Abstract
Background
Promising new drugs such as lenalidomide, an immunomodulatory agent, are available for the treatment of multiple myeloma. We describe the process of creating a provincial guideline for the use of lenalidomide, alone or in combination with other drugs, in relapsed, refractory, or newly diagnosed disease (including smoldering and symptomatic patients, and candidates and non-candidates for transplant) and in maintenance treatment (after transplant or non-transplant therapy); and for strategies to manage lenalidomide-related toxicities.
Methods
Outcomes of interest included overall survival, event-free survival, progression-free survival, time to progression, time to next treatment, response rate, and incidence of serious toxicity. The medline, embase, and Cochrane Library databases, as well as meeting abstracts and the Web sites of relevant organizations, were systematically searched for relevant literature.
Results
Recommendations were developed using the evidence from published studies and the clinical expertise of the working group and of the Cancer Care Ontario Hematology Disease Site Group.
Conclusions
Lenalidomide in combination with dexamethasone can be recommended for both previously untreated and treated patients with multiple myeloma. Guidelines for the management of cytopenias, venous thromboembolism, and second primary malignancies are discussed.
Keywords: Lenalidomide, multiple myeloma, imid, practice guideline
1. INTRODUCTION
Lenalidomide was first approved for use in multiple myeloma patients who had received at least 1 prior therapy, based on results from two large randomized trials comparing lenalidomide plus dexamethasone with placebo plus dexamethasone1,2. Since then, the role of lenalidomide has been investigated in expanded roles and novel combinations. Toxicities such as cytopenias, venous thromboembolism (vte), and the potential risk of second primary malignancies (spms) pose challenges in the management of patients receiving lenalidomide.
The Cancer Care Ontario (cco) Hematology Disease Site Group (dsg), in collaboration with the Program in Evidence-Based Care (pebc) developed recommendations for the use of lenalidomide in multiple myeloma as part of a trilogy of guidelines for the treatment of myeloma using three novel backbone agents: thalidomide, bortezomib, and lenalidomide. The recommendations presented here provide practical, evidence-based guidance concerning indications for use, dosing, combinations under investigation, and management of key toxicities.
2. METHODS
This practice guideline was developed by the Hematology dsg of cco’s pebc using the methods of the practice guidelines development cycle3. The pebc is editorially independent of cco and the Ontario Ministry of Health and Long-Term Care.
2.1. Questions
Does lenalidomide (alone or in combination with other therapies) improve outcomes in patients with previously untreated multiple myeloma (including smoldering and symptomatic patients, and candidates or non-candidates for transplant) compared with non-lenalidomide-containing treatments?
Does lenalidomide (alone or in combination with other therapies) improve outcomes in patients with relapsed or refractory multiple myeloma compared with non-lenalidomide-containing treatments?
Which multiple myeloma patients, both previously untreated and relapsed or refractory, are more or less likely to benefit from treatment with lenalidomide?
Are outcomes in myeloma patients improved with the use of lenalidomide as maintenance or consolidation treatment (after transplant and non-transplant treatments) compared with either non-lenalidomide-containing treatment or no maintenance or consolidation treatment?
What are the best strategies to manage lenalidomide-induced toxicity?
2.2. Target Population
The target population for this guideline is adult patients with previously untreated, relapsed, or refractory multiple myeloma.
2.3. Systematic Review
The literature was systematically searched using electronic databases (medline, embase, and the Cochrane Library; meeting proceedings of the American Society of Hematology, the American Society of Clinical Oncology, and the International Myeloma Workshops), relevant Web sites such as CancerGuidelines.ca, the U.S. National Guideline Clearinghouse, the Canadian Medical Association Infobase, the Physician Data Query database, and the American College of Physicians Journal Club; and reference lists of included articles.
2.4. Study Selection Criteria
Articles published from January 2000 to February 2012, inclusive, were selected for this systematic review if they were
studies of adult patients with multiple myeloma.
studies that tested the role of lenalidomide alone or in combination with other agents.
-
studies that reported results for any of the following outcomes:
overall survival (os)
event-free survival
progression-free survival (pfs)
time to progression (ttp)
time to next treatment
response rate (complete and partial)
incidence of serious toxicity (that is, grade 3 or 4 adverse events by the National Cancer Institute toxicity criteria)
studies that were systematic reviews or randomized controlled trials (rcts: phase ii or phase iii).
nonrandomized studies that were follow-ups or subanalyses of previous pivotal studies.
studies with a trial sample size of 30 or more.
studies published in English.
Narrative reviews, phase i trials, observational studies, case reports, noncomparative studies, and publication types such as commentaries, editorials, and letters were excluded. Conference abstracts that were reports of non-final analyses were also excluded. Cost-effectiveness and health-related quality of life were not outcomes of interest for this document.
2.5. Selection of Studies
Citations located in the search were screened independently by the methodologist and by two clinician members of the Working Group.
2.6. Development of Recommendations
The Hematology dsg developed draft recommendations based both on consensus and on evidence from the systematic review.
2.7. Internal and External Review
Before submission of the draft report for external review, the systematic review and practice guideline were reviewed by the pebc Report Approval Panel. The draft report was distributed to health care providers in the province of Ontario for feedback, the results of which can be found in the full guideline report on the cco Web site: https://www.cancercare.on.ca/toolbox/qualityguidelines/.
