Abstract
Background
The burden of axillary disease in patients with locally advanced breast cancer (labc) after neoadjuvant therapy (nat) has not been extensively described in a large modern cohort. Here, we describe the extent of nodal metastases after nat in patients with labc.
Methods
All patients with labc treated at a single institution during 2002–2007 were identified. Demographic, radiologic, and pathologic variables were extracted. To assess the extent of lymph node metastases after nat, patients were separated into two groups: those with and without clinical or radiologic evidence of lymph node metastases before nat. Axillary lymph nodes retrieved at surgery that had no evidence of metastases after hematoxylin and eosin (h&e) staining underwent further pathology evaluation.
Results
Of the 116 patients identified, 115 were female (median age: 48.5). Before nat, 26 patients were clinically and radiologically node-negative; of those 26, 14 were histologically negative on final pathology. After serial sectioning and immunohistochemistry, 9 of 26 (35%) were node-negative. Of the 90 patients who had clinical or radiologic evidence of lymph node metastases before nat, 23 (26%) had no evidence of lymph node metastases on h&e staining. After serial sectioning and immunohistochemistry, 19 (21%) had no further axillary lymph node metastases. Overall, 76% of patients had pathology evidence of lymph node metastases after nat.
Conclusions
Most patients with labc have axillary metastases after nat. Our findings support axillary lymph node dissection and locoregional radiation in most patients with labc after nat.
Keywords: Locally-advanced breast cancer, neoadjuvant therapy, axillary lymph node dissection, lymph node metastases
1. INTRODUCTION
Locally advanced breast cancer (labc) is defined as a breast cancer 5 cm or greater in size, involving the chest wall or skin, with matted axillary lymph nodes or with ipsilateral infraclavicular, supraclavicular, or internal mammary lymph node involvement, or both (stages iib, iiia, iiib, iiic)1. Fewer than 5% of patients with invasive breast cancer present with labc2. Of those patients, 70% will have metastatic spread of disease to the axillary lymph nodes at the time of diagnosis3. Importantly, the extent of axillary lymph node involvement after neoadjuvant therapy (nat) is recognized as the most significant prognosticator for labc patients4.
The effectiveness of nat appears to be less in eliminating disease in the lymph nodes than in the primary tumour5. In the National Surgical Adjuvant Breast and Bowel Project study B-27, 15.5% of patients with a pathologic complete response (pcr) in the breast still had cancer in the axillary lymph nodes6. Kuerer et al. also reported that 41% (78 of 191) of patients with a pcr in the breast still had positive axillary nodes after preoperative 5-fluorouracil, doxorubicin, and cyclophosphamide7.
In more recent work, pcr has been found to be associated with receptor status. Zambetti et al. found that patients with estrogen receptor (er)–negative tumours were more likely than those with er-positive tumours to achieve pcr (45.3% vs. 10.4%)8. Other authors have found that positivity for the human epidermal growth factor receptor 2 (her2/neu) and treatment with trastuzumab and anthracycline–taxane chemotherapy are also associated with a higher pcr than is treatment with chemotherapy alone for her2/neu–negative tumours (31.7% vs. 15.7%)9.
In our previous work, we demonstrated a 12% breast pcr for patients with labc treated with nat (6 of 48), and a 4% combined pcr (breast and axilla), suggesting that compared with patients having early breast cancer, those with labc have a lower pcr both in the breast and in the axillary nodes10. Our findings also suggested that lymph nodes are less likely than the breast to achieve a pcr when treated with nat, especially in patients with labc10.
A number of investigators have studied the extent of lymph node metastases after nat in patients with labc—often in the context of assessing the utility of sentinel lymph node biopsy (slnb) for this population. Chung et al. assessed slnb in 41 patients with breast cancers larger than 5 cm and demonstrated high accuracy (98%), a low false-negative rate (3%), and a nodal positivity rate of 75.6%11. A more recent study from France demonstrated a mapping rate of 90% and a false-negative rate of 11.5% in patients with labc (n = 195, either N0 or N1 before nat) who underwent slnb12. The nodal positivity rate after nat in that study was 26.6%10. In contrast, another recent study reviewed 77 patients who had labc treated with nat and who converted from node-positive to node-negative as determined clinically. The mapping rate was 92%; the false-negative rate, 13.7%; and the nodal positivity rate, 71.8%13.
