care, and society. N Eng J Med. 2011;365:1033–1041. doi: 10.1056/NEJMra1010517. [DOI] [PubMed] [Google Scholar]
- 50.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 51.Sandelowski M. Combining qualitative and quantitative sampling, data collection, and analysis techniques in mixed-method studies. Res Nurs Health. 2000;23:246–255. doi: 10.1002/1098-240X(200006)23:3<246::AID-NUR9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Beeson D. Nuance, complexity, and context: qualitative methods in genetic counseling research. J Genet Couns. 1997;6:21–43. doi: 10.1023/A:1025659701805. [DOI] [PubMed] [Google Scholar]
- 53.Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2. Thousand Oaks: Sage; 1994. [Google Scholar]
- 54.Wade CH, Shiloh S, Woolford SW, et al. Modelling decisions to undergo genetic testing for susceptibility to common health conditions: an ancillary study of the multiplex initiative. Psychol Health. 2012;27:430–444. doi: 10.1080/08870446.2011.586699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bunnik EM, Schermer MH, Janssens AC. Personal genome testing: test characteristics to clarify the discourse on ethical, legal and societal issues. BMC Med Ethics. 2011;12:11. doi: 10.1186/1472-6939-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster MW, Mulvihill JJ, Sharp RR. Evaluating the utility of personal genomic information. Genet Med. 2009;11:570–574. doi: 10.1097/GIM.0b013e3181a2743e. [DOI] [PubMed] [Google Scholar]
- 57.Lea DH, Kaphingst KA, Bowen D, Lipkus I, Hadley DW. Communicating genetic and genomic information: health literacy and numeracy considerations. Public Health Genomics. 2011;14:279–289. doi: 10.1159/000294191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walter FM, Emery J, Braithwaite D, Marteau TM. Lay understanding of familial risk of common chronic diseases: a systematic review and synthesis of qualitative research. Ann Fam Med. 2004;2:583–594. doi: 10.1370/afm.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Neill SC, McBride CM, Alford SH, Kaphingst KA. Preferences for genetic and behavioral health information: the impact of risk factors and disease attributions. Ann Behav Med. 2010;40:127–137. doi: 10.1007/s12160-010-9197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marteau TM, Lerman C. Genetic risk and behavioural change. BMJ. 2001;322:1056–1059. doi: 10.1136/bmj.322.7293.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon ES, Stack CB, Gharani N, Schmidlen TJ, Christman MF, Keller MA. Perceived risk, anxiety and sharing behavior in response to personalized risk information in a cohort study. In Presented at the 60th Annual Meeting of the American Society of Human Genetics, Washington DC, November 2010 (Abstract 1629).
- 62.Kaufman D, Murphy-Bollinger J, Devaney S, Scott J. A survey of 1,048 customers of three direct-to-consumer personal genomic testing companies about motivations, attitudes, and responses to testing. In Presented at the 60th Annual Meeting of the American Society of Human Genetics, Washington DC, November 2010 (Abstract 390).
- 63.Bloss CS, Darst BF, Topol EJ, Schork NJ. Direct-to-consumer personalized genomic testing. Hum Mol Genet. 2011;20:R132–R141. doi: 10.1093/hmg/ddr349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts JS, Christensen KD, Green RC. Using Alzheimer's disease as a model for genetic risk disclosure: implications for personal genomics. Clin Genet. 2011;80:407–414. doi: 10.1111/j.1399-0004.2011.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanderson SC, O'Neill SC, White DB, et al. Responses to online GSTM1 genetic test results among smokers related to patients with lung cancer: a pilot study. Cancer Epidemiol Biomarkers Prev. 2009;18:1953–1961. doi: 10.1158/1055-9965.EPI-08-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Daniel JM. The prospect of genome-guided preventive medicine: a need and opportunity for genetic counselors. J Genet Couns. 2010;19:315–327. doi: 10.1007/s10897-010-9302-4. [DOI] [PubMed] [Google Scholar]
- 67.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Eng J Med. 2010;363:166–176. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 68.National Center for Biotechnology Information. What is a Genome? Available at http://www.ncbi.nlm.nih.gov/About/primer/genetics_genome.html. Accessed 1 Dec 2011.