3. RESULTS AND RECOMMENDATIONS
3.1. Literature Search Results
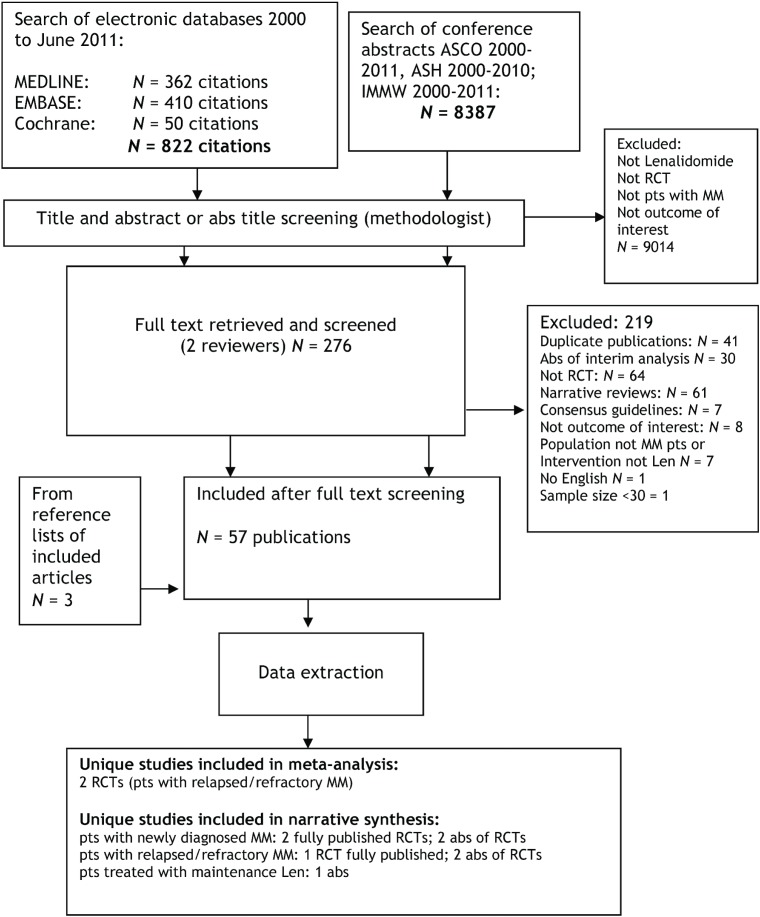
The search identified 9209 citations (Figure 1). After full text screening, fifty-two publications were included: two practice guidelines4,5; three systematic reviews6–8; ten unique primary studies, of which five were full-text publications1,2,9–11 and five were conference abstracts12–16. Forty-two publications were ancillary to the ten unique primary studies, including secondary analyses or follow-up data from the main publications (Table i).
FIGURE 1.
Study flow chart.
TABLE I.
Lenalidomide (len) in multiple myeloma (mm): primary and ancillary publications of identified randomized trials and their objectives



| Study name | Primary publication | Investigation | Additional publications | Investigation |
|---|---|---|---|---|
| MM-009 | Weber et al., 20071 | Compare len plus dexamethasone (dex) with placebo (plc) plus dex in relapsed or refractory mm | Zangari et al., 201017 | Pooled subgroup analysis investigating the effect of thromboembolic complications |
| Dimopoulos et al., 201018 | Pooled subgroup analysis of patients with impaired renal function | |||
| Harousseau et al., 201019 | Pooled subgroup analysis of patients with complete or very good partial response and those with partial response | |||
| Castaneda et al., 2010 (abstract)20 | Pooled subgroup analysis for the evaluation of peripheral neuropathy and adverse events | |||
| Baz et al., 2010 (abstract)21 | Pooled subgroup analysis for the evaluation of the occurrence of viral infections | |||
| MM-010 | Dimopoulos et al., 20072 | Compare len plus dex with plc plus dex in relapsed or refractory mm | Baz et al., 2009 (abstract)22 | Pooled subgroup analysis investigating the effect of len plus dex on levels of immunoglobulin A (IgA) |
| Lonial et al., 2009 (abstract)23 | Pooled subgroup analysis investigating the incidence, onset, and duration of neutropenia | |||
| Delforge et al., 2009 (abstract)24 | Pooled subgroup analysis of patients with and without a history of peripheral neuropathy | |||
| Dimopoulos et al., 2009 (abstract)25 | Pooled subgroup analysis of len dose adjustments to determine an optimal long-term treatment strategy | |||
| Dimopoulos et al., 2006 (abstract)26 | Pooled subgroup analysis investigating the effect of number of prior therapies | |||
| Dimopoulos et al., 200927 | Long-term follow-up of the two pivotal randomized trials | |||
| Wang et al., 200828 | Pooled subgroup analysis examining prior thalidomide exposure | |||
| Hazarika et al., 200829 | Regulatory perspectives; same data as original studies | |||
| San Miguel et al., 201130 | Pooled subgroup analysis investigating the effect on survival of continuing therapy after achievement of a partial response or better | |||
| Simpson et al., 2008 (abstract)31 | Pooled subgroup analysis evaluating the frequency of diarrhea in newly diagnosed patients from two studies conducted at the Mayo Clinic and relapsed or refractory patients from the MM009 and MM010 trials | |||
| Foa et al., 2007 (abstract)32 | Pooled subgroup analysis of IgA subtype of myeloma | |||
| San Miguel et al., 2007 (abstract)33 | Pooled subgroup analysis of the effect of dex dose reductions | |||
| Lonial et al., 2007 (abstract)34 | Pooled subgroup analysis of age categories | |||
| Chanan–Khan et al., 2006 (abstract)35 | Pooled subgroup analysis of age categories | |||
| Chanan–Khan et al., 2006 (abstract)36 | Pooled subgroup analysis of prior autologous stem-cell transplantation (asct) | |||
| Chanan–Khan et al., 2007 (abstract)37 | Pooled subgroup analysis of the effect of performance status | |||
| Niesvizky et al., 2006 (abstract)38 | Pooled subgroup analysis of the effect of thrombosis | |||
| Stadtmauer et al., 200939 | Pooled subgroup analysis of len at first relapse compared with later salvage | |||
| Richardson et al., 2011 (abstract)40 | Examination of the risk of secondary malignancies | |||
| Dimopoulos et al., 201141 | Examination of the risk of secondary primary malignancies | |||
| Dimopoulos et al., 201142 | Pooled subgroup analysis of patients who had not progressed and were still receiving len at 12 months | |||
| Dimopoulos et al., 2011 (abstract)43 | Pooled subgroup analysis at 48 months’ follow-up of patients with progression-free survival of 2 years or better | |||
| S0232 | Zonder et al., 20109 | Compare len plus dex with plc plus dex as first-line treatment formm | Zonder et al., 2008 (abstract)44. | Partial results of study and subgroup analysis of abnormal karyotype and high-risk cytogenetic abnormalities |
| Zonder et al., 2011 (abstract)45 | Extended results | |||
| E4A03 | Rajkumar et al., 201010 | Whether low-dose dex plus len is noninferior to and has lower toxicity than high-dose dex plus len in newly diagnosed mm | Siegel et al., 2010 (abstract)46 | Subgroup analysis based on age group and subsequent asct |
| Jacobus et al., 2010 (abstract)47 | Subgroup analysis of patients 70 years of age or older | |||
| Jacobus et al., 201148 | Subgroup analysis of patients with a fish-based genetic classification | |||
| Vesole et al., 201049 | Subgroup analysis of older patients | |||
| Harvey et al., 2011 (abstract)50 | Evaluate the impact of renal function on len dosing, cytopenias, and response | |||
| Richardson et al., 200611 | Evaluate 2 dose regimens of len for relapsed or refractory mm | |||
| RV-MM-PI-209 | Palumbo et al., 2011 (abstract)15 | To compare len plus melphalan (mel) plus prednisone with tandem mel [200 mg/m2 (mel200)] in newly diagnosed mm patients who received len during induction; 14 months’ follow-up data | Larocca et al., 201151 | Efficacy and safety of low-molecular weight heparin compared with asa as thromboprophylaxis in newly diagnosed patients less than 65 years of age, treated with len-based regimens |
| Palumbo et al., 2011 (abstract)52 | Results at 20 months’ follow-up | |||
| Palumbo et al., 2011 (abstract)53 | Results at 26 months’ follow-up | |||
| IFM 2005-02 | Attal et al., 2011 (abstract)14 | To investigate the efficacy of len for maintenance after transplantation | Attal et al., 2011 (abstract)54 | Presents additional data |
| Attal et al., 2010 (abstract)55 | Presents additional data | |||
