Abstract
Immunization registries are effective electronic tools for assessing vaccination coverage, but are only as good as the information reported to them. This review summarizes studies through August 2010 on vaccination coverage in registries and identifies key characteristics of successful registries. Based on the current state of registries, paper-based charts combined with electronic registry reporting provide the most cohesive picture of coverage. To ultimately supplant paper charts, registries must exhibit increased coverage and participation.
MeSH Key Words: Immunization/statistics & numerical data, Registries, Information Systems, Patient Compliance, Immunization Programs/utilization
Introduction
It is well known that vaccination is one of the most successful public health initiatives to date, and having a successful immunization program is paramount to preventing vaccine-preventable diseases (1–3). However, today’s vaccination delivery system is insufficient to keep up with the demands of an ever-changing landscape (3,4). To address this need and ensure adherence to recommended vaccination schedules, immunization registries are increasingly being utilized. This brief defines immunization registries (more recently known as immunization information systems) as population based electronic information systems that minimally capture and report vaccination events.
Studies have already demonstrated the use of regional registries increases vaccination coverage and documentation (5,6). Additionally, decision support capabilities, such as patient and provider reminders, provided through electronic information systems can improve up-to-date (UTD) rates (7). Participation in registries, or the number of children with vaccination records, has been steadily increasing (8). Despite this, the question remains among children recorded in the registry, are they UTD on their vaccinations? And, if a higher percentage of UTD vaccines indicate a more robust registry, what are the contributing factors to this increased coverage? This brief considers these two questions.
Methods
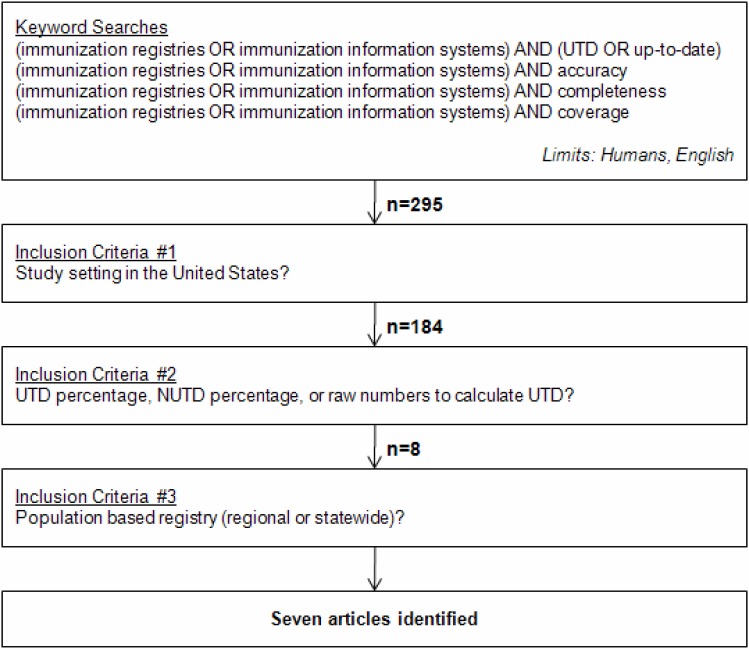
Relevant articles were identified via a PubMed search using the following keywords: (immunization registries OR immunization information systems) AND (UTD OR up-to-date). The keywords “accuracy,” “completeness,” and “coverage” were used in lieu of “UTD OR up-to-date” for subsequent searches and generated additional articles. Literature searches were limited to studies in humans and published in English. As no date limits were applied, studies available through August 2010 were eligible. Figure 1 is a graphical representation of the methodology.
Figure 1:
PubMed Search Methodology to Identify Literature on Vaccination Coverage in Immunization Registries
UTD = up-to-date, NUTD = not up-to-date
Three inclusion criteria were applied. First, the study setting was based in United States. Second, the study investigators included a vaccination up-to-date percentage, or the raw numbers from which the percentage was calculated, and third, the immunization registry was population based (that is, a hospital’s electronic medical record was excluded). These inclusion criteria yielded seven articles which we believe represent the body of published work on UTD coverage in an immunization registry at the time of writing (9–15).
Results
The seven articles were all unique studies covering various geographic regions, and consequently various regional and statewide immunization registries. Therefore these results are experiences with not a single registry, but myriad registries each with its own population of patients and providers. Table 1 summarizes the seven articles by study type, population, and setting; outcome measure; and UTD results.
