Abstract
A high mutation rate leading to tumor cell heterogeneity is a driver of malignancy in human cancers. Paradoxically, however, genomic instability can also render tumors vulnerable to therapeutic attack. Thus, targeting DNA repair may induce an intolerable level of DNA damage in tumor cells. BRCA2 mediates homologous recombination repair, and BRCA2 polymorphisms increase cancer risk. However, tumors with BRCA2 mutations respond better to chemotherapy and are associated with improved patient prognosis. Thymidylate synthase (TS) is also involved in DNA maintenance and generates cellular thymidylate. We determined that antisense downregulation of BRCA2 synergistically potentiated drugs with mechanisms of action related to BRCA2 function (cisplatin, melphalan), a phenomenon we named “complementary lethality.” TS knockdown induced complementary lethality to TS-targeting drugs (5-FUdR and pemetrexed) but not DNA cross-linking agents. Combined targeting of BRCA2 and TS induced complementary lethality to both DNA-damaging and TS-targeting agents, thus creating multidrug sensitive tumors. In addition, we demonstrated for the first time that simultaneous downregulation of both targets induced combined complementary lethality to multiple mechanistically different drugs in the same cell population. In this study, we propose and define the concept of “complementary lethality” and show that actively targeting BRCA2 and TS is of potential therapeutic benefit in multidrug treatment of human tumors. This work has contributed to the development of a BRCA2-targeting antisense oligdeoxynucleotide (ASO) “BR-1” which we will test in vivo in combination with our TS-targeting ASO “SARI 83” and attempt early clinical trials in the future.
Keywords: antisense, BRCA2, complementary lethality, DNA repair, drug sensitivity, TS
Introduction
Genomic instability and phenotypic heterogeneity are among the strongest drivers of cancer progression, malignancy, and recurrence.1,2 A high mutation rate appears to be a unifying hallmark of cancer, and one that enhances the rate at which malignant characteristics are generated in human cells to produce progressively more aggressive tumors.3,4 Next-generation sequencing technology has highlighted the extreme degree of genomic variability and mutation present in human malignancies.2,5,6 This underscores one of the reasons that cancer is a difficult disease to treat effectively—genomic plasticity and phenotypic heterogeneity allow for rapid adaptation and differential response to environmental selection pressures.3
However, continuing high mutation frequency and lack of genomic fidelity also leads to increased rates of cell death.7 Consequently, cancer cells may be closer to a maximum tolerated threshold of mutation than normal cells and are highly dependent for continued survival on cellular molecules that maintain at least some integrity of genetic information. This phenomenon has led to the concept of tumor cell “addiction” to some mediators of DNA repair.8 Therefore, targeting factors critical to DNA maintenance may be a useful strategy to promote the increase of deleterious mutations from which cancer cells cannot recover.
BRCA2, a protein intimately involved in homologous recombination repair of double-stranded DNA breaks (DSBs),9,10 is one such critical factor. Mutations in the BRCA2 gene induce a highly penetrant, autosomal dominant predisposition to cancers of the breast, ovary, and other organ systems.11,12,13 However, tumors with BRCA2 mutations respond better to chemotherapy than BRCA2-intact tumors in patients with sporadic cancer.14,15,16 This suggests that although responsible for increased cancer risk, reduced BRCA2 function may render cancer cells more vulnerable to chemotherapy regimens that damage DNA.
One way to exploit the inverse relationship between BRCA2 status and effectiveness of anticancer chemotherapy is to identify patients with BRCA2 mutations and tailor treatment accordingly.17,18 The prototypical example of such an approach is the development and use of PARP inhibitors, which are particularly effective in cells with pre-existing BRCA2 mutations—a phenomenon termed synthetic lethality.19,20 However, the incidence of mutated BRCA2 tumors among sporadic cancer patients is <3%, and the low incidence reduces the number of opportunities to therapeutically exploit the phenomenon.21 A potentially more valuable strategy is to actively disrupt BRCA2 function in tumors with intact BRCA2 to render them more sensitive to specific types of chemotherapy, similar to tumors with inactivating BRCA2 mutations.
This led us to formulate and test the concept of “complementary lethality,” defined generally as the synergistic enhancement of drug efficacy by downregulation of factors important for cellular resistance to the action of that specific drug. In other words, we propose to potentiate the effectiveness of chemotherapeutic drugs by targeting DNA repair mediators, such as BRCA2, which are functionally involved in the amelioration of specific types of drug-induced effects (e.g., DSBs produced by platinum or alkylating agents). This differs from synthetic lethality in that it depends neither on specific pre-existing genetic lesions in tumor cells, nor on the disruption of pathways capable of partially or completely replacing the function of a therapeutically targeted pathway. Rather, it depends on rational targeting of factors which, following abrogation of their function, synergistically potentiate the action of specific chemotherapeutics.
Another mediator of genomic integrity and DNA replication in the cell is thymidylate synthase (TS). This enzyme is the only de novo source of cellular thymidylate and is a well-established target of many approved anticancer drugs including fluoropyrimidines (e.g., 5-FU) and folate analogs (e.g., pemetrexed).22 We previously reported that TS antisense oligonucleotides (ASOs) sensitize human cancer cells to TS-targeting drugs,23,24 a phenomenon we now define as complementary lethality.
We hypothesized that BRCA2 could be downregulated in combination with TS downregulation to induce complementary lethality to a wider and different spectrum of drugs, thus creating multidrug sensitive tumors (Figure 1a). The association of both BRCA2 and TS with different aspects of DNA integrity suggests that combined downregulation of both targets could lead to enhanced cell death and potentiation of both TS-targeting and other drugs that induce or enhance accumulation of DNA damage.
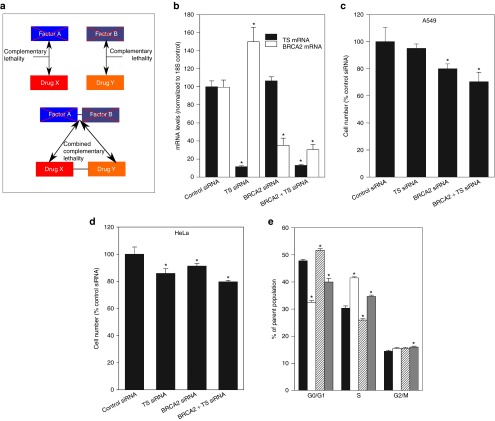
Figure 1.