| Zangari et al., 2003 (abstract)16 | Compare two doses of len in relapsed or refractory disease | = | = | |
| evolution | Kumar et al., 2011 (abstract)13 | To compare various combinations of bortezomib, dex, cyclophosphamide, and len in previously untreated mm | Kumar et al., 2009 (abstract)56 | Reports noncomparative data |
| Richardson et al., 2011 (abstract)12 | To evaluate the efficacy of elotuzumab plus len and low-dose dex in relapsed refractory mm | Lonial et al., 2011 (abstract)57 | Updated results (73 patients) | |
| Systematic reviews | ||||
| Carrier (thromboprophylaxis) | Carrier et al., 20118 | To determine the absolute rates of venous thromboembolism with and without various thromboprophylactic agents (asa, warfarin, low-molecular weight heparin) in newly diagnosed or previously treated mm patients receiving thalidomide- or len-based regimens | Not applicable | |
| Richardson (use of len) | Richardson et al., 20106 | To evaluate the evidence for the use of len in its current indication in relapsed or refractory mm, and additionally its investigational use for the treatment of newly diagnosed mm | Not applicable | |
| Boen (venous thromboembolism) | Boen et al., 2008 (abstract)7 | To compare the len/thalidomide/dex–associated venous thromboembolism rates pre– and post–fda approval | Not applicable | |
| Practice guidelines | ||||
| asco guideline (treatment of venous thromboembolism in cancer patients) | Lyman et al., 20074 | Recommendation for patients treated with len (p. 5501) | Not applicable | |
| nice guideline | nice, 20095 | Recommendations for the use of len in patients who have received at least 1 prior line of treatment | Hoyle et al., 200858 | Technology assessment |
fish = fluorescence in situ hybridization; asa = acetylsalicylic acid; fda = U.S. Food and Drug Administration; asco = American Society of Clinical Oncology; nice = U.K. National Institute for Health and Clinical Excellence.
A search for ongoing trials at ClinicalTrials.gov, performed April 2, 2012, identified 134 trials involving lenalidomide and multiple myeloma. Of those trials, thirty-four were rcts, either phase ii or phase iii. Details of the included trials can be found in the full guideline report at the cco Web site (https://www.cancercare.on.ca/toolbox/qualityguidelines/).
3.2. Question 1—Previously Untreated Patients
Does lenalidomide (alone or in combination with other therapies) improve outcomes in patients with previously untreated multiple myeloma (including smoldering and symptomatic patients, and candidates or non-candidates for transplant) compared with non-lenalidomide-containing treatments?
3.2.1. Recommendations
Previously Untreated Smoldering Multiple Myeloma:
In asymptomatic patients with no evidence of myeloma-related hypercalcemia, renal dysfunction, anemia, or bone disease (smoldering myeloma), the use of lenalidomide alone or in combination cannot be recommended.
Previously Untreated Symptomatic Multiple Myeloma:
Single-Agent Lenalidomide:
Lenalidomide alone cannot be recommended for standard use in this setting.
Lenalidomide and Dexamethasone:
The combination of lenalidomide and dexamethasone is an acceptable first-line treatment option for myeloma. Recommended dosing options for lenalidomide include either giving 25 mg daily on days 1–28 every 35-day cycle for the first 3 cycles, followed by 25 mg daily on days 1–21 every 28-day cycle thereafter; or proceeding directly to the 28-day cycle dosing at onset. The use of low-dose dexamethasone—40 mg daily on days 1, 8, 15, and 22 of a 28-day cycle—is preferred for safety; however, select patients with acute myeloma complications such as renal dysfunction, hypercalcemia, or hyperviscosity may benefit from high-dose dexamethasone (that is, 40 mg daily on days 1–4, 9–12, and 17–20 of a 28-day cycle).
Other Lenalidomide Combinations:
No other combinations can be recommended.
3.2.2. Key Evidence
Previously Untreated Smoldering Multiple Myeloma:
In asymptomatic smoldering myeloma, an ongoing randomized trial that is evaluating lenalidomide in combination with dexamethasone, compared with the conventional watch-and-wait approach until symptomatic disease progression, is showing promising preliminary results favouring the use of early lenalidomide59. However, no recommendations can be made for this population until the data have matured.
Previously Untreated Symptomatic Multiple Myeloma:
No rcts comparing lenalidomide alone with a non-lenalidomide regimen for first-line therapy both in candidates and in non-candidates for transplant were located.
The study by Zonder et al.9 showed an improved median 1-year pfs (78% vs. 52%, p = 0.002) and improved os (77% vs. 48%, p < 0.0001) in patients receiving lenalidomide plus dexamethasone compared with placebo plus dexamethasone. Rajkumar et al.10 demonstrated a longer median pfs for lenalidomide plus low-dose dexamethasone than for lenalidomide plus high-dose dexamethasone (25.3 months vs. 19.1 months, p = 0.026), with an improved safety profile (grade 3 or greater adverse events: p = 0.02 for neutropenia, p = 0.0003 for dvt, and p = 0.04 for infections) in favour of the low-dose dexamethasone arm.
No rcts of lenalidomide in combination with other agents in this setting were identified.
3.2.3. Qualifying Statements
The Zonder and Rajkumar studies9,10 have limitations: Both studies were stopped early because of observed benefit, and the Rajkumar study used overall response rate as a primary outcome. In the Rajkumar study, the improved safety profile and lower rate of early deaths with low-dose dexamethasone has led to widespread adoption of that approach. From a safety perspective, the Hematology dsg endorses it. It should be noted, however, that compared with low-dose dexamethasone, high-dose dexamethasone, although more toxic, was associated with higher response rates. Therefore, in select patient populations with acute myeloma-related complications, benefit might still be obtained from the robust efficacy of high-dose dexamethasone.
3.3. Question 2—Patients with Relapsed or Refractory Multiple Myeloma
Does lenalidomide (alone or in combination with other therapies) improve outcomes in patients with relapsed or refractory multiple myeloma compared with non-lenalidomide-containing treatments?
