Abstract
An article in a recent edition of Current Oncology explored the validation of progression-free survival (pfs) as an endpoint in clinical trials of antineoplastic agents for metastatic colorectal cancer, metastatic renal cell carcinoma, and ovarian cancer. The support for pfs as a surrogate endpoint for overall survival (os) was elucidated. As with the aforementioned tumour types, advanced non-small-cell lung cancer (nsclc) has seen a rise in active agents since the year 2000. Those agents range from improved cytotoxics such as pemetrexed, to targeted therapies such as tyrosine kinase inhibitors of the epidermal growth factor receptor and agents that target the EML4–ALK gene mutation. More recently, it has also become apparent that histology plays an important role in the response to and outcomes of treatment. With the therapeutic options for patients with advanced nsclc increasing, concerns are being raised that the efficacy of drugs measured by os may be diluted in clinical trials, thereby underestimating their true clinical benefit. That possibility, together with the need to have efficacious drugs available to patients earlier, has resulted in the search for a surrogate to the os endpoint in advanced nsclc. The present article follows up the recent article on pfs as a surrogate. Although advances in identifying pfs as a valid surrogate endpoint for os have been made in other tumour types, in advanced nsclc, such surrogacy has not been formally validated. Until it has, os should remain the primary endpoint of clinical trials in advanced nsclc.
Keywords: Progression-free survival, overall survival, endpoint, non-small-cell lung cancer
1. BACKGROUND
Lung cancer is the leading cause of cancer death among men and women in North America1. In advanced non-small-cell lung cancer (nsclc), median survival in untreated patients is only 4–5 months, with a survival rate at 1 year of 10%2. Since the year 2000, significant advances have been made in the treatment options for advanced nsclc, including cytotoxic agents and targeted therapies; and in a select group of patients, current treatments offer median survival rates that approach 2 years3.
Overall survival (os), defined as the time from randomization to death from any cause, is a direct measure of clinical benefit to a patient. Patients alive or lost to follow-up are censored4. Overall survival offers the greatest clinical gain, provided that quality of life (qol) is not compromised. As an endpoint, os is easily measured, unambiguous, objective, felt to be clinically significant, and unaffected by the timing of assessment. However, measuring os as an endpoint in clinical trials requires large patient numbers and increased length of follow-up, thus potentially delaying the approval of new agents.
With the therapeutic options for patients with advanced nsclc increasing, there are concerns that the efficacy of drugs measured by os may be diluted in clinical trials, thereby underestimating their true clinical benefit. That concern is based on the assumption that subsequent lines of therapy are more effective in the control arm than in the treatment arm, or that the biology of the treatment arm has changed in some way because of exposure to the study drug, making further treatments less effective—both of which are not supported by evidence5. Also, time to death unfortunately remains relatively short in advanced nsclc compared with time to death in breast and colorectal cancer, thus weakening the argument that os requires prolonged follow-up for lung cancer patients. Nonetheless, with the increased success of systemic treatments, there is a need to have efficacious drugs available to patients earlier, and the search for a more accessible endpoint and a surrogate to os is being sought.
Progression-free survival (pfs) is defined as time from randomization until first evidence of tumour progression or until death from any cause, whichever comes first. Patients who do not die or progress and those lost to follow-up are censored4,6. By definition, pfs events occur more quickly and more frequently than os events. As a result, pfs data become available much earlier than os data, and fewer patients are required to obtain those data7,8, potentially expediting the approval process for new agents. Also, pfs is not influenced by post-protocol therapy.
Although pfs is a popular endpoint, it comes with limitations of its own, including multiple types of bias, an inherent degree of subjectivity, and measurement error. Marginal differences in pfs observed between study arms might be a result of differences in subjective assessments of progression and might not represent clinically meaningful improvement, such as improvement of qol or performance status.
Despite those limitations, the use of pfs as a primary endpoint in clinical trials is increasing. Among all randomized controlled trials of systemic therapy in nsclc, breast cancer, and colorectal cancer published in five major journals from 1974 to 2009, the proportion of trials with pfs as the primary endpoint increased from 0% (1975–1984) to 20% (2005–2009)9. In an extensive literature search for all phase iii clinical trials evaluating systemic therapy for nsclc conducted since 1980, Sacher and Leighl found that 15% of all trials during 2001–2010 used pfs as the primary endpoint; before that, none did. At the same time, the magnitude of the pfs and os gains reported in positive trials was declining10.
Health authorities are also beginning to recognize pfs as a valid endpoint. A review of anticancer drug product approvals by the U.S. Food and Drug Administration (fda) between 2005 and 2007 found that 17% of drug approvals for new indications (9 of 53) were based on trials with pfs endpoints11.