In view of these mixed data with respect to nodal positivity rates in patients with labc after nat, the purpose of the present study was to describe the extent of nodal metastases after nat in a modern cohort of patients with labc who were both clinically and radiologically node-negative or -positive before nat.
2. METHODS
A prospectively kept database of all patients with labc attending the Edmund Odette Cancer Centre at Sunnybrook Health Sciences Centre was used to identify 116 consecutive patients treated for labc between 2002 and 2007. All patients had at least one of the following features: tumour larger than 5 cm, involvement of chest wall or skin, matted axillary lymph nodes, or infraclavicular or supraclavicular lymph node involvement. All patients were stage iib, iiia, iiib, or iiic. Demographic, radiologic, and pathologic data were collected, including age, stage of disease, sex, menopausal status, tumour type and size (as determined on physical examination), histologic grade, presence or absence of lymphovascular invasion, clinical assessment of axilla before nat, radiologic and pathologic assessment of axilla before nat, lymph node involvement in the final surgical specimen, hormone receptor and her2 status (determined before nat on core biopsy). All patients received anthracycline- or taxane-based chemotherapy (or both) or endocrine therapy (alone or in combination with chemotherapy) according to Canadian guidelines. Patients whose tumours were her2-positive received trastuzumab, and all underwent axillary lymph node dissection (alnd) as part of their surgical treatment. All patients were offered postoperative radiation as part of standard treatment.
Surgical specimens were processed according to standard institutional protocol: tissue was fixed in 10% buffered formalin, and lymph nodes were retrieved. All grossly negative nodes were entirely embedded. Lymph nodes not larger than 0.5 cm were embedded along the long axis; nodes 0.5–0.9 cm were bisected along the long axis; and nodes 1 cm or larger were serially sectioned perpendicular to the long axis at approximately 0.3-cm intervals. One section stained with h&e was evaluated from each block. All patients were signed out by dedicated breast pathologists.