3.3.1. Recommendations
Single-Agent Lenalidomide:
Lenalidomide alone cannot be recommended for standard use in the relapsed or refractory setting.
Lenalidomide and Dexamethasone:
The combination of lenalidomide and dexamethasone is recommended for myeloma patients who have received at least 1 prior line of therapy. The recommended dosing is lenalidomide 25 mg daily on days 1–21, plus dexamethasone (either low-dose 40 mg daily on days 1,8,15, and 22, or high-dose 40 mg daily on days 1–4, 9–12, and 17–20) in a 28-day cycle.
Other Lenalidomide Combinations:
No other combinations can be recommended.
3.3.2. Key Evidence
No randomized trials that compared lenalidomide as a single agent with a non-lenalidomide regimen in previously treated patients were located.
Two seminal studies1,2 showed an improved ttp for lenalidomide plus dexamethasone compared with dexamethasone plus placebo. Our meta-analysis of those two studies showed that, compared with a non-lenalidomide regimen, lenalidomide improved ttp [hazard ratio (hr): 0.35; 95% confidence interval (ci): 0.29 to 0.42; p < 0.00001], os (hr: 0.54; 95% ci: 0.36 to 0.80; p < 0.002), and overall response (hr: 0.50; 95% ci: 0.44 to 0.58; p < 0.00001).
Although high-dose dexamethasone in combination with lenalidomide was used in the two pivotal rcts of relapsed or refractory myeloma, low-dose weekly dexamethasone with lenalidomide appears less toxic when used in the first line10. From a safety perspective, the Hematology dsg considers low-dose dexamethasone a reasonable option for the relapsed or refractory setting. Again, select subgroups with acute myeloma complications may benefit from the greater response rates achievable with high-dose dexamethasone.
No rcts of lenalidomide in combination with other agents in this setting were identified.
3.3.3. Qualifying Statements
Both of the seminal studies1,2 were stopped at the first preplanned interim analysis for benefit and were funded by the drug’s manufacturer. However, the studies enrolled more than 300 patients before stopping and had a large number of events.
The recommendation to use low-dose dexamethasone with lenalidomide in the relapsed or refractory setting is generalized from the first-line Rajkumar study10 and is based primarily on improved safety. No comparative studies have evaluated low-dose dexamethasone dosing in the relapsed or refractory setting.
3.4. Question 3—Subgroups Most Likely to Benefit
Which multiple myeloma patients, both previously untreated and relapsed or refractory, are more or less likely to benefit from treatment with lenalidomide?
3.4.1. Recommendations
For patients with untreated myeloma, the evidence is insufficient to recommend lenalidomide in specific patient subgroups. When lenalidomide is combined with dexamethasone, the use of low-dose rather than high-dose dexamethasone may be preferable from a safety perspective, regardless of age.
For patients with relapsed or refractory multiple myeloma, lenalidomide plus dexamethasone is reasonable for the following patient subgroups:
Patients who have received at least 1 prior line of therapy. Patients who are less heavily treated (only 1 prior line of therapy vs. 2 or more) appear to benefit the most.
Patients who have received prior thalidomide or stem-cell transplantation.
Younger or older patients. Advanced age should not be an absolute contraindication to the use of lenalidomide. Careful monitoring for toxicities is recommended.
Patients with mild-to-moderate renal failure (creatinine clearance ≥30 mL/min and ≤60 mL/min). For patients with severe renal failure (creatinine clearance <30 mL/min), the Hematology dsg cautions about the use of lenalidomide until additional evidence for its use in this subgroup becomes available.
Patients with immunoglobulin A subtype, preexisting peripheral neuropathy, and varying levels of Eastern Cooperative Oncology Group performance status.
For patients with relapsed or refractory multiple myeloma, the following treatment guidelines for lenalidomide and dexamethasone may be considered:
Full-dose lenalidomide may be initiated (25 mg daily), but it is reasonable to consider dose reductions for use beyond 12 months.
A longer period of lenalidomide use, if possible until progression, is a reasonable target.
Dexamethasone dose reductions may be used as needed for improved tolerability.
3.4.2. Key Evidence
The subgroup analyses of data are derived primarily from the Rajkumar study10 in the first-line setting and from pooled data from the Weber and Dimopoulos studies1,2,10,18,34,35,39 in the relapsed or refractory setting. These data have been integrated with the clinical expertise of the Hematology dsg to provide support for the recommendations.
Evidence to recommend lenalidomide in specific subgroups of previously untreated patients is limited. When lenalidomide is combined with dexamethasone, the use of low-dose dexamethasone may be preferable in older and younger patients alike. Two subgroup analyses based on the age of patients participating in the Rajkumar study10 reported improved os in all age groups when treated with low-dose rather than high-dose dexamethasone47,49.
The recommendation for lenalidomide plus dexamethasone in patients who have received at least 1 prior line of therapy derives from study stratification results and a pooled subgroup analysis by Stadtmauer et al.39 from the Weber and Dimopoulos studies1,2.
The subgroup analysis by Wang et al.28 showed that there may be partial cross-resistance between thalidomide and lenalidomide, but that prior thalidomide exposure should not absolutely contraindicate the use of lenalidomide in the relapsed or refractory setting. The recommendation for patients who have undergone prior autologous stem-cell transplantation is based on study stratification results and a pooled subgroup analysis by Dimopoulos et al.26.
The recommendation for use of lenalidomide in older as well as younger patients is based on a subgroup analysis of the Weber and Dimopoulos studies1,2 reported by Chanan–Khan et al.35.
Lenalidomide is excreted primarily by the kidneys, posing a risk for cumulative drug-related toxicity with renal dysfunction. The recommendation for use in mild-to-moderate renal dysfunction (creatinine clearance ≥30 mL/min and ≤60 mL/min) is based on a subgroup analysis of pooled data from the pivotal relapsed or refractory studies1,2 reported by Dimopoulos et al.18 Given the small sample of patients with severe renal failure (creatinine clearance <30 mL/min) enrolled in the studies, the Hematology dsg cannot make recommendations for that population.
Lenalidomide and dexamethasone were found to be consistently superior to dexamethasone alone in several subgroup analyses24,32,37.
The guideline on dose reduction is supported by the Dimopoulos et al. subgroup analysis of patients remaining on lenalidomide beyond 12 months25. Those authors report that patients requiring dose reductions were able to stay on study longer, tolerated therapy as well as those not requiring dose reductions, and achieved a longer pfs.
The guideline suggesting continuing treatment until progression, if feasible, is based on two subgroup analyses by San Miguel et al.30 and Harousseau et al.19 suggesting that continued therapy after achievement of a partial response is beneficial, possibly by improving quality of response, which in turn prolongs survival.