2. THE SEARCH FOR A SURROGATE FOR THE OS ENDPOINT IN NSCLC
It has been proposed that, to be considered “valid,” a surrogate endpoint (for example, pfs) must show strong correlation with the clinical endpoint (for example, os), and the treatment effect on the surrogate endpoint must reliably predict the treatment effect on the clinical endpoint12,13.
Various intermediate endpoints—response rates, disease progression, disease control rates, and time-related endpoints such as time to progression and pfs—have been used in nsclc clinical trials to predict treatment effect on os. Response rates and disease progression describe changes in tumour burden defined by the Response Evaluation Criteria in Solid Tumors working group14. The intended use of response rate in clinical trials was to perform phase ii screening of drugs and to aid in identifying signals of activity in early drug development, with the expectation that obtaining such responses might result in a clinical benefit15. In a number of phase iii trials in advanced nsclc, improved response rates for one regimen over another failed to result in improved survival16,17, but disease control rate resulted in prolonged survival18. Furthermore, a pooled analysis from three Southwest Oncology Group trials of platinum-based chemotherapy regimens in nsclc reported that the disease control rate (rate of nonprogression) was a stronger predictor of os than tumour response rates were, particularly at 8 weeks19.
Mandrekar et al.20 investigated the relationship of disease progression with os in phase ii studies. Their pooled analysis, which used individual patient data from four first-line phase ii trials, demonstrated that the progression status of a patient, when considered as a time-dependent covariate to account for time to disease progression, was significantly associated with os. In particular, pfs status at 12 weeks was superior to tumour response as an endpoint for predicting subsequent survival in advanced nsclc. Based on those results, the authors concluded that pfs should be used in place of tumour response in phase ii trials in advanced nsclc.
The foregoing studies are hypothesis-generating, but if pfs is to be considered “valid” based on the relationship proposed earlier, a statistical analysis using independent patient data or publication-based data from multiple controlled phase iii clinical trials needs to determine that pfs and os are correlated in advanced nsclc12. With the exception of one study by Buyse et al.21,22 that tried to correlate pfs with os in the specific setting of advanced nsclc treated with docetaxel or vinca alkaloids in the first line, no such large-scale validation study exists for nsclc.
Despite that lack, the fda gives consideration to pfs as the primary endpoint in advanced nsclc for demonstration of efficacy for drug approval, which is based on the magnitude of the effect and the risk–benefit profile of the drug. The fda does state that, because of the subjectivity of pfs as a surrogate endpoint, and because assessments depend on frequency, accuracy, reproducibility, and completeness, the observed magnitude of the effect must be substantial and robust; the degree of improvement in pfs and the definitions for “substantial” and “robust” are not stated, however4. Nevertheless, os remains the “gold standard” for drug approval by the fda. In its most recent recommendations for clinical trial endpoints in nsclc, the fda stated that os should be considered the standard clinical benefit endpoint and that it should be used to establish the efficacy of a treatment in patients with advanced and metastatic nsclc4.
3. PFS AS A SURROGATE FOR THE OS ENDPOINT IN ADVANCED NSCLC
3.1. PFS As a Surrogate for the OS Endpoint in First-Line Treatment of Advanced NSCLC
To be able to use pfs as a surrogate endpoint, the “surrogate threshold effect,” defined as the minimum effect of a treatment on pfs that would predict a statistically significant effect of that treatment on os, must be clarified. To look at the prediction of survival benefit arising from pfs in patients with advanced nsclc, Buyse et al.21,22 pooled data from 2838 patients in seven randomized controlled trials comparing docetaxel with vinca alkaloids in the first-line setting. Variation in the definition of pfs was noted across trials; the trial-specific definitions of pfs were used and were not recalculated. The authors concluded that the surrogate threshold effect was a pfs hazard ratio of 0.70, indicating that a relative improvement of 30% in pfs would predict for an os advantage in first-line treatment of advanced nsclc. However, most first-line studies in advanced nsclc have failed to show that degree of pfs benefit (Table i).
TABLE I.