Table 1:
Summary of Articles for Vaccination Coverage in Immunization Registries Review
| Author (Year) | Study Type, Population & Setting | Outcome Measure Reviewed | Results |
|---|---|---|---|
| Kolasa et al. (2006) | Cross-sectional survey in Philadelphia, PA of private practices where children were at risk for underimmunization. Children age 19 to 35 months. | UTD for 4:3:1:3 series in registry versus provider charts. | 62% UTD coverage in registry; 80% UTD coverage in chart. |
| Boyd et al. (2002) | Cross-sectional survey in Bexar County, TX of clinics participating in the Vaccine for Children program. Children aged 12 to 35 months | UTD for 4:3:1 series in registry versus clinic provider charts. | 64.1% coverage in registry; 39.8% coverage in clinic charts. |
| Stille et al. (2000) | Cohort study in Hartford, CT of infants younger than 1 month tracked through 7 months. Source population was three primary care facilities serving >80% Medicaid population. | UTD at seven months defined as 3 DTaP, 2 polio, 3 Hib, 2 Hep B in provider charts versus charts plus registry. | 53% UTD at chart review for cohort; 58% UTD after chart plus registry review for cohort. |
| Davidson et al. (2003) | Cohort study in Denver, CO at Denver Health Medical Center. Two birth cohorts from 1993 and 1998. | UTD for 3:2:3 series in registry assessed at 12 months. | 83.1% UTD for 1993 cohort; 78.9% UTD for 1998 cohort. |
| Khare et al. (2006) | Cross-sectional survey of four mature registries located in the US. Children aged 19 to 35 months. | UTD for 4:3:1:3 series in registry versus provider charts. | 31.7%, 65.4%, 71.9%, and 61.8% coverage at each site based on registry; 65.6%, 78.8%, 81.6%, and 77.0% UTD coverage at each site based on charts, respectively. |
| Callahan et al. (2004) | Cohort study in Syracuse, New York at University Hospital. Patients < 11 years presenting in the Emergency Department. | UTD per Advisory Committee on Immunization Practices, depending on age. | 61% UTD in cohort. |
| Kolasa et al. (2009) | Cross-sectional survey in Philadelphia, PA of all children born between November 1, 2003 and October 1, 2004, and living in areas served by two community-based outreach organizations. Study population was NUTD according to the registry at 10 months of age. | UTD at 10 months defined as 3 DTaP, 2 polio, 2 Hib, 2 Hep B (+1 at birth not recorded) in registry. | 64% UTD post-outreach, despite being marked as NUTD in registry. |
UTD = up-to-date, NUTD = not up-to-date; DTaP = Diptheria, Tetanus, acellular Pertusis, Hib = Haemophilus influenzae type b, Hep B = Hepatitis B
When determining immunization status, the study investigators have used a subset of the U.S. Department of Health and Human Services recommended vaccinations (16) depending on various factors, such as subject age, ability to track antigens in the registry, antigen availability, and local preferences. While the studies reviewed mainly used different vaccination series dependent mostly on age, a direct comparison is still valid as we are tracking immunization completeness in the registry, and are not interested in comparing antigens tracked per registry. Supplementing U.S. vaccination policy, the World Health Organization (WHO) has issued the Global Immunization Vision and Strategy aiming to achieve a 90% national coverage and 80% local coverage (17). Although desirable to evaluate the results in Table 1 against WHO criteria, a direct comparison may be misleading as children in registries represent a specific subset of the total population.
The two most common series based on the study subjects age included the 4:3:1(:3) series and the 3:2:2 series. The 4:3:1(:3) includes 4 Diptheria, Tetanus, acellular Pertusis (DTaP); 3 Polio (oral or inactivated); 1 Measles, Mumps, Rubella (MMR); and 3 Haemophilus influenzae type b (Hib). The 3:2:2 series includes 3 DTaP, 2 Polio, and 2 Hib. The 3:2:2 series may also be recorded as 3:2:3 indicating a third Hib vaccination, depending on the age and eligibility of the child. Additionally, for both series, the Hepatitis B vaccination may or may not be considered in the study.