To determine whether patients who were found to be node-negative after axillary node dissection would also be node-negative if their nodes were processed according to the institutional protocol for sentinel nodes, we identified all blocks of axillary lymph nodes from the subset of patients who were histologically node-negative on final pathology. Sections were obtained from 4 additional levels on each block: 2 for h&e staining, 1 for pan-cytokeratin immunohistochemistry [ihc (mouse monoclonal antibody, clone AE1/AE3: Dako, Carpentaria, CA, U.S.A.)], and 1 additional level for use as a negative control. The nodal status was classified as ypN0i–, ypN0i+, ypN1mic, or ypN1 based on the American Joint Committee on Cancer staging manual, 7th edition14.
Descriptive statistics, including medians, ranges, and frequencies were performed using the Statistical Package for Social Sciences (SPSS, Chicago, IL, U.S.A.). The study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre.
3. RESULTS
3.1. Demographics and Tumour Characteristics
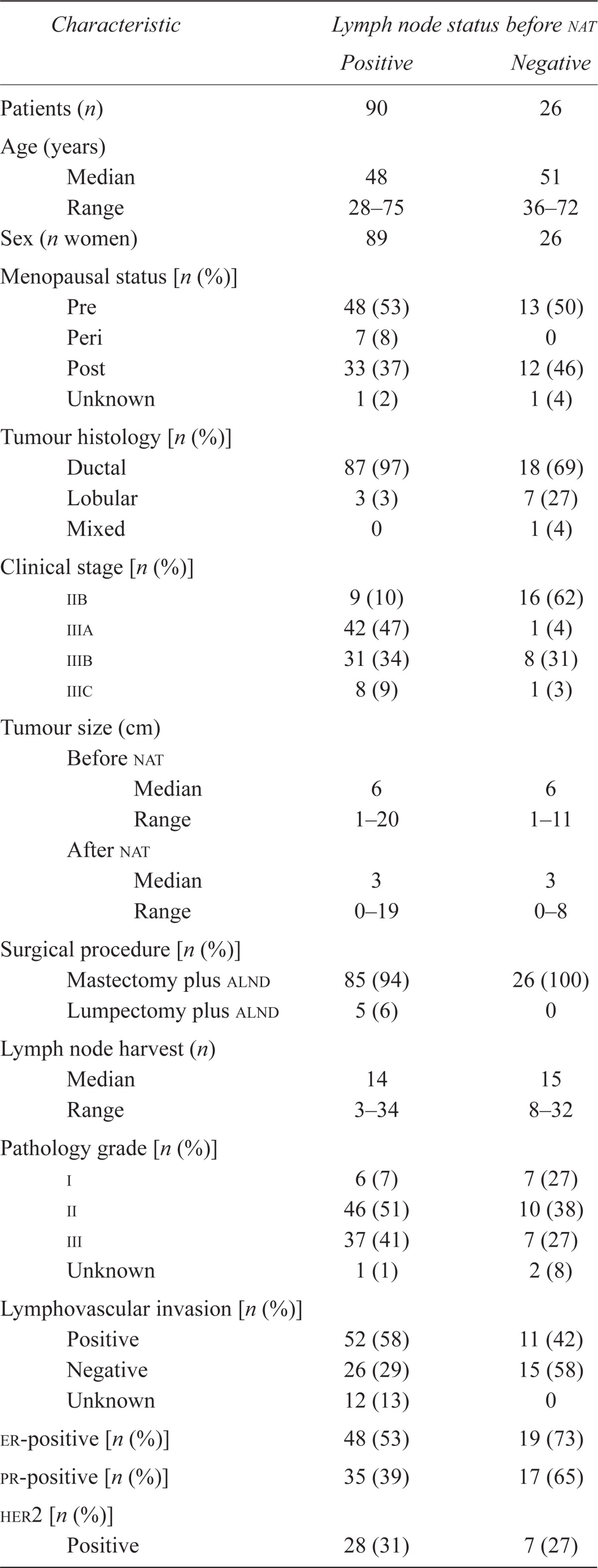
The 116 patients from our prospective database, including 1 man who met the selection criteria for labc, had a median age of 48.5 years (range: 28–75 years), and 62 patients (53%) were premenopausal (Table i). The diagnosis in 106 patients (91%) was invasive ductal carcinoma. In 62% of patients, the tumour was er- or progesterone receptor (pr)–positive; in 33%, her2-positive; and in 24%, er-, pr-, and her2-negative (triple-negative). Clinical stage distribution was iib in 25 patients (22%), iiia in 43 patients (37%), iiib in 39 patients (34%), and iiic in 9 patients (8%). The median tumour size before nat was 6 cm (range: 1–20 cm). All patients received neoadjuvant chemotherapy, including epirubicin–docetaxel (n = 45), doxorubicin–cyclophosphamide–docetaxel (n = 26), 5-fluorouracil–epirubicin–cyclophosphamide (n = 24), 5-f luorouracil–epirubicin–cyclophosphamide–docetaxel (n = 10), doxorubicin–cyclophosphamide (n = 6), docetaxel only (n = 4), and doxorubicin–cyclophosphamide–docetaxel plus trastuzumab (n = 1). Of the 116 patients, 114 (98%) received postoperative radiation. No patients received preoperative radiation. The surgical procedure in 111 patients (96%) was mastectomy and alnd, and in the remaining 5 (4%), it was lumpectomy and alnd. Based on pathology data, the median tumour size after nat was 3 cm (range: 0–19 cm), and the median number of lymph nodes removed during surgery was 14 (range: 3–34; mean: 15.5). The median follow-up was 50 months (range: 0–94 months).
TABLE I.