The guideline on dexamethasone reduction for tolerability is based on a subgroup analysis by San Miguel et al.33 that associated dose reductions of dexamethasone with improved survival outcomes.
3.4.3. Qualifying Statements
All subgroup analyses upon which the recommendations are based are retrospective post hoc analyses. In isolation, they represent a weak evidence base and therefore have been integrated with the expert opinion and clinical experience of the Hematology dsg. Validation of these recommendations through further clinical investigation is required.
3.5. Question 4—Maintenance or Consolidation Treatment
Are outcomes in myeloma patients improved with the use of lenalidomide as maintenance or consolidation treatment (after transplant and non-transplant treatments) compared with either non-lenalidomide-containing treatment or no maintenance or consolidation treatment?
3.5.1. Recommendations
Non-Candidates for Transplant:
The evidence is insufficient to support the use of lenalidomide maintenance or consolidation treatment after initial non-transplant therapy.
Candidates for Transplant:
In the absence of a final full publication of supporting trials in the post-transplant setting (currently published as conference abstracts14,54,55,60), the Hematology dsg recommends that lenalidomide maintenance at 10–15 mg daily continuously until progression is a reasonable option.
3.5.2. Key Evidence
In non-candidates for transplant, a randomized trial reported by Palumbo et al.61 evaluating lenalidomide as maintenance after melphalan, prednisone, and lenalidomide therapy showed promising preliminary results, but did not provide adequate mature evidence to support a recommendation for use.
In three companion abstract publications14,54,55, a significant improvement in pfs (p < 0.0001) was reported with maintenance compared with no maintenance after transplant. In addition, an ongoing randomized study, presented in preliminary form, strongly supported the benefit of post-transplant maintenance, with an os advantage. The median ttp was 43.6 months compared with 21.5 months, and pfs was also favourable for the lenalidomide group (hr: 0.43; one-sided unadjusted p < 0.0001)60. These combined data provide emerging support for the use of lenalidomide maintenance post transplant, which the Hematology dsg considers a reasonable post-transplant option.
3.5.3. Qualifying Statements
The Palumbo62, Attal63, and McCarthy64 maintenance trials were recently published in full, but were not captured within our search cut-off dates. Therefore all recommendations in the present guidelines are based on ongoing trial data available at the time of the literature search. Data from the study by Attal et al.14,54,55 is based on a final analysis of the completed data, but presented in abstract form. That study was stopped early for benefit. Data from the study by McCarthy et al.60 is based on an interim analysis that had already shown os benefit. Although the abstract data are adequately compelling for the Hematology dsg to recommend maintenance in post-transplant patients, evaluation of the full publications with further maturation is required before full recommendations can be made.
3.6. Question 5—Management of Toxicity
What are the best strategies to manage lenalidomideinduced toxicity?
3.6.1. Recommendations
VTE:
For newly diagnosed patients, and for relapsed or refractory patients who are not at high risk for bleeding or vte, either daily low-dose acetylsalicylic acid (asa) 100 mg given orally or daily enoxaparin (low molecular weight heparin) 40 mg given subcutaneously can be used in patients treated with lenalidomide-based therapy to prevent vte. For patients at high risk of vte or bleeding, the evidence is insufficient to support a specific thromboprophylactic approach. If asa in a 100 mg dose form is not available, the Hematology dsg suggests that replacement with the 81 mg dose form is reasonable.
Cytopenias:
The evidence is insufficient to recommend a uniform approach for the management of cytopenias. Lenalidomide dose reductions can be considered for patients who have responded to full-dose lenalidomide. For those who require the full dose of lenalidomide for efficacy, the use of granulocyte colony–stimulating factor can be considered for neutropenia support.
For the management of anemia and thrombocytopenia, there are no randomized data evaluating approaches in the lenalidomide setting, including the use of erythropoiesis-stimulating agents (esas).
SPMs:
The evidence to date is insufficient to confirm or refute the association of spm with lenalidomide or to identify specific subgroups of patients at risk of spm when treated with lenalidomide.
3.6.2. Key Evidence
The recommendation on vte is based on a published substudy of patients participating in a lenalidomide-based study for first-line use, randomized to either oral asa 100 mg daily or subcutaneous enoxaparin 40 mg daily for vte prophylaxis51. Equally low rates of vte, with no major hemorrhagic complications were reported for both trial arms, and therefore the Hematology dsg recommends either option as reasonable. Given the favourable safety profile of both asa and enoxaparin in prophylactic dosing, generalization of the recommendation to the relapsed or refractory setting is not unreasonable until randomized data in that setting become available. Patients at high risk for vte (prior deep vein thrombosis in the preceding 12 months) or bleeding were not enrolled in the supporting study; the recommendation therefore cannot be extended to that subgroup.
The cytopenia recommendation for lenalidomide dose reductions was based on a subgroup analysis of data pooled from the Weber and Dimopoulos trials1,2,25 suggesting that dose reductions might allow patients to tolerate therapy longer, leading to prolonged pfs. However, in the pivotal rcts, routine use of granulocyte colony–stimulating factor with full-dose lenalidomide was mandated as initial management for severe neutropenia1,2. Based on those data and the clinical expertise of the Hematology dsg, the recommended options include either dose reductions in patients with responsive disease or granulocyte colony–stimulating factor support if full-dose lenalidomide is required for efficacy.
3.6.3. Qualifying Statements
The Larocca et al. superiority trial51 showed no difference between two drugs for the prevention of vte; untreated patients were 65 years of age or younger, the power of the study ranged from 47% to 80%, and for ethical reasons, a placebo arm was not used. However, this trial was the only rct to study vte prophylaxis in patients with myeloma treated with lenalidomide. Despite the study limitations, the Hematology dsg felt that, based on clinical experience, the results could be generalized to other patient groups.
In the absence of evidence for esa use specific to lenalidomide, the Hematology dsg suggests that published evidence-based guidelines for esa use in cancer may be applied65,66. In a subgroup analysis of the Weber and Dimopoulos studies published as a letter to the editor and excluded from our systematic review, the rates of vte were significantly higher with concomitant use of esa and lenalidomide than with lenalidomide without esa67. Because these observations mandate further validation, the Hematology dsg advises consideration of risks and benefits before initiating esa with lenalidomide, followed by careful monitoring for vte.