Discussion
The current state of registry coverage was ascertained from the available literature. Registry completeness ranged from 31.7% to 83.1%, whereas paper charts ranged from 39.8% to 81.6%. In studies where registry data were compared to provider charts, the charts were more inclusive of vaccination events. In all cases, when provider charts were supplemented with registry data, the UTD percent increased. This is an important finding; registries can augment the patient chart to provide a more inclusive look into vaccination UTD rates, particularly for children that have switched providers who may have incomplete histories.
Many factors can affect vaccination coverage both in paper charts and electronically, and it is currently not clear which have the greatest effect. One study that attempted to determine which factors were associated with low coverage found that the level of coverage was not well predicted by number of providers per capita, a common assumption (18). Specific reasons were attributed to registry incompleteness. Kolasa observed lack of electronic data submission resulted in a disparity of completeness between the registry and charts (9). Studies have shown electronic submission results in greater accuracy (8,9,19). Additionally, Kolasa found that hospital-based practices, which typically have a more robust infrastructure, have a higher UTD percentage versus smaller practices. Davidson posited the infancy of a registry explains why historical immunization events captured in provider charts are not electronically accessible (12). It stands to reason over time that electronic immunization data will increase.
Legislation may also affect differences in registry coverage. A survey of state-level immunization information system legislation found wide variability in whether or not states had laws authorizing an immunization information system, mandating reporting to that system, addressing sharing of immunization information (and healthcare information in general) and the type of consent required to share information (20).In cases where switching providers was common, or registry use was mandated, UTD completeness of the registry eclipsed paper charts (10,12).
There are several limitations to these studies. First, by consulting a registry, the practitioner assumes accuracy of the reported data. Callahan notes “[t]his has been shown to be a problem with registries in their current state of development” (14,p300). Indeed, Kolasa found among children listed in the Philadelphia registry as being not UTD on their vaccinations, 64% were found to be UTD from charts (15). Stille had similar qualms with the Connecticut state registry; specifically vaccinations received by un-identified providers cannot be tracked and children who have relocated outside the reaches of the registry may be erroneously reported as not UTD (11). Second, study populations were frequently drawn from underimmunized, “at risk” populations who visit public providers, potentially affecting the external validity of the results. However, since the underlying technology of the registry is the same regardless of the provider, it is reasonable to expect the findings to generalize. An opportunity exists for additional studies to assess particular “at risk” populations, such as low birth weight and immunocompromised children, and use of electronic registries to decrease mortality. Last, UTD percent may not be an accurate metric for studying immunization coverage. In a study by Robison et al., the UTD measure served as a general guide, but does not provide a reason for the low coverage (21).
There are limitations to the methods used to conduct this literature review. The registries considered for inclusion had to be population based. By disqualifying a hospital’s electronic medical record, we feel the population is more inclusive of the total vaccinated population, not just children that have presented to a hospital likely for other indications. Next, our search strategy may have excluded relevant articles, although we feel the searches incorporated the totality of keywords used to index these articles. Finally, only studies that were U.S.-based were eligible. Given the immunization policy and tracking differences observed between countries, this allowed us to draw conclusions specific to the U.S.
Percentages of UTD children in immunization registries are lagging compared to provider charts. To accurately assess a child’s true vaccination status, a combination of the registry and providers charts provides the best picture. Registries that offered decision support, broad participation, and efficient electronic reporting of vaccination events tended to have a higher proportion of UTD children.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention (CDC) Impact of vaccines universally recommended for children--United States, 1990–1998. MMWR Morb Mortal Wkly Rep. 1999 Apr 2;48(12):243–8. [PubMed] [Google Scholar]
