Abstract
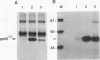
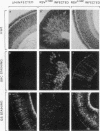
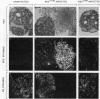
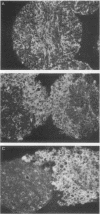
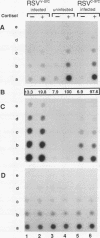
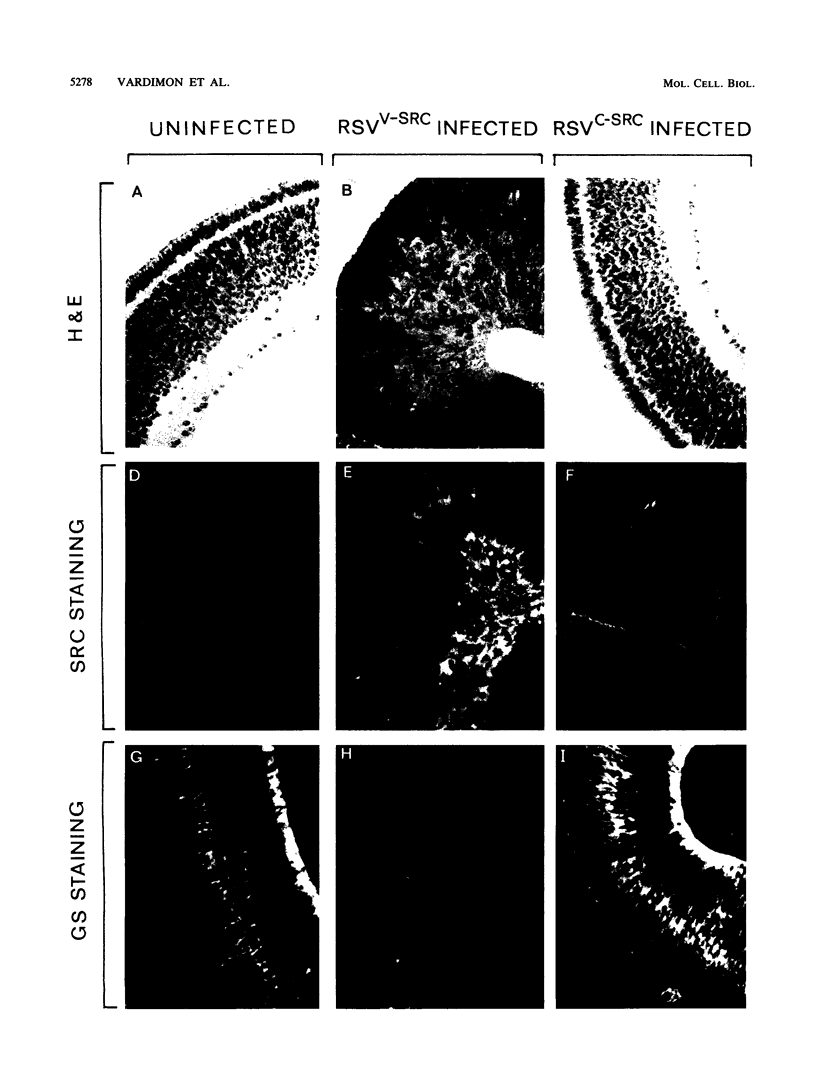
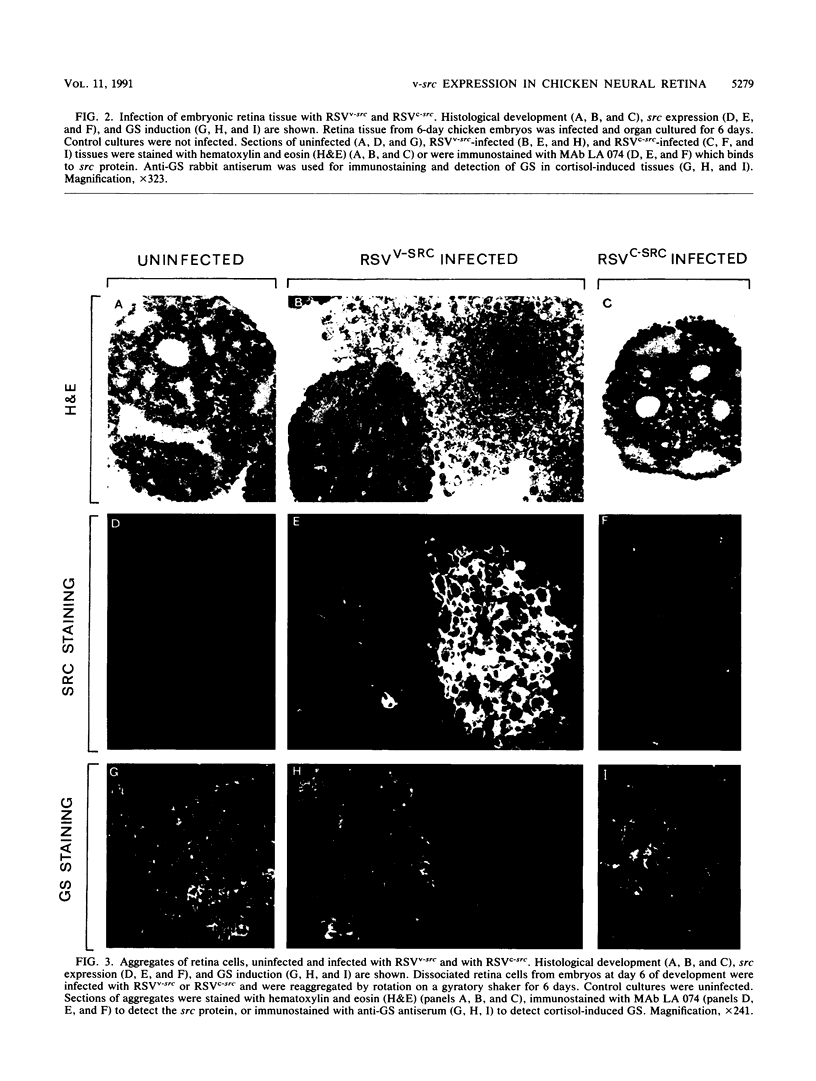
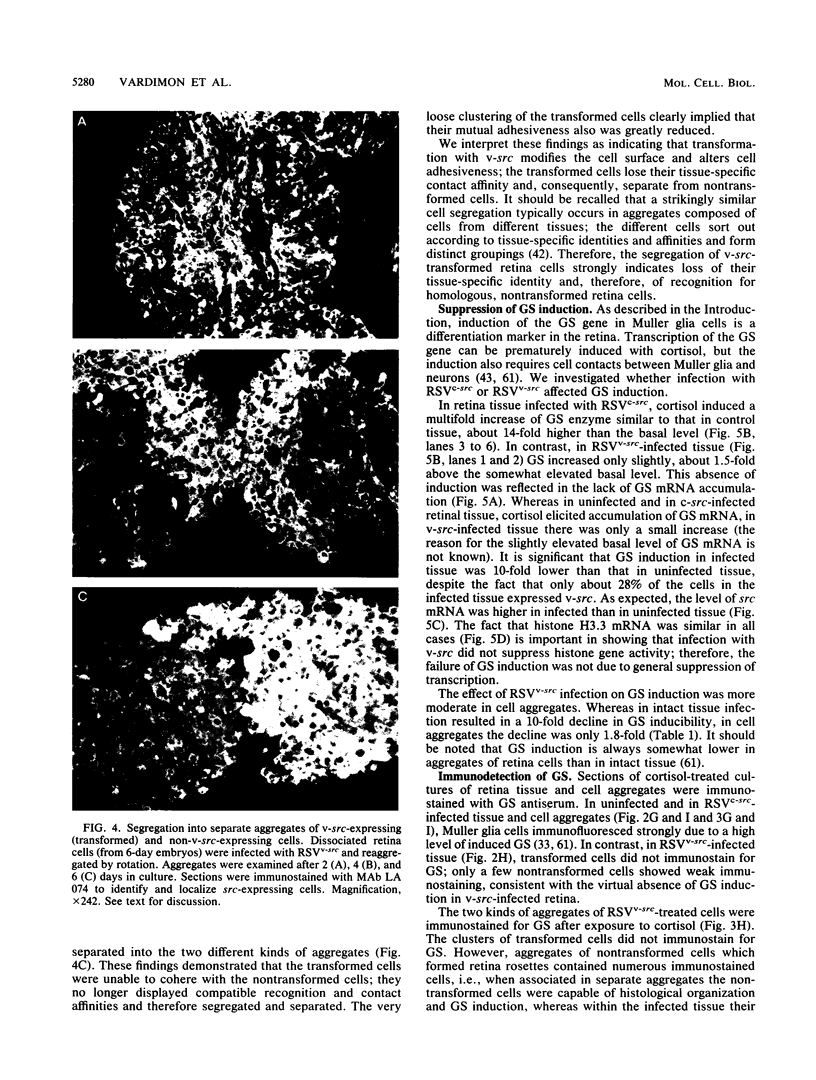
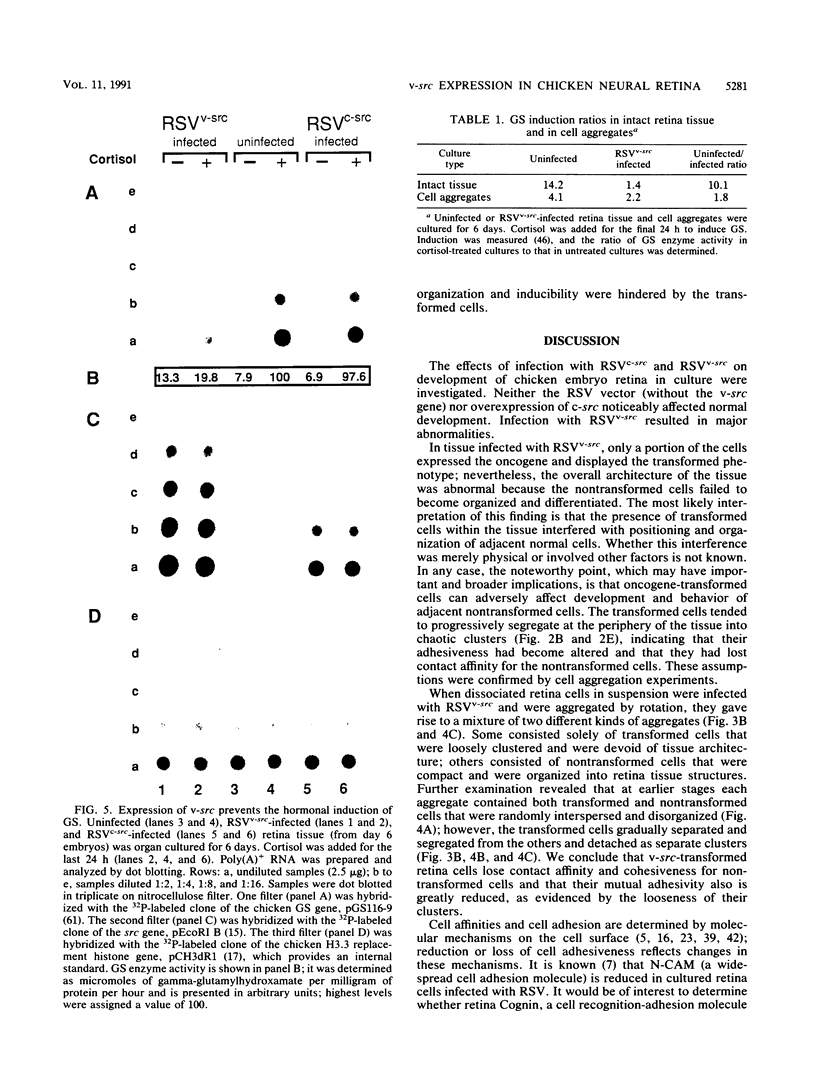
Using Rous sarcoma virus as the vector, v-src or c-src genes were introduced into 6-day chicken embryo retina tissue in organ culture and their effects on retina development were investigated. Overexpression of c-src in many of the cells had no noticeable effect on retina development. In contrast, infection with v-src resulted in abnormal histogenesis and inhibition of differentiation. Although only a portion of the cells in infected tissue expressed the oncogene and displayed the transformation phenotype, the other cells were also hindered from becoming normally positioned and organized. Therefore, presence of oncogene-transformed cells within the tissue hindered organization and development of adjacent nontransformed cells. Failure of normal cell relationships impeded induction by cortisol of glutamine synthetase in Muller glia, which requires contact associations of the glia cells with neurons. The transformed cells tended to assemble into chaotic clusters, suggesting that their adhesiveness and contact affinities had become altered. This was confirmed by aggregation experiments with dissociated cells which showed that adhesiveness of transformed cells was greatly reduced and that they had lost the ability to cohere with nontransformed cells. In binary mixtures of transformed and nontransformed cells, the two sorted out into separate aggregates. Transformed cells formed loose clusters devoid of tissue architecture; aggregates of nontransformed cells became organized into retinotypic structures, and glutamine synthetase was inducible. Our findings suggest that the mechanisms of cell adhesion and cell affinities are a key target of v-src activity in infected cells and that modification of the cell surface may be a leading factor in other cellular changes characteristic of the v-src transformation phenotype.