Combined BRCA2 siRNA and TS siRNA inhibits A549 and HeLa cell proliferation and differentially affects cell cycle progression. (a) Drug X is potentiated by the downregulation of Factor A, and Drug Y is potentiated by the inhibition of Factor B. This phenomenon, which we define as complementary lethality, occurs because Factor A and B are involved in resistance to separate classes of drugs (X and Y). Combined complementary lethality to both drugs can therefore be achieved by the simultaneous inhibition of both Factor A and B. (b) A549 and HeLa cells were transfected with control siRNA, TS siRNA and/or BRCA2 siRNA. mRNA was isolated 24 hours later. TS mRNA (black bars) and BRCA2 mRNA (white bars) levels were measured relative to 18S endogenous control. *Different from treatment with control siRNA (Student's t-test, P < 0.05). Representative data from one of two independent experiments is shown (mean ± SD). (c) Effect of siRNA treatment on A549 cell proliferation, 96 hours after transfection. *Different from treatment with control siRNA (Student's t-test, P < 0.05). Representative data from one of three independent experiments is shown (mean ± SD). (d) Effect of siRNA treatment on HeLa cell proliferation, 96 hours after transfection. *Different from treatment with control siRNA (Student's t-test, P < 0.05). Representative data from one of three independent experiments is shown (mean ± SD). (e) A549 cells were transfected with control siRNA (black bars), TS siRNA (white bars), BRCA2 siRNA (white bars with pattern), or TS siRNA and BRCA2 siRNA (grey bars) and the number of cells in different cell cycle stages measured 48 hours after transfection. *Different from treatment with control siRNA (Student's t-test, P < 0.05). Representative data from one of two independent experiments is shown (mean ± SD).
In this report, we demonstrate that actively targeting DNA repair pathways is useful therapeutically and induces sensitization to specific chemotherapeutics, a phenomenon we labeled complementary lethality. In particular, we show that BRCA2 is valuable as a therapeutic target in addition to TS, and that combined downregulation of BRCA2 and TS can sensitize cells to a panel of chemotherapeutic drugs with different mechanisms of action. We used siRNAs targeting TS and BRCA2 to promote sensitization to separate classes of chemotherapeutics in the same human tumor population, and induced a state of combined complementary lethality which resulted in potential therapeutic benefit.
Results
siRNA-mediated knockdown of BRCA2 and TS reduces cancer cell growth and affects cell cycle progression
Target mRNA downregulation was confirmed by RT-qPCR 24 hours after transfection. There were no synergistic or inhibitory effects on mRNA levels when both targets were downregulated at the same time (Figure 1b). Given the potential for baseline DNA damage normally accrued by mammalian cells and repaired by complexes in which BRCA2 is important, it was hypothesized that BRCA2 siRNA would have an effect on cell growth even in the absence of an exogenous damaging agent. Ninety-six hours after transfection, A549 cells treated with BRCA2 siRNA (10 nmol/l) exhibited a significant decrease in cell number (20 ± 9%, P = 0.036) compared with cells treated with control siRNA (10 nmol/l) (Figure 1c). Cells transfected with TS siRNA (2.5 nmol/l) did not appear to be negatively affected by this treatment compared with cells transfected with control, nontargeting siRNA. Combination treatment with BRCA2 siRNA (10 nmol/l) and TS siRNA (2.5 nmol/l) decreased A549 cell number to a similar level as BRCA2 siRNA alone (29.6 ± 7.0%, P = 0.04 versus control siRNA; no difference versus BRCA2 siRNA)(Figure 1c), suggesting that these are separate and nonoverlapping pathways. In HeLa cells tested under the same conditions, both BRCA2 and TS siRNA, as well as their combination, induced decreases in cell number (Figure 1d).
To assess the possibility that the difference in cell number following siRNA treatment was due to cell cycle changes, A549 cells were transfected with BRCA2 and TS siRNA and then stained with propidium iodide for cell cycle analysis. Forty-eight hours after transfection, BRCA2 siRNA induced a small increase in the number of cells in G0/G1 phase (8.15 ± 2.14%, P = 0.007), and a concomitant decrease in the number of cells in S phase (14.8 ± 3%, P = 0.009) compared with treatment with nontargeting siRNA (Figure 1e).
TS siRNA treatment exhibited the opposite effect, with an increase in S phase (26.7 ± 1.1%, P = 0.0002) and a decrease in G0/G1 (32 ± 2.21%, P = 0.00004). Combined downregulation of both TS and BRCA2 led to a cell cycle profile that reflected the opposite contributions of the individual siRNA treatments in terms of G0/G1 and S phase frequency which, as a result, yielded cell cycle frequencies that were intermediate when compared with control siRNA treatment (G0/G1 change: −19.8 ± 4.9%, P = 0.005; S change: +13.8 ± 1.9%, P = 0.01). However, the G2/M phase frequency in BRCA2- and TS siRNA-treated cells was increased compared with control (10.4 ± 3%, P = 0.04) (Figure 1e).
BRCA2 siRNA synergistically potentiates cisplatin, melphalan, and 5-FUdR, but not pemetrexed
We investigated whether BRCA2 siRNA altered the capacity of A549 cells to proliferate after treatment with DNA-damaging chemotherapeutic drugs such as cisplatin. Cisplatin binds to DNA and induces inter- and intrastrand crosslinks which progress to DSBs and are repaired by homologous recombination.25 In A549 cells treated with BRCA2 siRNA and an IC25 concentration of cisplatin, drug treatment was 43 ± 3% (P = 0.0001) more effective when compared with cells transfected with nontargeting control siRNA (Figure 2a).
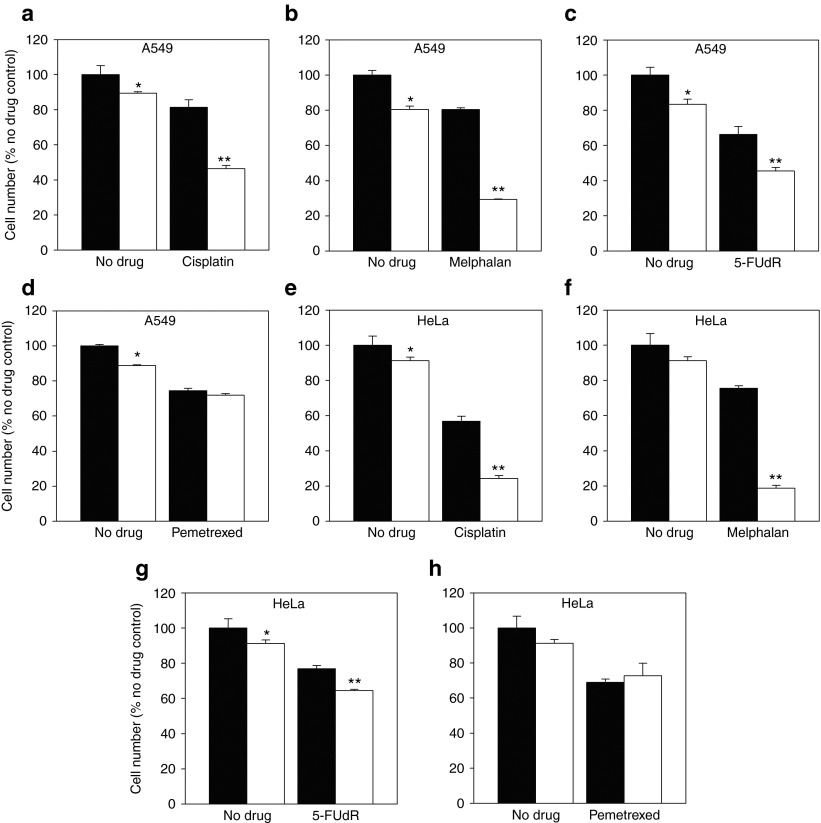
Figure 2.