- 2.Stern AM, Markel H. The history of vaccines and immunization: familiar patterns, new challenges. Health Aff (Millwood) 2005 May-Jun;24(3):611–21. doi: 10.1377/hlthaff.24.3.611. [DOI] [PubMed] [Google Scholar]
- 3.Orenstein WA, Douglas RG, Rodewald LE, Hinman AR. Immunizations in the United States: success, structure, and stress. Health Aff (Millwood) 2005 May-Jun;24(3):599–610. doi: 10.1377/hlthaff.24.3.599. [DOI] [PubMed] [Google Scholar]
- 4.Hammer LD, Curry ES, Harlor AD, et al. Increasing immunization coverage. Pediatrics. 2010 Jun;125(6):1295–304. doi: 10.1542/peds.2010-0743. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox SA, Koepke CP, Levenson R, Thalheimer JC. Registry-driven, community-based immunization outreach: a randomized controlled trial. Am J Public Health. 2001 Sep;91(9):1507–11. doi: 10.2105/ajph.91.9.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempe A, Steiner JF, Renfrew BL, Lowery E, Haas K, Berman S. How much does a regional immunization registry increase documented immunization rates at primary care sites in rural colorado? Ambul Pediatr. 2001 Jul-Aug;1(4):213–6. doi: 10.1367/1539-4409(2001)001<0213:hmdari>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson VJ, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003941. doi: 10.1002/14651858.CD003941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Progress in immunization information systems - United States, 2008. MMWR Morb Mortal Wkly Rep. 2010 Feb 12;59(5):133–5. [PubMed] [Google Scholar]
- 9.Kolasa MS, Chilkatowsky AP, Clarke KR, Lutz JP. How complete are immunization registries? The Philadelphia story. Ambul Pediatr. 2006 Jan-Feb;6(1):21–4. doi: 10.1016/j.ambp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Boyd TD, Linkins RW, Mason K, Bulim I, Lemke B. Assessing immunization registry data completeness in Bexar County, Texas. Am J Prev Med. 2002 Apr;22(3):184–7. doi: 10.1016/s0749-3797(01)00427-5. [DOI] [PubMed] [Google Scholar]
- 11.Stille CJ, Christison-Lagay J. Determining immunization rates for inner-city infants: statewide registry data vs medical record review. Am J Public Health. 2000 Oct;90(10):1613–5. doi: 10.2105/ajph.90.10.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson AJ, Melinkovich P, Beaty BL, et al. Immunization registry accuracy: improvement with progressive clinical application. Am J Prev Med. 2003 Apr;24(3):276–80. doi: 10.1016/s0749-3797(02)00638-4. [DOI] [PubMed] [Google Scholar]
- 13.Khare M, Piccinino L, Barker LE, Linkins RW. Assessment of immunization registry databases as supplemental sources of data to improve ascertainment of vaccination coverage estimates in the national immunization survey. Arch Pediatr Adolesc Med. 2006 Aug;160(8):838–42. doi: 10.1001/archpedi.160.8.838. [DOI] [PubMed] [Google Scholar]
- 14.Callahan JM, Reed D, Meguid V, Wojcik S, Reed K. Utility of an immunization registry in a pediatric emergency department. Pediatr Emerg Care. 2004 May;20(5):297–301. doi: 10.1097/01.pec.0000125657.05196.55. [DOI] [PubMed] [Google Scholar]
- 15.Kolasa MS, Lutz JP, Cofsky A, Jones T. Provider chart audits and outreach to parents: impact in improving childhood immunization coverage and immunization information system completeness. J Public Health Manag Pract. 2009 Nov-Dec;15(6):459–63. doi: 10.1097/PHH.0b013e3181abbee6. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Recommended immunization schedules for persons aged 0 through 18 years---United States, 2010. MMWR Morb Mortal Wkly Rep. 2010 Jan 8;58(51&52):1–4. [Google Scholar]
- 17.World Health Organization Immunization, Vaccines and Biologicals > Global Immunization Vision and Strategy. Available at: http://www.who.int/immunization/givs/goals/en/index.html. Accessed May 19, 2011.
- 18.Rosenthal J, Rodewald L, McCauley M, et al. Immunization coverage levels among 19- to 35-month-old children in 4 diverse, medically underserved areas of the United States. Pediatrics. 2004 Apr;113(4):e296–302. doi: 10.1542/peds.113.4.e296. [DOI] [PubMed] [Google Scholar]
- 19.Kolasa MS, Cherry JE, Chilkatowsky AP, Reyes DP, Lutz JP. Practice-based electronic billing systems and their impact on immunization registries. J Public Health Manag Pract. 2005 Nov-Dec;11(6):493–9. doi: 10.1097/00124784-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, National Center of Immunization and Respiratory Diseases Survey of State Immunization Information System Legislation. Available at: http://www.cdc.gov/vaccines/programs/iis/privacy/legsurv.htm. Accessed May 19, 2011.
- 21.Robison SG, Kurosky SK, Young CM, Gallia CA, Arbor SA. Immunization milestones: a more comprehensive picture of age-appropriate vaccination. J Biomed Biotechnol. 2010;2010:916525. doi: 10.1155/2010/916525. [DOI] [PMC free article] [PubMed] [Google Scholar]