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Boettiger D., Focht R. J., Holtzer H., Pacifici M. Regulation of the synthesis of extracellular matrix components in chondroblasts transformed by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1982 Sep;30(2):373–384. doi: 10.1016/0092-8674(82)90235-5. [DOI] [PubMed] [Google Scholar]
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Azarnia R., Mitcho M., Shalloway D., Loewenstein W. R. Junctional intercellular communication is cooperatively inhibited by oncogenes in transformation. Oncogene. 1989 Oct;4(10):1161–1168. [PubMed] [Google Scholar]
- Ben-Shaul Y., Hausman R. E., Moscona A. A. Visualization of a cell surface glycoprotein, the retina cognin, on embryonic cells by immuno-latex labeling and scanning electron microscopy. Dev Biol. 1979 Sep;72(1):89–101. doi: 10.1016/0012-1606(79)90100-3. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Roby K., Brumbaugh J., Biehl J., Holtzer H. Transformation of chicken embryo retinal melanoblasts by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1977 Aug;11(4):881–890. doi: 10.1016/0092-8674(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Brackenbury R., Greenberg M. E., Edelman G. M. Phenotypic changes and loss of N-CAM-mediated adhesion in transformed embryonic chicken retinal cells. J Cell Biol. 1984 Dec;99(6):1944–1954. doi: 10.1083/jcb.99.6.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Calothy G., Poirier F., Dambrine G., Pessac B. A transformation defective mutant of Rous sarcoma virus inducing chick embryo neuroretinal cell proliferation. Virology. 1978 Aug;89(1):75–84. doi: 10.1016/0042-6822(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisanti-Combes P., Lorinet A. M., Girard A., Pessac B., Wasseff M., Calothy G. Expression of neuronal markers in chick and quail embryo neuroretina cultures infected with Rous sarcoma virus. Cell Differ. 1982 Jan;11(1):45–54. doi: 10.1016/0045-6039(82)90016-1. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Sugarman B. J., Dodgson J. B. A chicken histone H3 gene contains intervening sequences. Nature. 