Clinicopathologic characteristics of patients with locally advanced breast cancer (labc)
| Characteristic | Lymph node status before nat | |
|---|---|---|
| Positive | Negative | |
| Patients (n) | 90 | 26 |
| Age (years) | ||
| Median | 48 | 51 |
| Range | 28–75 | 36–72 |
| Sex (n women) | 89 | 26 |
| Menopausal status [n (%)] | ||
| Pre | 48 (53) | 13 (50) |
| Peri | 7 (8) | 0 |
| Post | 33 (37) | 12 (46) |
| Unknown | 1 (2) | 1 (4) |
| Tumour histology [n (%)] | ||
| Ductal | 87 (97) | 18 (69) |
| Lobular | 3 (3) | 7 (27) |
| Mixed | 0 | 1 (4) |
| Clinical stage [n (%)] | ||
| iib | 9 (10) | 16 (62) |
| iiia | 42 (47) | 1 (4) |
| iiib | 31 (34) | 8 (31) |
| iiic | 8 (9) | 1 (3) |
| Tumour size (cm) | ||
| Before nat | ||
| Median | 6 | 6 |
| Range | 1–20 | 1–11 |
| After nat | ||
| Median | 3 | 3 |
| Range | 0–19 | 0–8 |
| Surgical procedure [n (%)] | ||
| Mastectomy plus alnd | 85 (94) | 26 (100) |
| Lumpectomy plus alnd | 5 (6) | 0 |
| Lymph node harvest (n) | ||
| Median | 14 | 15 |
| Range | 3–34 | 8–32 |
| Pathology grade [n (%)] | ||
| i | 6 (7) | 7 (27) |
| ii | 46 (51) | 10 (38) |
| iii | 37 (41) | 7 (27) |
| Unknown | 1 (1) | 2 (8) |
| Lymphovascular invasion [n (%)] | ||
| Positive | 52 (58) | 11 (42) |
| Negative | 26 (29) | 15 (58) |
| Unknown | 12 (13) | 0 |
| er-positive [n (%)] | 48 (53) | 19 (73) |
| pr-positive [n (%)] | 35 (39) | 17 (65) |
| her2 [n (%)] | ||
| Positive | 28 (31) | 7 (27) |
| her2 [n (%)] | ||
| Unknown | 1 (1) | 2 (7) |
| Patients [n (%)] with | ||
| Positive lymph nodesc | 67 | 12 |
| 1 Node | 16 (24) | 4 (33) |
| 2 Nodes | 4 (6) | 1 (8) |
| 3 Nodes | 9 (13) | 2 (17) |
| 4 nodes | 38 (57) | 5 (42) |
| Negative lymph nodesa | 23 | 14 |
| Follow-up (months) | ||
| Median | 48 | 58 |
| Range | 0–86 | 14–94 |
| Disease status [n (%)] | ||
| Disease-free | 48 (53) | 20 (77) |
| Palliative | 30 (36) | 4 (16) |
| Deceased | 3 (3) | 0 |
| Lost to follow-up | 9 (10) | 2 (8) |
By hematoxylin and eosin staining.
nat = neoadjuvant therapy; alnd = axillary lymph node dissection; er = estrogen receptor; pr = progesterone receptor; her2 = human epidermal growth factor receptor 2.
3.2. Pre-NAT Clinical and Radiologic Assessment of Axillary Lymph Node Involvement
Among the 90 patients who had clinical or radiologic evidence of lymph node metastases (or both) before nat, 53% were er- or pr-positive (or both), 27% were her2-positive, and 34% were triple-negative. On clinical examination, 67 patients had suspicious axillary lymph nodes, and on ultrasonography or magnetic resonance imaging, 81 had suspicious lymph nodes. Of these 90 patients, 28 (31%) underwent imaging-guided pre-nat fine-needle aspiration, with 25 (89%) being positive for metastatic disease and with 3 (11%) having a negative or inconclusive biopsy, but positive disease on final pathology. Biopsies were not completed on all patients with suspicious lymph nodes because this practice was not common at our institution until later in the study period.
Before nat, 26 patients had no clinical or radiologic evidence of lymph node metastases. Of those 26 patients, all underwent axillary ultrasonography, all underwent mammography, and 22 underwent magnetic resonance imaging as part of their radiologic assessment.
3.3. Post-NAT Pathology Assessment of Lymph Nodes
Overall, pathology assessment of h&e-stained alnd specimens demonstrated that 79 patients (68%) had metastases in the axillary lymph nodes. Of those 79 patients, 20 (25%) had 1 positive lymph node, 5 (6%) had 2 positive nodes, 11 (14%) had 3 positive nodes, and 43 (54%) had 4 or more positive nodes. The mean number of positive nodes was 9. Of patients who had a high volume of axillary metastases (>4 nodes) after nat, 60% were er- or pr-positive (or both), 23% were her2-positive, and 26% were triple-negative. Among the 28 patients who underwent imaging-guided fine-needle aspiration before nat, 4 (14%) had axillary lymph nodes that initially tested positive on h&e staining; after alnd, they tested negative (Figure 1). Of those 4 patients, 3 had triple-negative disease; the 4th had triple-positive disease.