4. CONCLUSIONS
Lenalidomide is an active agent for the treatment of multiple myeloma. There is sufficient evidence to recommend lenalidomide for myeloma patients who are previously untreated, who have received at least 1 prior line of therapy, and who are post-transplant, for maintenance. Data to provide guidance for patient selection, dosing, and management of toxicities are increasing. There is, however, an ongoing need for studies comparing lenalidomide-based regimens with regimens utilizing other novel agents such as bortezomib, and studies evaluating rational lenalidomide combinations, novel roles such as consolidation or maintenance, strategies for the management of toxicities, and the long-term risks with lenalidomide. In this rapidly evolving field, vigilant scrutiny of the literature on an ongoing basis is required.
5. REVIEW AND UPDATE
Practice guidelines developed by the pebc are reviewed and updated regularly. Please visit the cco Web site (http://www.cancercare.on.ca) for the full evidence-based series report (https://www.cancercare.on.ca/toolbox/qualityguidelines/) and subsequent updates.
6. ACKNOWLEDGMENTS
The authors thank the members of the Hematology dsg for their contributions to the development of this practice guideline. The guideline was created by the pebc, a provincial initiative of cco, supported by the Ontario Ministry of Health and Long-Term Care through cco. The full version of this guideline is published on the cco Web site at https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=137819.
7. CONFLICT OF INTEREST DISCLOSURES
Among the authors of this report, CC has received research support from Celgene, and MC is a site investigator on trial MM020, sponsored by Celgene. Among the members of the dsg, Dr. Donna Reece has received trial support from Celgene, BMS, Janssen, Johnson & Johnson, Otsuka, Novartis, and Merck; Dr. Ralph Meyer has received research funding from Celgene and is an independent research assessment committee member in a Celgene-sponsored trial, receiving an annual honorarium of less than $5000; Dr. Andre Schuh is a principal investigator on a clinical trial of lenalidomide; Dr. Tom Kouroukis is a member of the Celgene Canadian Advisory Board and a local principal investigator in the first study, sponsored by Celgene. All the other members of the Working Group and of the dsg declared no conflict of interest.
All members of the Report Approval Panel declared no conflict of interest.
Among the four external reviewers constituting the technical expert panel, Dr. Vishal Kukreti declared honoraria for acting as a consultant to Celgene, Roche, and Janssen-Ortho that exceeded $5,000 in one year. Dr. Kukreti also declared principal investigator status in a phase iii lenalidomide trial. All the other member of the panel declared no conflict of interest.
8. REFERENCES
- 1.Weber DM, Chen C, Niesvizky R, et al. on behalf of the Multiple Myeloma (009) Study Investigators Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–42. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos M, Spencer A, Attal M, et al. on behalf of the Multiple Myeloma (010) Study Investigators Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 3.Browman GP, Levine MN, Mohide EA, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13:502–12. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 4.Lyman GH, Khorana AA, Falanga A, et al. on behalf of the American Society of Clinical Oncology American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence (nice) Lenalidomide for the treatment of multiple myeloma in people who have received at least one prior therapy. London, UK: NICE; 2009. [Available online at: http://www.nice.org.uk/nicemedia/pdf/TA171Guidance.pdf; cited December 15, 2011] [Google Scholar]
- 6.Richardson P, Mitsiades C, Laubach J, et al. Lenalidomide in multiple myeloma: an evidence-based review of its role in therapy. Core Evid. 2010;4:215–45. doi: 10.2147/ce.s6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boen M, McKoy J, West D, et al. Thalidomide (thal), lenalidomide (len), and dexamethasone (dex)–associated venous thromboembolism (vte) and reported vte rates pre- and post-fda approval: optimal prophylaxis strategies are still unclear [abstract] Blood. 2008;112:A2403. [Google Scholar]
- 8.Carrier M, Le Gal G, Tay J, Wu C, Lee AY. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost. 2011;9:653–63. doi: 10.1111/j.1538-7836.2011.04215.x. [DOI] [PubMed] [Google Scholar]
- 9.Zonder JA, Crowley J, Hussein MA, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232) Blood. 2010;116:5838–41. doi: 10.1182/blood-2010-08-303487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus lowdose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–64. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson PGG, Moreau P, Jakubowiak AJ, et al. Elotuzumab with lenalidomide and low-dose dexamethasone in patients with relapsed multiple myeloma: a randomized phase ii study [abstract 8014] J Clin Oncol. 2011;29 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=102&abstractID=80348; cited January 10, 2013] [Google Scholar]
- 13.Kumar A, Flinn I, Richardson P, et al. Final results from the multi-center, randomized, phase 2 evolution study of combinations of bortezomib (v), dexamethasone (d), cyclophosphamide (c), and lenalidomide (r) in previously untreated multiple myeloma (mm) Haematologica. 2011;96(suppl 1):S100. [Google Scholar]
- 14.Attal M, Olivier P, Lauwers VC, et al. Maintenance treatment with lenalidomide after transplantation for myeloma: analysis of secondary malignancies within the IFM 2005-02 trial [abstract] Haematologica. 2011;96(suppl 1):S23. [Google Scholar]
- 15.Palumbo A, Cavallo F, Nagler A, et al. Melphalan, prednisone, lenalidomide versus melphalan (200 mg/m2) and autologous transplantation in newly diagnosed myeloma patients: a phase iii trial [abstract O272] Bone Marrow Transplant. 2011;46(suppl 1):S38. [Google Scholar]
- 16.Zangari M, Barlogie B, Jacobson J, et al. Revlimid 25 mg (REV 25) × 20 versus 50 mg (REV 50) × 10 q 28 days with bridging of 5 mg × 10 versus 10 mg × 5 as post-transplant salvage therapy for multiple myeloma [abstract 1642] Blood. 2003;102:1642. [Google Scholar]
- 17.Zangari M, Tricot G, Polavaram L, et al. Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high-dose dexamethasone. J Clin Oncol. 2010;28:132–5. doi: 10.1200/JCO.2009.23.0169. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos M, Alegre A, Stadtmauer EA, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010;116:3807–14. doi: 10.1002/cncr.25139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harousseau JL, Dimopoulos MA, Wang M, et al. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010;95:1738–44. doi: 10.3324/haematol.2009.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castaneda C, Weiss L, Minton NA, et al. Peripheral neuropathy in multiple myeloma patients treated with lenalidomide [abstract 5024] Blood. 2010;116:5024. [Google Scholar]