1982 Jun 3;297(5865):434–436. doi: 10.1038/297434a0. [DOI] [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Activation of cellular genes by avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5351–5354. doi: 10.1073/pnas.77.9.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Rous sarcoma virus activates embryonic globin genes in chicken fibroblasts. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4464–4468. doi: 10.1073/pnas.72.11.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B. J., DeFranco D. Glucocorticoid and cAMP induction mechanisms are differentially affected by the p85gag-mos oncoprotein. Proc Natl Acad Sci U S A. 1989 Jan;86(2):597–601. doi: 10.1073/pnas.86.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Purification and characterization of the retina-specific cell-aggregating factor. Proc Natl Acad Sci U S A. 1975 Mar;72(3):916–920. doi: 10.1073/pnas.72.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Multiple tropomyosin polypeptides in chicken embryo fibroblasts: differential repression of transcription by Rous sarcoma virus transformation. Mol Cell Biol. 1984 Sep;4(9):1823–1833. doi: 10.1128/mcb.4.9.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Kosik E. Mutagenesis of the region between env and src of the SR-A strain of Rous sarcoma virus for the purpose of constructing helper-independent vectors. Virology. 1984 Jul 15;136(1):89–99. doi: 10.1016/0042-6822(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Hyrc K., Rose B. The action of v-src on gap junctional permeability is modulated by pH. J Cell Biol. 1990 Apr;110(4):1217–1226. doi: 10.1083/jcb.110.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Jove R., Hanafusa H. Lack of induction of neuroretinal cell proliferation by Rous sarcoma virus variants that carry the c-src gene. Mol Cell Biol. 1985 Oct;5(10):2856–2859. doi: 10.1128/mcb.5.10.