BRCA2 siRNA induces complementary lethality to treatment with cisplatin, melphalan and 5-FUdR, but not pemetrexed. A549 cells were transfected with control siRNA (black bars) or BRCA2 siRNA (white bars) and treated with (a) cisplatin (IC25), (b) melphalan (IC25), (c) 5-FUdR (IC25), or (d) pemetrexed (IC25). HeLa cells were treated under the same conditions and with (e–h) the same drugs. Effects on cell growth were assessed 96 hours after transfection, as a percentage of cells treated with control nontargeting siRNA alone. *Different from treatment with control siRNA alone (Student's t-test, P < 0.05). **Different from treatment with drug and control siRNA (Student's t-test, P < 0.05). Representative data from one of three independent experiments is shown (mean ± SD).
We then determined whether potentiation of cisplatin treatment by BRCA2 siRNA was due to the induction of DNA strand cross-linking and subsequent DSBs, by using another anticancer agent with a related mechanism of action. Melphalan is a DNA alkylator but not a platinum-based agent and is therefore structurally distinct from cisplatin.26 A549 cells transfected with BRCA2 siRNA 48 hours before melphalan treatment (IC25) were 63 ± 0.3% (P = 9.5 × 10−7) more sensitive to melphalan-mediated growth inhibition than cells treated with control nontargeting siRNA (Figure 2b).
We next tested whether siRNA-mediated knockdown of BRCA2 potentiated the effects of a folate antimetabolite (pemetrexed) and a fluoropyrimidine (5-FUdR), both approved for use in treatment of a broad range of human tumors.27,28 These drugs are not DNA alkylators and do not share a mechanism of action with cisplatin and melphalan. Rather, they inhibit the action of TS and consequently starve cells of the thymidylate necessary for DNA replication and repair.22 Cells transfected with BRCA2 siRNA (10 nmol/l) were 31.2 ± 3.1% (P = 0.001) more sensitive to 5-FUdR than cells tranfected with control siRNA (Figure 2c) 96 hours after transfection. However, this did not induce synergy of the same magnitude as cisplatin and melphalan treatment. BRCA2 siRNA treatment did not sensitize A549 cells to pemetrexed and cell numbers were similar to control siRNA-transfected cells 96 hours after transfection (Figure 2d). These results were broadly reproducible in HeLa cells tested under the same conditions, and BRCA2 inhibition induced complementary lethality to cisplatin, melphalan, and to a lesser extent 5-FUdR (Figure 2e–h).
TS siRNA synergistically potentiates 5-FUdR and pemetrexed, but not cisplatin or melphalan
A549 cells were transfected with control and/or TS siRNA, and then treated with 5-FUdR (IC25) 24 hours later. As predicted, TS siRNA sensitized cells to 5-FUdR, reducing cell proliferation by 55.3 ± 4.9% (P = 0.0006) relative to cells treated with control siRNA (Figure 3a). In addition, TS siRNA also induced complementary lethality to pemetrexed (IC25) 96 hours after transfection and decreased cell number by 56.0 ± 3.5% relative to cells treated with 5-FUdR and control siRNA (P = 0.0003)(Figure 3b).
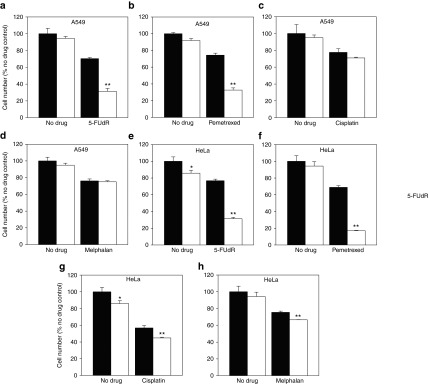
Figure 3.
TS siRNA induces complementary lethality to 5-FUdR and pemetrexed but not cisplatin or melphalan. A549 cells were transfected with control siRNA (black bars) or TS siRNA (white bars) and treated with (a) 5-FUdR (IC25), (b) pemetrexed (IC25), (c) cisplatin (IC25), or (d) melphalan (IC25). HeLa cells were treated under the same conditions and with (e–h) the same drugs. Effects on cell growth were assessed 96 hours after transfection, as percentages of cells treated with control nontargeting siRNA alone. *Different from cells treated with control nontargeting siRNA alone (Student's t-test, P < 0.05).**Different from cells treated with control nontargeting siRNA and drug. Representative data from one of three independent experiments is shown (mean ± SD).
We next determined whether TS siRNA sensitized A549 cells to drugs which do not target the TS pathway. A549 cells transfected with TS siRNA (2.5 nmol/l) did not exhibit increased sensitivity to cisplatin (IC25)(Figure 3c) or melphalan (IC25)(Figure 3d) compared with cells treated with drug and control siRNA. This suggested that TS siRNA-mediated potentiation of drug efficacy is limited to drugs that target the TS pathway and not drugs that induce specific types of DNA damage. These results were reproduced in HeLa cells under the same experimental conditions (Figure 3e–h), and though TS siRNA induced a statistically significant difference in cell number following cisplatin and melphalan treatment, this was an additive and not synergistic response driven by the action of TS siRNA alone on cell number. Therefore, it was not suggestive of complementary lethality.
Simultaneous siRNA-mediated knockdown of BRCA2 and TS induces complementary lethality to cisplatin, melphalan, and both TS-targeting drugs
As single siRNA treatment promoted the efficacy of different sets of drugs (e.g., TS siRNA and pemetrexed, BRCA2 siRNA and cisplatin), we assessed whether transfecting BRCA2 and TS siRNA simultaneously sensitized A549 cells to the entire panel of four chemotherapeutics. Our aim was to investigate whether combination siRNA treatment could be utilized to induce complementary lethality to both alkylating drugs and TS targeting agents, and whether the magnitude of sensitization would exceed that induced by each individual siRNA to its “noncomplementary” drug partner (e.g., TS siRNA and cisplatin).
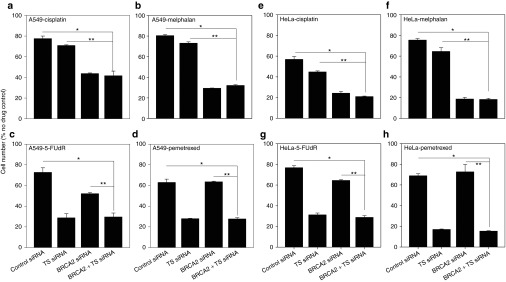
A549 cells were transfected with control siRNA, BRCA2 siRNA, and/or TS siRNA and subsequently treated with cisplatin 24 hours later. Ninety-six hours after transfection the combination siRNA treatment potentiated the effects of cisplatin by 46.3 ± 9.9% (P = 0.002) compared with control siRNA treatment and was 41.2 ± 10.9% (P = 0.002) more effective at growth inhibition following drug treatment than TS siRNA alone (Figure 4a).