- 21.Baz R, Lonial S, Hussein M, Swern AS, Dimopoulos MA. Lenalidomide (len) therapy in combination with dexamethasone (dex) is associated with a low incidence of viral infections [abstract 1950] Blood. 2010;116:1950. [Google Scholar]
- 22.Baz R, Dimopoulos M, Richardson P, Yu Z, Hussein M, Chanan–Khan A. Lenalidomide-based therapy leads to improvement of humoral immune system in relapsed or refractory multiple myeloma patients who respond to therapy [abstract 0395] Haematologica. 2009;94(suppl 2):159. [Google Scholar]
- 23.Lonial S, Baz R, Swern AS, Weber D, Dimopoulos MA. Neutropenia is a predictable and early event in affected patients with relapsed/refractory multiple myeloma treated with lenalidomide in combination with dexamethasone [abstract 2978] Blood. 2009;114:2878. [Google Scholar]
- 24.Delforge M, Facon T, Bravo ML, Dimopoulos MA. Lenalidomide plus dexamethasone has similar tolerability and efficacy in treatment of relapsed/refractory multiple myeloma patients with or without history of neuropathy [abstract 3873] Blood. 2009;114:A3873. [Google Scholar]
- 25.Dimopoulos MA, Hussein M, Swern AS, Weber D. Full dose of lenalidomide for 12 months followed by a lower maintenance dose improves progression-free survival in patients with relapsed/refractory multiple myeloma [abstract 2874] Blood. 2009;114:2874. [Google Scholar]
- 26.Dimopoulos MA, Anagnostopoulos A, Prince M, et al. Lenalidomide (Revlimid) combination with dexamethasone (Dex) is more effective than Dex alone in patients with relapsed or refractory multiple myeloma and independent of number of previous treatments [abstract 0494] Haematologica. 2006;91(suppl 1):181. [Google Scholar]
- 27.Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase iii trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23:2147–52. doi: 10.1038/leu.2009.147. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–51. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 29.Hazarika M, Rock E, Williams G, et al. Lenalidomide in combination with dexamethasone for the treatment of multiple myeloma after one prior therapy. Oncologist. 2008;13:1120–7. doi: 10.1634/theoncologist.2008-0077. [DOI] [PubMed] [Google Scholar]
- 30.San Miguel JF, Dimopoulos MA, Stadtmauer EA, et al. Effects of lenalidomide and dexamethasone treatment duration on survival in patients with relapsed or refractory multiple myeloma treated with lenalidomide and dexamethasone. Clin Lymphoma Myeloma Leuk. 2011;11:38–43. doi: 10.3816/CLML.2010.n.120. [DOI] [PubMed] [Google Scholar]
- 31.Simpson L, Rajkumar SV, Dispenzieri A, et al. High incidence of diarrhea in patients on long term therapy with lenalidomide and dexamethasone for multiple myeloma [abstract 8586] J Clin Oncol. 2008;26(suppl 15) [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=35993; cited January 12, 2013] [Google Scholar]
- 32.Foa R, Weber D, Dimopoulos M, et al. Lenalidomide/dexamethasone improves response and prolongs time to progression, even in patients with IgA multiple myeloma: a sub-analysis of the MM-009/010 studies [abstract 284b] Blood. 2007;110:284b. [Google Scholar]
- 33.San Miguel JF, Dimopoulos M, Weber D, et al. Dexamethasone dose adjustments seem to result in better efficacy and improved tolerability in patients with relapsed/refractory multiple myeloma who are treated with lenalidomide/dexamethasone (MM009/010 sub-analysis) [abstract] Blood. 2007;110:2712. [Google Scholar]
- 34.Lonial S, Knight R, Dimopolous M, et al. Effect of len/dex in mm in different age groups [abstract] Hematologica. 2007;92(suppl 2):171. [Google Scholar]
- 35.Chanan–Khan A, Weber D, Dimopolous M, et al. Lenalidomide (l) in combination with dexamethasone (d) improves survival and time to progression in elderly patients (pts) with relapsed or refractory (rel/ref) multiple myeloma (mm) [abstract 3553] Blood. 2006;108:1014. [Google Scholar]
- 36.Chanan–Khan AA, Yu Z, Weber D, et al. Lenalidomide (l) in combination with dexamethasone (d) significantly improves time to progression (ttp) in non-stem cell transplant patients (pts) with relapsed or refractory (rel/ref) multiple myeloma (mm): analysis from MM-009 and MM-010 randomized phase iii clinical trials. Blood. 2006;108:1015. [Google Scholar]
- 37.Chanan–Khan AA, Dimopoulos M, Weber D, et al. ecog performance status affects efficacy, but not safety, of lenalidomide/dexamethasone in relapsed/refractory multiple myeloma (MM009/010 sub-analysis) [abstract 2721] Blood. 2007;110:2721. [Google Scholar]
- 38.Niesvizky R, Spencer A, Wang M, et al. Increased risk of thrombosis with lenalidomide in combination with dexamethasone and erythropoietin [abstract 7506] J Clin Oncol. 2006;24(suppl 18) [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=40&abstractID=34300; cited January 12, 2013] [Google Scholar]
- 39.Stadtmauer EA, Weber DM, Niesvizky R, et al. Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. Eur J Haematol. 2009;82:426–32. doi: 10.1111/j.1600-0609.2009.01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson P, Orlowski RZ, San Miguel J, et al. Risk of second primary malingnacies (spm) in relapsed/refractory multiple myeloma (rrmm) patients (pts) treated with lenalidomide and dexamethasone (len+dex): analysis of MM-009/010 spm incidence rate (ir) Haematologica. 2011;96(suppl 1):S25. doi: 10.3324/haematol.2011.041608. [DOI] [Google Scholar]
- 41.Dimopoulos MA, Orlowski RZ, Niesvizky R, Lonial S, Brandenburg NA, Weber DM. Lenalidomide and dexamethasone (len plus dex) treatment in relapsed/refractory multiple myeloma (rrmm) patients (pts) and risk of second primary malignancies (spm): analysis of MM-009/010 [abstract 8009] J Clin Oncol. 2011;2 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=102&abstractID=84539; cited January 12, 2013] [Google Scholar]
- 42.Dimopoulos MA, Hussein M, Swern AS, Weber D. Impact of lenalidomide dose on progression-free survival in patients with relapsed or refractory multiple myeloma. Leukemia. 2011;25:1620–6. doi: 10.1038/leu.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimopoulos MA, Hussein M, Swern AS, Weber DM. Long-term outcomes and safety of continuous lenalidomide plus dexamethasone (len+dex) treatment in patients (pts) with relapsed or refractory multiple myeloma (rrmm) [abstract] Blood. 2011;118:2929. doi: 10.1182/blood-2011-05-355115. [DOI] [Google Scholar]