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi R., Salmons B., Muellener D., Groner B. The v-mos and H-ras oncogene expression represses glucocorticoid hormone-dependent transcription from the mouse mammary tumor virus LTR. EMBO J. 1986 Oct;5(10):2609–2616. doi: 10.1002/j.1460-2075.1986.tb04541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer P. G., Willbold E. Embryonic chicken retinal cells can regenerate all cell layers in vitro, but ciliary pigmented cells induce their correct polarity. Cell Tissue Res. 1989 Nov;258(2):233–242. doi: 10.1007/BF00239443. [DOI] [PubMed] [Google Scholar]
- Levy J. B., Dorai T., Wang L. H., Brugge J. S. The structurally distinct form of pp60c-src detected in neuronal cells is encoded by a unique c-src mRNA. Mol Cell Biol. 1987 Nov;7(11):4142–4145. doi: 10.1128/mcb.7.11.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Hormonal induction of glutamine synthetase in cultures of embryonic retina cells: requirement for neuron-glia contact interactions. Dev Biol. 1983 Apr;96(2):529–534. doi: 10.1016/0012-1606(83)90190-2. [DOI] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Induction of glutamine synthetase in embryonic neural retina: localization in Müller fibers and dependence on cell interactions. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- Martinez R., Mathey-Prevot B., Bernards A., Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987 Jul 24;237(4813):411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Inhibition of chicken embryo lens differentiation and lens junction formation in culture by pp60v-src. Mol Cell Biol. 1988 Apr;8(4):1414–1420. doi: 10.1128/mcb.8.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. G., Adam M. A., Miller A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990 Aug;10(8):4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatani S., Shimamura K., Hatta M., Nagafuchi A., Nose A., Matsunaga M., Hatta K., Takeichi M. Neural cadherin: role in selective cell-cell adhesion. Science. 1989 Aug 11;245(4918):631–635. doi: 10.1126/science.2762814. [DOI] [PubMed] [Google Scholar]
- Morris J. E., Moscona A. A. Induction of glutamine synthetase in embryonic retina: its dependence on cell interactions. Science. 1970 Mar 27;167(3926):1736–1738. doi: 10.1126/science.167.3926.1736. [DOI] [PubMed] [Google Scholar]