Figure 4.
Simultaneous siRNA-mediated knockdown of BRCA2 and TS induces complementary lethality to cisplatin, melphalan, and both TS-targeting drugs. A549 cells were transfected with control, TS and/or BRCA2 siRNA and, at 24 hours after transfection, treated with (a) cisplatin (IC25), (b) melphalan (IC25), (c) 5-FUdR (IC25), or (d) pemetrexed (IC25). HeLa cells were treated under the same conditions and with (e–h) the same drugs. Effects on cell proliferation were determined by direct cell counting at 96 hours and calculated as a percentage of nondrug-treated, control siRNA-transfected cells.*Different from cells treated with control nontargeting siRNA and drug (Student's t-test, P < 0.05). **Different from cells treated with single siRNA and drug (Student's t-test, P < 0.05). Representative data from one of three independent experiments is shown (mean ± SD).
In addition, simultaneous BRCA2 and TS siRNA treatment sensitized human A549 cancer cells to melphalan treatment (IC25) by 60 ± 0.8% (P = 2.4 × 10−7) more than control siRNA treatment and 56 ± 1% (P = 1.0 × 10−6) more than TS siRNA (Figure 4b).
Combination siRNA treatment sensitized A549 cells to 5-FUdR by 59.2 ± 5.1% (P = 0.0001) and was 43.0 ± 7.1% (P = 0.0005) more effective at sensitization than single BRCA2 siRNA treatment (Figure 4c), even though BRCA2 siRNA also potentiated 5-FUdR toxicity on its own as shown previously.
Furthermore, combined BRCA2 and TS siRNA transfection potentiated the efficacy of pemetrexed (IC25) by 56 ± 3.3% (P = 0.0004) more than control siRNA treatment and was 56.4 ± 3.3% (P = 1.0 × 10−5) more effective at drug sensitization than BRCA2 siRNA alone (Figure 4d). The above experiments were repeated in HeLa cells and yielded results of similar magnitude and scope (Figure 4e–h). These results show that combination BRCA2 and TS siRNA treatment induces complementary lethality to a broader panel of chemotherapeutic agents and creates multidrug sensitive tumors. They also suggest that combination siRNA treatment and combination drug treatment may be an effective way to maximize chemotherapeutic efficacy of different classes of drugs.
Simultaneous downregulation of BRCA2 and TS in the same cell population induces complementary lethality to two different sets of drugs and increases overall therapeutic effectiveness
Combined treatment with TS-targeting and platinum-based anticancer drugs is standard of care for a variety of common and important human cancers.29,30 Therefore, antisense-mediated enhancement of these drug combinations is desirable and potentially valuable therapeutically. Given that complementary lethality was separately induced to cross-linking agents and TS-targeting drugs by combined TS and BRCA2 siRNA treatment, we next determined whether combined complementary lethality to both types of drugs could be induced simultaneously in the same cell population and if the magnitude of drug potentiation could present a potential therapeutic benefit.
A549 cells were transfected with control siRNA and combination BRCA2 and TS siRNA. Twenty-four hours after transfection, the cells were treated with either cisplatin (IC25), 5-FUdR (IC25) or both drugs simultaneously, and effects on proliferation were enumerated 96 hours after transfection.
Combination siRNA treatment potentiated the effects of combination cisplatin and 5-FUdR treatment by 64.1 ± 7% (P = 0.0001) relative to control siRNA treatment. This showed that complementary lethality to two different drugs could be induced in the same cell population with combination siRNA treatment. Furthermore, the overall magnitude of this approach was more effective at reducing overall cell number compared with nondrug-treated control than combination siRNA transfection and treatment with any single drug (P ≤ 0.05), suggesting that there is potential therapeutic benefit to sensitizing to cisplatin and 5-FUdR in the same cell population (Figure 5a).
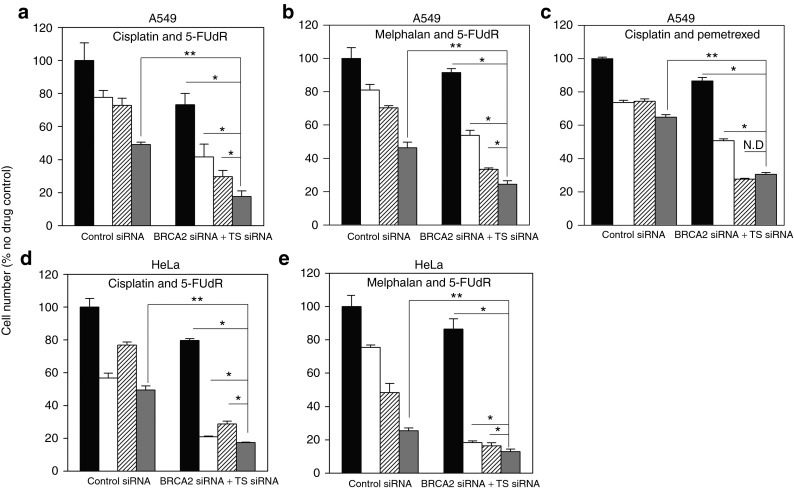
Figure 5.
Combined complementary lethality to two different drugs in the same cell population can be achieved by combined BRCA2 and TS siRNA transfection. A549 cells were transfected with control, TS and/or BRCA2 siRNA and treated with (a) cisplatin, 5-FUdR, or cisplatin and 5-FUdR; (b) melphalan, 5-FUdR or melphalan and 5-FUdR; and (c) cisplatin, pemetrexed, or cisplatin and pemetrexed. HeLa cells were treated under the same condition and with (d) cisplatin, 5-FUdR, or cisplatin and 5-FUdR; and (e) melphalan 5-FUdR, or melphalan and 5-FUdR. Black bars = cells untreated with drugs (a–e), white bars = treatment with (a,c,d) cisplatin or (b,e) melphalan. Patterned white bars = cells treated with (a,b,d,e) 5-FUdR or (c) pemetrexed; dark grey bars = cells treated with (a–e) combinations of drugs. Cell proliferation was measured 96 hours after transfection and is shown as a percentage of cells treated with control nontargeting siRNA alone. **Different from cells treated with drug combination and control nontargeting siRNA (Student's t-test, P < 0.05). *Different from cells treated with combined siRNA, and one drug or no drug (one-way analysis of variance, P < 0.05). Representative data from one of two independent experiments is shown (mean ± SD).
To determine whether the same phenomenon could be reproduced with another drug whose toxicity was potentiated by BRCA2 siRNA, the experiment was repeated with melphalan substituted for cisplatin. Again, combined siRNA treatment effectively sensitized to combined drug treatment relative to control siRNA (47.4 ± 4.6%, P = 0.0006) and the final outcome in terms of cell proliferation was superior to treatment with combination siRNA and any single drug (P ≤ 0.05)(Figure 5b).