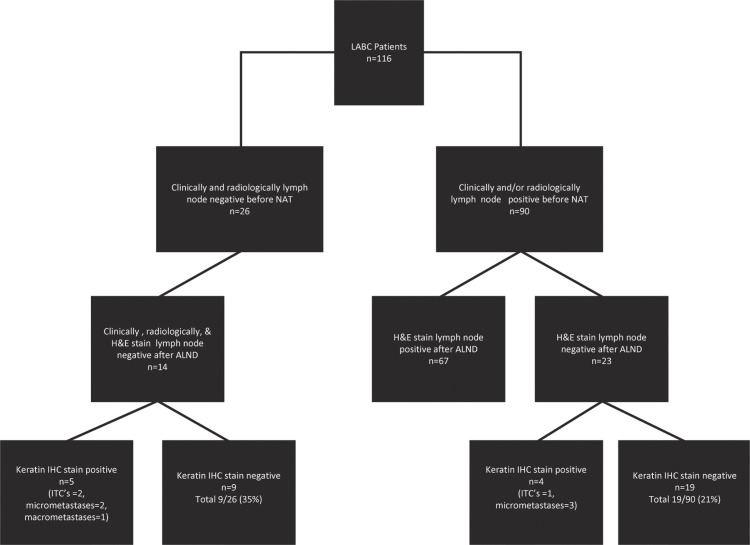
FIGURE 1.
Pathology assessment of axillary lymph nodes in patients with and without clinical and radiologic evidence of lymph node metastases before neoadjuvant therapy.
3.4. Pre-NAT Pathology Assessment of Axillary Lymph Nodes from Patients with No Clinical and Radiologic Evidence of Lymph Node Metastases
Before nat, 26 patients had no clinical or radiologic evidence of lymph node metastases. Of those 26 patients, 14 had no evidence of lymph node metastases on the final standard pathology assessment of their axillary lymph node dissection specimens. However, further pathology analysis using keratin ihc staining and serial sectioning demonstrated that 5 patients had evidence of lymph node metastases when a sentinel node protocol was applied (n = 2, isolated tumour cells; n = 2, micrometastases; n = 1, macrometastases). The average number of lymph nodes undergoing additional ihc analysis was 15.3 nodes.
In summary, 8 of 26 patients (31%) were clinically and radiologically node-negative before nat and, by pathology analysis, had negative nodes by both h&e staining and ihc. Those 8 patients had a median age of 51 years (range: 38–71 years), and 3 (38%) were premenopausal, with 6 (75%) being er-positive, 5 (63%) being pr-positive, and 2 (25%) being her2-positive. When the characteristics of this group (clinically and radiologically node-negative and negative by h&e and ihc staining) were compared with the characteristics of the overall group, median age was older (51 vs. 48 years), frequency of lymphovascular invasion in the tumour was less (33% vs. 55%), and frequency of her2 positivity was lower (17% vs. 33%).
3.5. Pre-NAT Pathology Assessment of Patients with Evidence of Lymph Node Metastases
Of the 90 patients who had clinical or radiologic evidence of lymph node metastases before nat, 67 (74%) were confirmed to have lymph node metastases on standard pathology evaluation of h&e stained samples from the excised axillary lymph nodes.
The 23 patients with no evidence of lymph node metastases in the alnd by standard processing underwent further pathology analysis, and in 4, lymph node metastases were identified (n = 1, isolated tumour cells; n = 3, micrometastases). In total, 19 of 90 patients (21%) who had clinical or radiologic evidence of disease before nat had no evidence of lymph node metastases after nat when the excised axillary lymph nodes were serially sectioned and examined by ihc.
In 4 of 26 patients (15%) whose fine-needle aspiration specimen initially tested positive by h&e staining before nat, a full pathology analysis after alnd yielded a negative result.
3.6. Outcomes
Median follow-up was 50 months (range: 0–94 months). At last follow-up, 68 patients (59%) were disease-free, 34 (29%) had metastatic disease, 3 (3%) were deceased, and 11 (9%) were lost to follow-up. In the subgroup of patients who were node-negative after nat (n = 28), median follow-up was 50 months (range: 0–94 months). At last follow-up, 18 (64%) were disease-free, 4 (14%) had metastatic disease, 1 (4%) was deceased, and 5 (18%) were lost to follow-up.
4. DISCUSSION
In this study, we found that 68% of patients (n = 79) with labc who received nat had evidence of lymph node metastases on standard h&e assessment. With more detailed pathology assessment using a sentinel node protocol, an additional 9 patients with lymph node metastases were identified, so that, in total, 76% (88 of 116) of patients with labc treated with nat had evidence of lymph node metastases on final pathology assessment. Furthermore, of the patients who had metastatic disease in their lymph nodes, more than half had metastases in 4 or more lymph nodes at final histopathology, which reflects the severity of disease in this population and a lack of response to nat.