- Moscona A. A., Degenstein L. Normal development and precocious induction of glutamine synthetase in the neural retina of the quail embryo. Dev Neurosci. 1981;4(3):211–219. doi: 10.1159/000112758. [DOI] [PubMed] [Google Scholar]
- Moscona A. A., Linser P. Developmental and experimental changes in retinal glia cells: cell interactions and control of phenotype expression and stability. Curr Top Dev Biol. 1983;18:155–188. doi: 10.1016/s0070-2153(08)60582-7. [DOI] [PubMed] [Google Scholar]
- Moscona A. A., Moscona M. H., Saenz N. Enzyme induction in embryonic retina: the role of transcription and translation. Proc Natl Acad Sci U S A. 1968 Sep;61(1):160–167. doi: 10.1073/pnas.61.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona M., Degenstein L., Byun K. Y., Moscona A. A. Development of differential affinities and positional information in embryonic retina cells: inhibition by BrdU. Cell Differ. 1981 Dec;10(6):317–327. doi: 10.1016/0045-6039(81)90023-3. [DOI] [PubMed] [Google Scholar]
- Moscona M., Moscona A. A. The development of inducibility for glutamine synthetase in embryonic neural retina: inhibition by BrdU. Differentiation. 1979;13(3):165–172. doi: 10.1111/j.1432-0436.1979.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Moss P. S., Honeycutt N., Pawson T., Martin G. S. Viral transformation of chick myogenic cells. The relationship between differentiation and the expression of the SRC gene. Exp Cell Res. 1979 Oct 1;123(1):95–105. doi: 10.1016/0014-4827(79)90425-7. [DOI] [PubMed] [Google Scholar]
- Notter M. F., Navon S. E., Fung B. K., Balduzzi P. C. Infection of neuroretinal cells in vitro by avian sarcoma viruses UR1 and UR2: transformation, cell growth stimulation, and changes in transducin levels. Virology. 1987 Oct;160(2):489–493. doi: 10.1016/0042-6822(87)90023-7. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Patejunas G., Young A. P. Tissue-specific regulation of avian glutamine synthetase expression during development and in response to glucocorticoid hormones. Mol Cell Biol. 1987 Mar;7(3):1070–1077. doi: 10.1128/mcb.7.