Interestingly, though combination siRNA transfection still sensitized to combination cisplatin and pemetrexed treatment (52.8 ± 2.9%, P = 5.6 × 10−5), it did not result in a larger decrease in cell proliferation than combination siRNA transfection and pemetrexed treatment alone (Figure 5c). Concomitant cisplatin and pemetrexed treatment also failed to induce an additive effect on cell growth, unlike that seen with cisplatin and 5-FUdR, and melphalan and 5-FUdR.
The experiments with cisplatin and 5-FUdR, and melphalan and 5-FUdR were repeated in HeLa cells to establish the generalizability of the phenomenon. The results obtained using HeLa cells mirrored those in A549 cells and again showed that combined complementary lethality to two separate drugs in the same cell population is of potential treatment benefit.
Discussion
Genomic instability, mutation, and the accompanying heterogeneity represent common traits among most human cancers.31 However, and despite the fact that a high mutation rate is a driving factor behind malignant progression, lack of genomic fidelity can act as an “Achilles heel” to be exploited by therapeutic intervention.32 We propose that inhibiting proteins involved in DNA repair and maintenance, such as BRCA2, is a method to induce complementary lethality to DNA damage-inducing chemotherapeutics and enhance the probability of catastrophic and lethal toxicity in tumor cells. We show here, for the first time, that the induction of complementary lethality with combined targeting of DNA repair factors involved in cellular response to the mechanism of action of different drugs can be a viable strategy to improve cancer treatment.
We hypothesized that BRCA2 knockdown would synergistically potentiate cisplatin and melphalan treatment because both drugs induce DSBs,17 and BRCA2 is an integral part of the complex responsible for homologous recombination33,34. Our data support this assertion, in agreement with a recent study highlighting increased sensitivity to alkylating agents in BRCA2 knockout mice,25 and a report describing glioma cell sensitization to temozolomide following downregulation of BRCA2 and RAD51.35
We were surprised to find that BRCA2 knockdown potentiated 5-FUdR, albeit to a smaller degree than cisplatin and melphalan. We expected that BRCA2 knockdown would have no effect on 5-FUdR toxicity given the lack of effect with pemetrexed. On the face of it, BRCA2 does not appear directly related to the mechanism of action of 5-FUdR as the drug targets TS. However, upsetting the balance of intracellular nucleotide pools through treatment with drugs like 5-FU can stall replication fork progression.36 BRCA2 has been shown to maintain stability of stalled replication forks37,38 which may explain why BRCA2 knockdown potentiates the activity of 5-FUdR. The fact that BRCA2 knockdown sensitizes to 5-FUdR but not pemetrexed may mean there are differences in the mechanism of action of the drugs beyond TS inhibition, and shows that BRCA2 does not sensitize to all drugs regardless of mechanism.
As expected from previously published work, TS downregulation potentiated the effects of both 5-FUdR and pemetrexed. This may in large part be due to the fact that knockdown of TS mRNA in combination with pharmacological inhibition of TS protein are complementary functions.24,39 TS siRNA, however, did not appear to induce complementary lethality to either cisplatin or melphalan, suggesting that TS is not intimately involved in cellular response to those drugs.
Cumulatively, these results suggest that BRCA2 and TS are involved in resistance to different classes of drugs, and that they may in fact be involved in largely separate pathways within the cell. The cell cycle data further supports this assertion because it shows that BRCA2 and TS downregulation differentially affects cell cycle progression and that simultaneously targeting both factors has the potential to induce two discrete blocks in cell cycle. This renders an anti-BRCA2 and anti-TS combination therapy particularly intriguing from an acquired resistance perspective, and we hypothesize the likelihood of cells developing resistance to a therapy targeting two separate pathways is very low.
Simultaneous downregulation of BRCA2 and TS potentiated the effects of all four tested drugs, thus creating multidrug sensitive tumors. These experiments demonstrated that tumors can be sensitized to different classes of chemotherapeutics by targeting separate factors critical to cellular response to those drugs. An important determination was that there were no antagonistic effects observed during combined siRNA treatment. These findings are particularly germane in the context of chemotherapy, because drugs are rarely administered in isolation.40
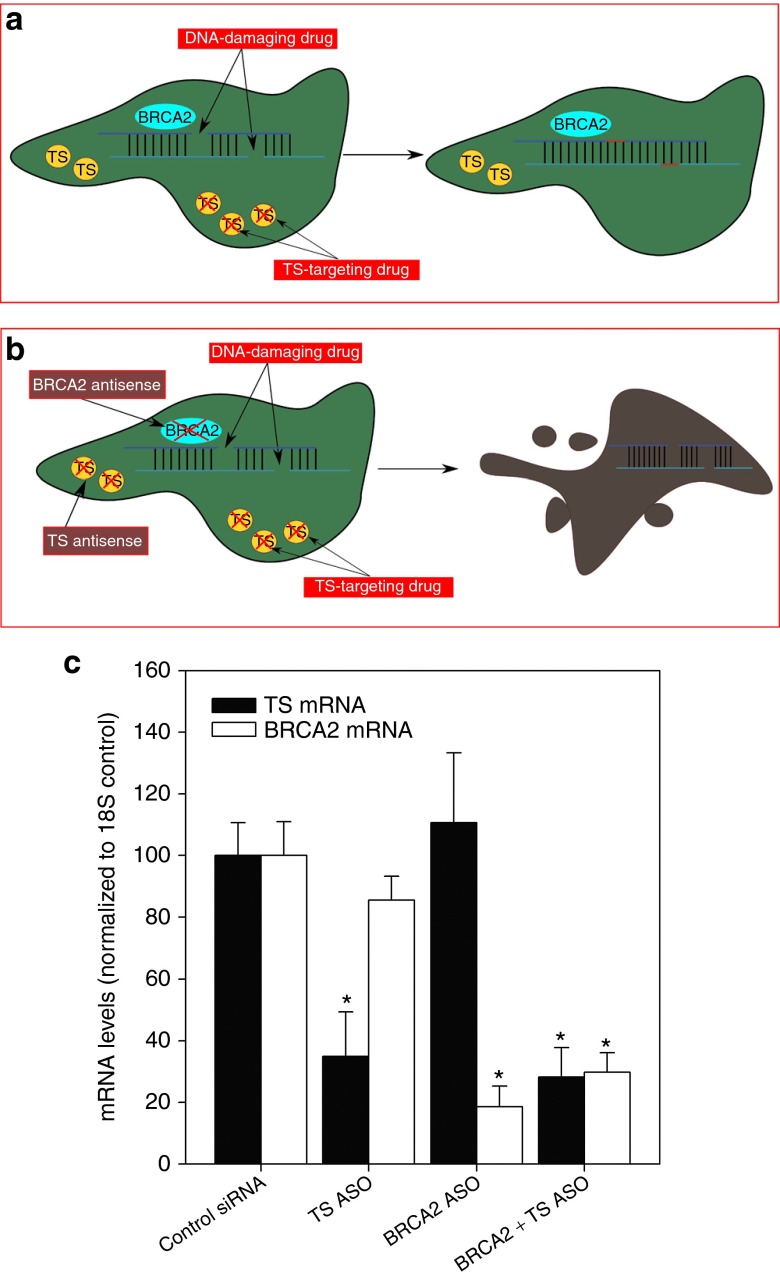
We tested whether combined complementary lethality to two drugs could be achieved simultaneously in the same cell population, because this would be most closely related to current and future clinical scenarios. An important point to consider is that differential pharmacokinetic and pharmacodynamic factors relevant to different drugs result in unequal uptake into tumor cells; that is, cells in different locations within tumors, and at different sites in the body, are likely to be accessible to different drugs in different ways.41,42 As tumor cells may take up drugs at rates different from those administered (and some drugs may accumulate in tumors at low concentrations that are difficult to predict or plan for) then sensitizing tumor cells to all drugs in a treatment cocktail can be an advantage. Treatment with combined siRNAs targeting multiple mRNAs to induce complementary lethality to different drugs can have this desirable affect (Figure 6a,b).