Our finding of upstaging with the use of a sentinel node protocol (serial sectioning and ihc staining) for assessment is consistent with results in previous reports, which demonstrated that whether a patient received or did not receive chemotherapy before surgery, metastatic disease was identified in an additional 9%–31% of patients originally diagnosed with node-negative disease by standard pathology examination15–20. We acknowledge that the volume of disease for 8 of the 9 patients who were upstaged was low: 3 had isolated tumour cells only, and 5 had micrometastases. Nonetheless, these patients had already received chemotherapy and, consequently, the presence of lymph node metastases may be clinically significant.
In our cohort, 21% of patients were converted from clinical or radiologic suspicion of lymph node metastases to no evidence of lymph node metastases after nat. Even fewer patients who were biopsied and who proved to have lymph node metastases were converted to node-negative status (15%) after nat. Regression of tumour growth and sterilization in primary lesions and lymph nodes has been well described to be a result of nat; however, our rate of 15% is much lower than the 35%–40% reported in the literature for neoadjuvant chemotherapy21,22. In our series, 3 of these patients were triple-negative3, and 1 was triple-positive. This lower response rate despite the use of similar treatment regimens is likely related to the greater burden of disease in our population of patients strictly with labc21. We do not currently support offering slnb to patients with biopsy-proven lymph node metastases before nat. The false-negative rate for slnb when metastases are present in the axillary lymph nodes before nat is currently unknown; what that rate might be is currently the focus of a number of clinical trials.
Importantly for operative planning and patient education, we have found that 35% of patients with no clinical or radiologic evidence of metastases before nat are actually free of nodal macrometastases on final pathology assessment. Consequently, a very select group of patients with labc who are clinically and radiologically node-negative before nat could potentially benefit from a slnb. We strongly advocate for a completion alnd if the sentinel nodes demonstrate metastases, because no evaluation of the comparative efficacy of postoperative radiation or endocrine therapy (or both) on locoregional control has been conducted in the setting of residual axillary disease in nonsentinel nodes after nat.
There are a number of concerns about slnb in the labc population after nat. A few studies have looked specifically at patients with labc and slnb. In those studies, the false-negative rate of slnb after nat in the labc population has been reported to be between 3% and 13.7%9–11. False-negative slnbs are of particular concern in the labc setting, because any residual disease is chemotherapy-resistant. Consequently, at the present time, we suggest they patients with lymph node metastases before nat should be offered an alnd. Postoperative locoregional radiation is our standard treatment for all patients with labc.
This single-centre retrospective study of a well-defined group of patients with labc has limitations. The major limitation is the lack of biopsies for all suspicious lymph nodes before nat, because biopsy as a standard procedure was not normal clinical practice during part of the study period. However, a subset of patients did undergo biopsy, and a conversion rate of 15% from node-positive to node-negative was noted. Another limitation is the size of the cohort (n = 116), which does not allow for an exploration of the varying effects of nat on malignancies with different receptor profiles (er-positive and -negative, her2-negative and -positive). The small cohort consequently does not allow for a more tailored recommendation for axillary lymph node assessment by receptor profile.
Overall, in our cohort, 76% of labc patients had evidence of lymph node metastases after nat, a rate that is comparable to those reported in the literature. We support slnb only in labc patients who have no evidence of disease after nat; however, if disease is identified in the sentinel nodes, then the relevant patients should have a completion alnd and locoregional radiation to optimize locoregional control.
5. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
6. REFERENCES
- 1.Olivotto IA, Chua B, Allan SJ, Speers CH, Chia S, Ragaz J. Long-term survival of patients with supraclavicular metastases at diagnosis of breast cancer. J Clin Oncol. 2003;21:851–4. doi: 10.1200/JCO.2003.11.105. [DOI] [PubMed] [Google Scholar]
- 2.Seidman H, Gelb SK, Silverberg E, LaVerda N, Lubera JA. Survival experience in the Breast Cancer Detection Demonstration Project. CA Cancer J Clin. 1987;37:258–90. doi: 10.3322/canjclin.37.5.258. [DOI] [PubMed] [Google Scholar]
- 3.Reintgen D, Cox C, Greenberg H, et al. The medical legal implications of following mammographic breast masses. Am Surg. 1993;59:99–105. [PubMed] [Google Scholar]
- 4.McCready DR, Hortobagyi GN, Kau SW, Smith TL, Buzdar AU, Balch CM. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surgery. 1989;124:21–5. doi: 10.1001/archsurg.1989.01410010027005. [DOI] [PubMed] [Google Scholar]
- 5.Chung A, Giuliano A. Axillary staging in the neoadjuvant setting. Ann Surg Oncol. 2010;17:2401–10. doi: 10.1245/s10434-010-1001-8. [DOI] [PubMed] [Google Scholar]
- 6.Mamounas EP. Sentinel lymph node biopsy after neoadjuvant systemic therapy. Surg Clin North Am. 2003;83:931–42. doi: 10.1016/S0039-6109(03)00032-X. [DOI] [PubMed] [Google Scholar]
- 7.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumour and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–9. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 8.Zambetti M, Mansutti M, Gomez P, et al. Pathological complete response rates following different neoadjuvant chemotherapy regimens for operable breast cancer according to er status, in two parallel, randomized phase ii trials with an adaptive design (ecto ii) Breast Cancer Res Treat. 2012;132:843–51. doi: 10.1007/s10549-011-1660-6. [DOI] [PubMed] [Google Scholar]
- 9.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in her2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–31. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 10.Wright FC, Zubovits J, Gardner S, et al. Optimal assessment of residual disease after neo-adjuvant therapy for locally advanced and inflammatory breast cancer—clinical examination, mammography or magnetic resonance imaging? J Surg Oncol. 2010;101:604–10. doi: 10.1002/jso.21559. [DOI] [PubMed] [Google Scholar]
- 11.Chung MH, Ye W, Giuliano AE. Role for sentinel lymph node dissection in the management of large (> or +5 cm) invasive breast cancer. Ann Surg Oncol. 2001;8:688–92. doi: 10.1007/s10434-001-0688-y. [DOI] [PubMed] [Google Scholar]
- 12.Classe JM, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of GanglionSentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009;27:726–32. doi: 10.1200/JCO.2008.18.3228. [DOI] [PubMed] [Google Scholar]
- 13.Ozmen V, Unal ES, Muslumanoglu ME, et al. Axillary sentinel node biopsy after neoadjuvant chemotherapy. Eur J Surg Oncol. 2010;36:23–9. doi: 10.1016/j.ejso.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer–Verlag; 2009. pp. 432–3. [Google Scholar]
- 15.Cohen LF, Breslin TM, Kuerer HM, Ross MI, Hunt KK, Sahin AA. Identification and evaluation of axillary sentinel lymph nodes in patients with breast carcinoma treated with neoadjuvant chemotherapy. Am J Surg Pathol. 2000;24:1266–72. doi: 10.1097/00000478-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZL, Wen DR, Coulson WF, Giuliano AE, Cochran AJ. Occult metastases in the axillary lymph nodes of patients with breast cancer node negative by clinical and histologic examination and conventional histology. Dis Markers. 1991;9:239–48. [PubMed] [Google Scholar]
- 17.Prognostic importance of occult axillary lymph node micro-metastases from breast cancers. International (Ludwig) Breast Cancer Study Group. Lancet. 1990;335:1565–8. [PubMed] [Google Scholar]
- 18.Trojani M, de Mascarel I, Bonichon F, Coindre JM, Delsol G. Micrometastases to axillary lymph nodes from carcinoma of breast: detection by immunohistochemistry and prognostic significance. Br J Cancer. 1987;55:303–6. doi: 10.1038/bjc.1987.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner RR, Ollila DW, Stern S, Giuliano AE. Optimal histopathologic examination of the sentinel lymph node for breast cancer staging. Am J Surg Pathol. 1999;23:263–7. doi: 10.1097/00000478-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang PJ, Reisner RM, Nangia R, Edge SB, Brooks JJ. Effectiveness of multiple-level sectioning in detecting axillary nodal micrometastasis in breast cancer. Arch Pathol Lab Med. 1998;122:687–90. [PubMed] [Google Scholar]
- 21.Kuerer HM, Sahin AA, Hunt KK, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230:72–8. doi: 10.1097/00000658-199907000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bear HD, Anderson S, Brown A, et al. The effect of tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–74. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]