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F. Evidence the pp60src, the product of the Rous sarcoma virus src gene, undergoes autophosphorylation. J Virol. 1982 Jan;41(1):1–7. doi: 10.1128/jvi.41.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M., Hamilton B. J., DeFranco D. v-mos oncoproteins affect the nuclear retention and reutilization of glucocorticoid receptors. Mol Endocrinol. 1989 Aug;3(8):1279–1288. doi: 10.1210/mend-3-8-1279. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Gallis B., Bornstein P. Coordinate transcriptional regulation of type I procollagen genes by Rous sarcoma virus. J Biol Chem. 1981 May 25;256(10):5022–5028. [PubMed] [Google Scholar]
- Simon M. A., Drees B., Kornberg T., Bishop J. M. The nucleotide sequence and the tissue-specific expression of Drosophila c-src. Cell. 1985 Oct;42(3):831–840. doi: 10.1016/0092-8674(85)90279-x. [DOI] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Tereba A., Murti K. G. A very sensitive biochemical assay for detecting and quantitating avian oncornaviruses. Virology. 1977 Jul 1;80(1):166–176. doi: 10.1016/0042-6822(77)90389-0. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Fox L. E., Moscona A. A. Accumulation of c-src mRNA is developmentally regulated in embryonic neural retina. Mol Cell Biol. 1986 Nov;6(11):4109–4111. doi: 10.1128/mcb.6.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L., Fox L. E., Moscona A. A. Developmental regulation of glutamine synthetase and carbonic anhydrase II in neural retina. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9060–9064. doi: 10.1073/pnas.83.23.9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L., Fox L. L., Degenstein L., Moscona A. A. Cell contacts are required for induction by cortisol of glutamine synthetase gene transcription in the retina. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5981–5985. doi: 10.1073/pnas.85.16.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. L., Nelson W. J. Nonmitogenic morphoregulatory action of pp60v-src on multicellular epithelial structures. Mol Cell Biol. 1987 Apr;7(4):1326–1337. doi: 10.1128/mcb.7.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]