Figure 6.
Schematic representation of combined complementary lethality and efficacy of candidate ASO drugs to downregulate target mRNA. (a) DNA-damaging drugs such as cisplatin induce DSBs in cellular DNA, while TS-targeting agents inhibit the function of TS. However, the cell can be rescued from these drugs if it expresses functional BRCA2 and maintains residual TS activity. (b) When DNA-damaging drugs and TS-targeting drugs are administered in combination with antisense molecules targeting both resistance factors, the cell will be more susceptible to drug-induced effects; this is the essence of combined complementary lethality. (c) A549 cells were transfected with 20 nmol/l control ASO, SARI 83 ASO, BR-1 ASO, or a combination of SARI 83 ASO + BR-1 ASO. mRNA was isolated 24 hours later. TS mRNA (black bars) and BRCA2 mRNA (white bars) levels were measured relative to 18S endogenous control. *Different from treatment with control siRNA (Student's t-test, P < 0.05). Representative data from one of two independent experiments is shown (mean ± SD).
Interestingly, we observed some antagonism when cisplatin and pemetrexed were used in a combined treatment, and antisense-mediated complementary lethality was not as effective in this treatment group. These results have potential implications for therapy because cisplatin and pemetrexed combinations are currently used to treat a variety of different cancers, including non-small cell lung cancer.43
The induction of complementary lethality in the context of DNA repair provides an alternative perspective to the focus on personalized medicine in cancer therapy. It contrasts with the idea of synthetic lethality, which relies upon identifying pre-existing deficiencies in cancer cell genomes and exploiting them for therapeutic benefit.20 A prototypical example of synthetic lethality with therapeutic applications is the discovery and development of poly(ADP-ribose) polymerase 1 (PARP1) inhibitors, many of which have and are currently undergoing clinical trials with varying degrees of success.44,45 The key to this strategy, however, is the identification of a pre-existing weakness (e.g., BRCA deficiency) in a cancer cell, and tailoring a therapy designed to exploit or exacerbate that vulnerability.
As a result, synthetic lethality is dependent upon one of the enduring challenges to cancer therapy—the need to assay for specific genetic lesions conferring vulnerability to targeted therapies. Induction of complementary lethality via targeting DNA repair theoretically nullifies the need to understand the distinct genotype of a targeted tumor. Instead, this concept relies on a unifying principle: the fact that tumors need to maintain a minimum level of genomic stability to survive, and that this must occur in the face of chemotherapy and radiation.
Classically, one of the barriers to antisense-mediated therapy has been the inability to adequately deliver and maintain potency of these molecules in vivo.46 However, modified ss-siRNAs can be used to efficiently downregulate target mRNA in vivo, without need for a delivery vehicle.47 In addition, many candidate ASO-based agents are currently undergoing various phases of clinical trials.48,49 As a result of these studies, we are actively pursuing the development of a novel, second generation BRCA2-targeting ASO named “BR-1”, which we hope to combine with our TS-targeting ASO “SARI 83” (Figure 6c). ASOs exhibit several advantages over siRNAs for in vivo use, including increased stability in serum and decreased need for a delivery or carrier vehicle.50 Future studies in this laboratory will determine the efficacy of combined complementary lethality to create multidrug sensitive tumors in vivo using BR-1 and SARI 83.
With these current studies in mind, it remains to be determined how normal noncancerous cells will respond to a strategy that actively targets DNA repair. However, we hypothesize that they will be less sensitive than cancerous cells due to highly regulated and redundant DNA repair pathways, and a much high level of genomic stability. In addition, the idea of complementary lethality needs to be explored in the context of a larger panel of drugs, and with different DNA repair targets to identify potential therapeutic avenues for clinical application.
Materials and Methods
Cell lines. A549 and HeLa cells were obtained from the American Type Culture Collection and grown in AMEM or DMEM (Wisent, St. Bruno, Quebec, Canada) medium supplemented with 10% fetal bovine serum (Gibco—Life Technologies, Burlington, Ontario, Canada) at 37 °C in a humidified 5% CO2 atmosphere. Cell culture plastic-ware was obtained from Invitrogen, Fisher Scientific (Unionville, Ontario, Canada), and VWR International (Mississauga, Ontario, Canada).
siRNA transfection. siRNA transfection was performed according to the following protocol. The concentrations of siRNAs targeting human BRCA2 (OnTarget Plus BRCA2 siRNA #4 or OnTarget Plus SMARTPool BRCA2 (Dharmacon RNAi Technologies, Thermo Fisher Scientific, Lafayette, CO)) and human TS (OnTarget Plus TS siRNA #2; (Dharmacon)) that reduced target mRNAs by approximately 70% at 24 hours after transfection were determined (10 nmol/l for BRCA2 siRNA, and 2.5 nmol/l for TS siRNA). To apply equal amounts of siRNA to A549 cells in studies where BRCA2 and TS were knocked down individually or collectively, control nontargeting siRNA (Dharmacon control siRNA #2 or control SMARTPool) was added to BRCA2 siRNA or TS siRNA so that the total siRNA concentration applied in every case was 12.5 nmol/l. BRCA2 siRNA (10 nmol/l and 2.5 nmol/l control nontargeting siRNA), TS siRNA (2.5 nmol/l and 10 nmol/l control nontargeting siRNA), for BRCA2 siRNA and TS siRNA (10 nmol/l and 2.5 nmol/l, respectively) were diluted in serum-free AMEM and incubated with diluted Lipofectamine 2000 (LFA2K, Invitrogen—Life Technologies) for 20 min. The siRNA:LFA2K mix was then added to A549 cells seeded, in triplicate, at a density of 2.0 × 105 cells in 25 cm2 flasks 24 hours before transfection. Medium was exchanged for fresh AMEM and 10% fetal bovine serum 4 hours after transfection and the effects of siRNA treatments on target mRNA levels and sensitivity to cytotoxic drugs assessed as described below. A549 cells were transfected with BR-1, SARI 83, and control ASOs (synthesized by Eurogentec, Seraing, Belgium) according to the same protocol outlined for siRNA; however, they were used at 20 nmol/l each (for a total concentration of 40 nmol/l).