- 44.Zonder JA, Crowley JJ, Bolejack V, et al. A randomized Southwest Oncology Group study comparing dexamethasone (d) to lenalidomide plus dexamethasone (ld) as treatment of newly-diagnosed multiple myeloma (ndmm): impact of cytogenetic abnormalities on efficacy of ld, and updated overall study results [abstract 8521] J Clin Oncol. 2008;26 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=36278; cited January 12, 2013] [Google Scholar]
- 45.Zonder JA, Crowley J, Hussein M, et al. Extended results of Southwest Oncology Group protocol S0232: durable responses achieved with lenalidomide (l) plus high-dose dexamethasone (d) as first-line therapy for multiple myeloma. Haematologica. 2011;96(suppl 1):S79. [Google Scholar]
- 46.Siegel DS, Jacobus S, Rajkumar SV, et al. Outcome with lenalidomide plus dexamethasone followed by early autologous stem cell transplantation in the ecog E4A03 randomized clinical trial [abstract] Blood. 2010;116:38. doi: 10.1038/bcj.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobus S, Callander N, Siegel D, et al. Outcome of elderly patients 70 years and older with newly diagnosed myeloma in the ecog randomized trial of lenalidomide/high-dose dexamethasone (rd) versus lenalidomide/low-dose dexamethasone (Rd) [abstract 370] Haematologica. 2010;95(suppl 2):149. [Google Scholar]
- 48.Jacobus SJ, Kumar S, Uno H, et al. Impact of high-risk classification by fish: an Eastern Cooperative Oncology Group (ecog) study E4A03. Br J Haematol. 2011;155:340–8. doi: 10.1111/j.1365-2141.2011.08849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vesole DH, Jacobus S, Rajkumar SV, et al. Lenalidomide plus low-dose dexamethasone (ld): superior one and two year survival regardless of age compared to lenalidomide plus high-dose dexamethasone (ld) [abstract 308] Blood. 2010;116:308. doi: 10.1182/blood-2010-04-278507. [DOI] [PubMed] [Google Scholar]
- 50.Harvey RD, Jacobus SJ, Rajkumar SV, Greipp PR, Lonial S. Renal function measures improve on lenalidomide and dexamethasone and compete with patient characteristics to predict lenalidomide dose density and hematologic toxicity: an E4A03 analysis [abstract] Blood. 2011;118:1882. [Google Scholar]
- 51.Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for newly-diagnosed multiple myeloma patients treated with lenalidomide. Blood. 2012;119:933–9. doi: 10.1182/blood-2011-03-344333. [DOI] [PubMed] [Google Scholar]
- 52.Palumbo A, Cavallo F, Di Raimondo F, et al. A phase 3 study comparing melphalan–prednisone–lenalidomide (mpr) with high-dose melphalan and autologous transplantation (MEL200) in newly diagnosed patients with multiple myeloma (mm) [abstract] Eur J Cancer. 2011;47(suppl AS639):S639. doi: 10.1016/S0959-8049(11)72465-0. [DOI] [Google Scholar]
- 53.Palumbo A, Cavallo F, Hardan I, et al. Melphalan/prednisone/lenalidomide (mpr) versus high-dose melphalan and autologous transplantation (MEL200) in newly diagnosed multiple myeloma (mm) patients < 65 years: results of a randomized phase iii study [abstract] Blood. 2011;118:3069. doi: 10.1182/blood-2011-06-358812. [DOI] [Google Scholar]
- 54.Attal M. Lenalidomide (Revlimid) maintenance therapy for multiple myeloma after transplantation: the ifm 2005-02 trial, final analysis. Pharmacol Ther. 2011;36:102. [Google Scholar]
- 55.Attal M, Lauwers–Cances V, Marit G, et al. Maintenance treatment with lenalidomide after transplantation for myeloma: final analysis of the ifm 2005-02 [abstract] Blood. 2010;116:310. [Google Scholar]
- 56.Kumar S, Flinn IW, Hari PN, et al. Novel three- and four-drug combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide, for newly diagnosed multiple myeloma: encouraging results from the multi-center, randomized, phase 2 evolution study [abstract 127] Blood. 2009;114:127. doi: 10.1182/blood-2009-04-205013. [DOI] [Google Scholar]
- 57.Lonial S, Jakubowiak AJ, Jagannath S, et al. A phase 2 study of elotuzumab in combination with lenalidomide and low-dose dexamethasone in patients with relapsed/refractory multiple myeloma [abstract] Blood. 2011;118:303. [Google Scholar]
- 58.Hoyle M, Rogers G, Garside R, Moxham T, Stein K. The Clinical- and Cost-Effectiveness of Lenalidomide for Multiple Myeloma in People Who Have Received At Least One Prior Therapy: An Evidence Review of the Submission from Celgene. Exeter, UK: Peninsula College of Medicine and Dentistry; 2008. [Available online at: http://www.hta.ac.uk/erg/reports/1746.pdf; cited January 2, 2011] [Google Scholar]
- 59.Mateos MV, Lopez–Corral L, Hernandez M, et al. Smoldering multiple myeloma (smm) at high-risk of progression to symptomatic disease: a phase iii, randomized, multicenter trial based on lenalidomide–dexamethasone (len–dex) as induction therapy followed by maintenance therapy with len alone vs no treatment. [abstract 991] Blood. 2011;118:991. doi: 10.1182/blood-2011-04-345801. [DOI] [Google Scholar]
- 60.McCarthy PL, Owzar K, Anderson K, et al. Phase iii intergroup study of lenalidomide versus placebo maintenance therapy following single autologous stem cell transplant (asct) for multiple myeloma (mm): calgb ecog bmt–cnt 100104. Haematologica. 2011;96(suppl 1):S23. doi: 10.3324/haematol.2011.041319. [DOI] [Google Scholar]
- 61.Palumbo AP, Delforge M, Catalano J, et al. Incidence of second primary malignancy (spm) in melphalan–prednisone–lenalidomide combination followed by lenalidomide maintenance (mpr-r) in newly diagnosed multiple myeloma patients (pts) age 65 or older [abstract 8007] J Clin Oncol. 2011;29 doi: 10.1200/JCO.2010.31.6844. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=102&abstractID=82234; cited January 31, 2013] [DOI] [Google Scholar]
- 62.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–69. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 63.Attal M, Lauwers–Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–91. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 64.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–81. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guideline summary: use of epoetin and darbepoetin in patients with cancer—2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Oncol Pract. 2008;4:48–52. doi: 10.1200/JOP.0818502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shehata N, Walker I, Quirt K, Imrie K, Haynes AE, Trudeau M, on behalf of the Hematology Disease Site Group . Treatment of Anemia with Erythropoietic Agents in Patients with Cancer—Adaption/Adoption of ash–asco Guidelines. Toronto, ON: Cancer Care Ontario; 2010. Evidence-based series #6–11. [Google Scholar]
- 67.Knight R, DeLap RJ, Zeldis JB. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med. 2006;354:2079–80. doi: 10.1056/NEJMc053530. [DOI] [PubMed] [Google Scholar]