siRNA-mediated reduction of target mRNAs in A549 cells. Twenty-four hours after transfection of siRNA, RNA was isolated from cells using Trizol reagent according to manufacturer's instructions (Ambion—Life Technologies) and reverse-transcribed to generate cDNA using M-MLV RT enzyme (Invitrogen—Life Technologies). cDNA (1 µg) was used in conjunction with BRCA2, TS, and 18S rRNA qPCR probe and primers and Taqman master mix (Applied Biosystems—Life Technologies) to generate fluorescently labeled target cDNA. Quantification of cDNA to infer levels of TS and BRCA2 mRNAs and 18S rRNA was performed using a Perkin Elmer ViiA 7 Real-time PCR System (Life Technologies). TS and BRCA2 mRNA levels were determined relative to cellular 18S rRNA levels.
Cytotoxic drug treatment. Twenty-four hours after transfection of siRNA, medium was removed from cells and replaced with cytotoxic drug in AMEM + 10% fetal bovine serum. Cells were allowed to proliferate in the presence of drug for 72 hours followed by assessment of cell numbers. Control cells were incubated with AMEM + 10% fetal bovine serum. The concentration of cisplatin, 5-FUdR, melphalan, or pemetrexed that reduced proliferation by 25% (the IC25) was determined, and that concentration was used for most experiments where drugs were applied alone or in combination to A549 cells and HeLa cells.
Cell proliferation assay. Ninety-six hours after transfection of siRNA, cells were washed with PBS, trypsinized, and resuspended in isotonic saline (20 ml). The diluted cells were then counted (twice per sample) using a Coulter Z-1 Particle Counter (Beckman Coulter, Mississauga, Ontario, Canada). Cell number was calculated as a percentage of medium-treated control cells.
Cell cycle analysis. Cells were transfected with siRNA as described previously. 48 and 72 hours after transfection they were harvested, washed with PBS, and fixed with 70% ethanol for at least 2 hours. Cells were washed with PBS and resuspended in 1 ml of a propidium iodide (20 µg/ml) (Sigma Aldrich, St Louis, MO) and 0.1% Triton X-100 (BDH Chemicals, Toronto, Ontario, Canada) staining solution with RNAse A (Bioshop Canada, Burlington, Ontario, Canada) for 15 minutes at 37 °C. Cell cycle enumeration was performed using a BD FACSCalibur flow cytometer (BD Biosciences, Mississauga, Ontario, Canada) and analyzed using Flow Jo software (Tree Star, Ashland, OR).
Statistical analysis. Single comparisons were made using a parametric Student's t-test and multiple comparisons using a nonparametric one-way analysis of variance. All distributions of data were determined to be normal, and post hoc tests were therefore not employed. A confidence of 95% was selected a priori as the benchmark for rejection of the null hypothesis.
Acknowledgments
This work was performed under the auspices of a grant from the Canadian Institutes of Health Research to J.K. and M.V. (MOP82720). M.R. and S.M.V. are scholars of the CIHR CaRTT Program. The authors declare no conflict of interest.
References
- Youn, A and Simon, R (2012). Estimating the order of mutations during tumorigenesis from tumor genome sequencing data. Bioinformatics 28: 1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasance, ED, Cheetham, RK, Stephens, PJ, McBride, DJ, Humphray, SJ, Greenman, CD et al. (2010). A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb, LA (2011). Human cancers express mutator phenotypes: origin, consequences and targeting. Nat Rev Cancer 11: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy, LD and Venkitaraman, AR (2012). Genome instability mechanisms and the structure of cancer genomes. Curr Opin Genet Dev 22: 10–13. [DOI] [PubMed] [Google Scholar]
- Lee, W, Jiang, Z, Liu, J, Haverty, PM, Guan, Y, Stinson, J et al. (2010). The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature 465: 473–477. [DOI] [PubMed] [Google Scholar]
- Russnes, HG, Navin, N, Hicks, J and Borresen-Dale, AL (2011). Insight into the heterogeneity of breast cancer through next-generation sequencing. J Clin Invest 121: 3810–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, HH, Choe, J and Loeb, LA (2004). Protein tolerance to random amino acid change. Proc Natl Acad Sci USA 101: 9205–9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen, M, Allen, C, Nickoloff, JA and Hromas, R (2011). Synthetic lethality: exploiting the addiction of cancer to DNA repair. Blood 117: 6074–6082. [DOI] [PubMed] [Google Scholar]
- Holloman, WK (2011). Unraveling the mechanism of BRCA2 in homologous recombination. Nat Struct Mol Biol 18: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira, A, Hilario, J, Amitani, I, Baskin, RJ, Shivji, MK, Venkitaraman, AR et al. (2009). The BRC repeats of BRCA2 modulate the DNA-binding selectivity of RAD51. Cell 136: 1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooster, R, Bignell, G, Lancaster, J, Swift, S, Seal, S, Mangion, J et al. (1995). Identification of the breast cancer susceptibility gene BRCA2. Nature 378: 789–792. [DOI] [PubMed] [Google Scholar]
- Tan, DS, Rothermundt, C, Thomas, K, Bancroft, E, Eeles, R, Shanley, S et al. (2008). “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol 26: 5530–5536. [DOI] [PubMed] [Google Scholar]
- Hughes-Davies, L, Huntsman, D, Ruas, M, Fuks, F, Bye, J, Chin, SF et al. (2003). EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell 115: 523–535. [DOI] [PubMed] [Google Scholar]
- Vencken, PM, Kriege, M, Hoogwerf, D, Beugelink, S, van der Burg, ME, Hooning, MJ et al. (2011). Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol 22: 1346–1352. [DOI] [PubMed] [Google Scholar]
- Ben David, Y, Chetrit, A, Hirsh-Yechezkel, G, Friedman, E, Beck, BD, Beller, U et al.; National Israeli Study of Ovarian Cancer. (2002). Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J Clin Oncol 20: 463–466. [DOI] [PubMed] [Google Scholar]
- Chetrit, A, Hirsh-Yechezkel, G, Ben-David, Y, Lubin, F, Friedman, E and Sadetzki, S (2008). Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol 26: 20–25. [DOI] [PubMed] [Google Scholar]
- Evers, B, Schut, E, van der Burg, E, Braumuller, TM, Egan, DA, Holstege, H et al. (2010). A high-throughput pharmaceutical screen identifies compounds with specific toxicity against BRCA2-deficient tumors. Clin Cancer Res 16: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, H, McCabe, N, Lord, CJ, Tutt, AN, Johnson, DA, Richardson, TB et al. (2005). Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434: 917–921. [DOI] [PubMed] [Google Scholar]
- Ashworth, A (2008). A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 26: 3785–3790. [DOI] [PubMed] [Google Scholar]
- Rehman, FL, Lord, CJ and Ashworth, A (2010). Synthetic lethal approaches to breast cancer therapy. Nat Rev Clin Oncol 7: 718–724. [DOI] [PubMed] [Google Scholar]
- Malone, KE, Daling, JR, Doody, DR, Hsu, L, Bernstein, L, Coates, RJ et al. (2006). Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res 66: 8297–8308. [DOI] [PubMed] [Google Scholar]
- Berg, RW, Ferguson, PJ, DeMoor, JM, Vincent, MD and Koropatnick, J (2002). The means to an end of tumor cell resistance to chemotherapeutic drugs targeting thymidylate synthase: shoot the messenger. Curr Drug Targets 3: 297–309. [DOI] [PubMed] [Google Scholar]
- Di Cresce, C, Figueredo, R, Ferguson, PJ, Vincent, MD and Koropatnick, J (2011). Combining small interfering RNAs targeting thymidylate synthase and thymidine kinase 1 or 2 sensitizes human tumor cells to 5-fluorodeoxyuridine and pemetrexed. J Pharmacol Exp Ther 338: 952–963. [DOI] [PubMed] [Google Scholar]
- Ferguson, PJ, DeMoor, JM, Vincent, MD and Koropatnick, J (2001). Antisense-induced down-regulation of thymidylate synthase and enhanced cytotoxicity of 5-FUdR in 5-FUdR-resistant HeLa cells. Br J Pharmacol 134: 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing, Y, Yamazoe, M, Hirota, K, Dejsuphong, D, Sakai, W, Yamamoto, KN et al. (2011). The epistatic relationship between BRCA2 and the other RAD51 mediators in homologous recombination. PLoS Genet 7: e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, BS, Sondhi, SM and Lown, JW (1999). Synthetic DNA minor groove-binding drugs. Pharmacol Ther 84: 1–111. [DOI] [PubMed] [Google Scholar]
- Schmitz, JC, Chen, TM and Chu, E (2004). Small interfering double-stranded RNAs as therapeutic molecules to restore chemosensitivity to thymidylate synthase inhibitor compounds. Cancer Res 64: 1431–1435. [DOI] [PubMed] [Google Scholar]
- de Boer, RH, Arrieta, Ó, Yang, CH, Gottfried, M, Chan, V, Raats, J et al. (2011). Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 29: 1067–1074. [DOI] [PubMed] [Google Scholar]
- Scagliotti, GV, Parikh, P, von Pawel, J, Biesma, B, Vansteenkiste, J, Manegold, C et al. (2008). Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26: 3543–3551. [DOI] [PubMed] [Google Scholar]
- Metro, G, Chiari, R, Mare, M, Giannarelli, D, Tofanetti, FR, Minotti, V et al. (2011). Carboplatin plus pemetrexed for platinum-pretreated, advanced non-small cell lung cancer: a retrospective study with pharmacogenetic evaluation. Cancer Chemother Pharmacol 68: 1405–1412. [DOI] [PubMed] [Google Scholar]
- Lord, CJ and Ashworth, A (2012). The DNA damage response and cancer therapy. Nature 481: 287–294. [DOI] [PubMed] [Google Scholar]
- Di Cresce, C, Way, C, Rytelewski, M, Vareki, SM, Nilam, S, Vincent, MD et al. (2012). Antisense Technology: From Unique Laboratory Tool to Novel Anticancer Treatments. In: Erdmann, VA Barciszewski J (eds). From Nucleic Acids Sequences to Molecular Medicine. Springer: Berlin Heidelberg. pp. 145–189. [Google Scholar]
- Jensen, RB, Carreira, A and Kowalczykowski, SC (2010). Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 467: 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira, A and Kowalczykowski, SC (2011). Two classes of BRC repeats in BRCA2 promote RAD51 nucleoprotein filament function by distinct mechanisms. Proc Natl Acad Sci USA 108: 10448–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros, S, Roos, WP and Kaina, B (2011). Rad51 and BRCA2–New molecular targets for sensitizing glioma cells to alkylating anticancer drugs. PLoS ONE 6: e27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlacher, K, Wu, H and Jasin, M (2012). A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell 22: 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonosov, M, Anand, S, Sangrithi, M, Davies, R and Venkitaraman, AR (2003). Stabilization of stalled DNA replication forks by the BRCA2 breast cancer susceptibility protein. Genes Dev 17: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlacher, K, Christ, N, Siaud, N, Egashira, A, Wu, H and Jasin, M (2011). Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 145: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, Y, Nakajima, G, Schmitz, JC, Chu, E and Ju, J (2006). Multi-level gene expression profiles affected by thymidylate synthase and 5-fluorouracil in colon cancer. BMC Genomics 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabner, BA and Roberts, TG Jr (2005). Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer 5: 65–72. [DOI] [PubMed] [Google Scholar]
- Stephen, RL, Novak, JM, Jensen, EM, Kablitz, C and Buys, SS (1990). Effect of osmotic pressure on uptake of chemotherapeutic agents by carcinoma cells. Cancer Res 50: 4704–4708. [PubMed] [Google Scholar]
- Eichholtz-Wirth, H and Hietel, B (1986). The relationship between cisplatin sensitivity and drug uptake into mammalian cells in vitro. Br J Cancer 54: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares, L, de Marinis, F, Dediu, M, Thomas, M, Pujol, JL, Bidoli, P et al. (2012). Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 13: 247–255. [DOI] [PubMed] [Google Scholar]
- Villanueva, T (2010). Expanding the horizons of PARP inhibitors. Nat Rev Cancer 10: 814. [DOI] [PubMed] [Google Scholar]
- Krishnakumar, R and Kraus, WL (2010). The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell 39: 8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenski, DM, Butora, G, Willingham, AT, Cooper, AJ, Fu, W, Qi, N et al. (2012). siRNA-optimized Modifications for Enhanced In Vivo Activity. Mol Ther Nucleic Acids 1: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, WF, Prakash, TP, Murray, HM, Kinberger, GA, Li, W, Chappell, AE et al. (2012). Single-stranded siRNAs activate RNAi in animals. Cell 150: 883–894. [DOI] [PubMed] [Google Scholar]
- Bogdahn, U, Hau, P, Stockhammer, G, Venkataramana, NK, Mahapatra, AK, Suri, A et al.; Trabedersen Glioma Study Group. (2011). Targeted therapy for high-grade glioma with the TGF-ß2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro-oncology 13: 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, S, Moore, JO, Boyd, TE, Larratt, LM, Skotnicki, A, Koziner, B et al. (2007). Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 25: 1114–1120. [DOI] [PubMed] [Google Scholar]
- Bennett, CF and Swayze, EE (2010). RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol 50: 259–293. [DOI] [PubMed] [Google Scholar]