Selected trials in the first-line setting
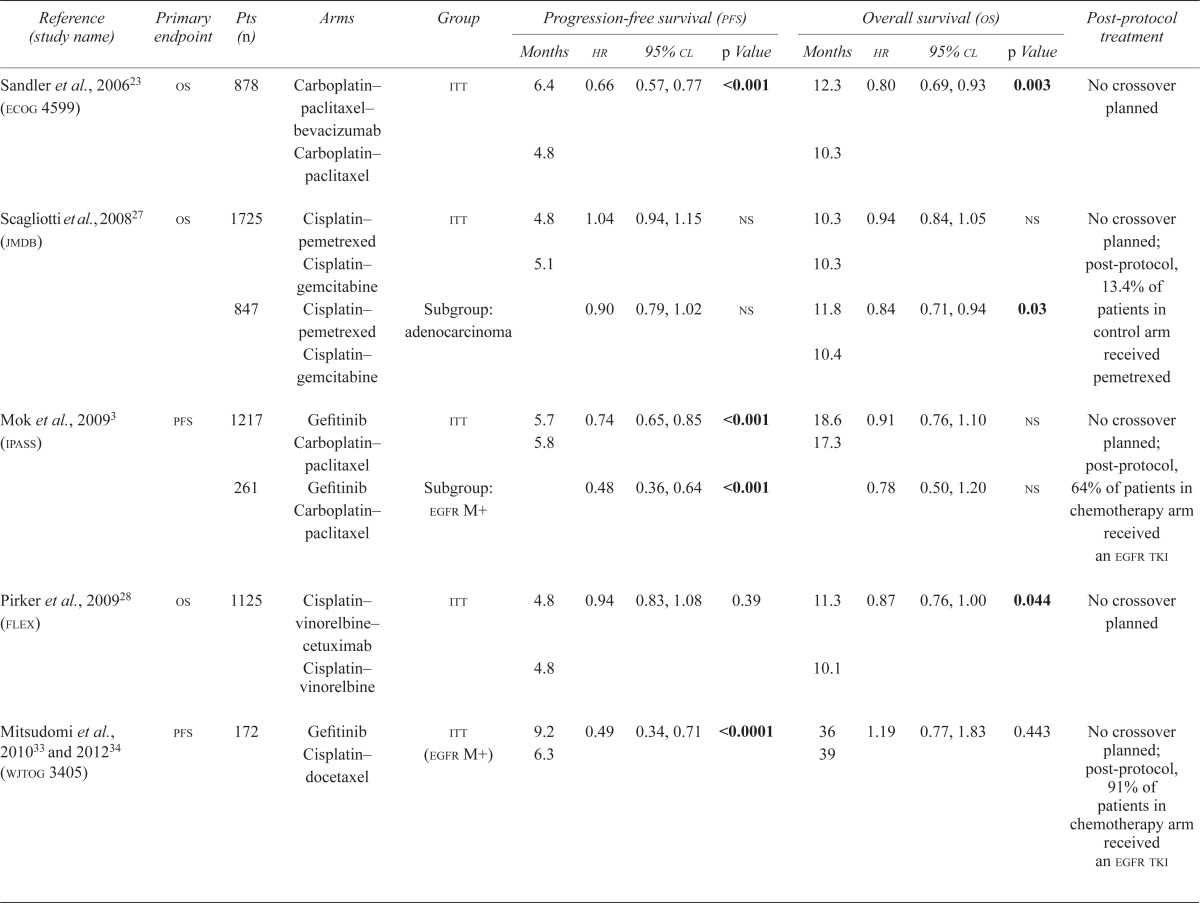
| Reference (study name) | Primary endpoint | Pts ( n ) | Arms | Group | Progression-free survival (pfs) | Overall survival (os) | Post-protocol treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Months | hr | 95% cl | p Value | Months | hr | 95% cl | p Value | ||||||
| Sandler et al., 200623 (ecog 4599) | os | 878 | Carboplatin–paclitaxel–bevacizumab | itt | 6.4 | 0.66 | 0.57, 0.77 | <0.001 | 12.3 | 0.80 | 0.69, 0.93 | 0.003 | No crossover planned |
| Carboplatin–paclitaxel | 4.8 | 10.3 | |||||||||||
| Scagliotti et al., 200827 (jmdb) | os | 1725 | Cisplatin–pemetrexed | itt | 4.8 | 1.04 | 0.94, 1.15 | ns | 10.3 | 0.94 | 0.84, 1.05 | ns | No crossover planned; post-protocol, 13.4% of patients in control arm received pemetrexed |
| Cisplatin–gemcitabine | 5.1 | 10.3 | |||||||||||
| 847 | Cisplatin–pemetrexed | Subgroup: adenocarcinoma | 0.90 | 0.79, 1.02 | ns | 11.8 | 0.84 | 0.71, 0.94 | 0.03 | ||||
| Cisplatin–gemcitabine | 10.4 | ||||||||||||
| Mok et al., 20093 (ipass) | pfs | 1217 | Gefitinib Carboplatin–paclitaxel | itt | 5.7 | 0.74 | 0.65, 0.85 | <0.001 | 18.6 | 0.91 | 0.76, 1.10 | ns | No crossover planned; post-protocol, 64% of patients in chemotherapy arm received an egfr tki |
| 5.8 | 17.3 | ||||||||||||
| 261 | Gefitinib Carboplatin–paclitaxel | Subgroup: egfr M+ | 0.48 | 0.36, 0.64 | <0.001 | 0.78 | 0.50, 1.20 | ns | |||||
| Pirker et al., 200928 (flex) | os | 1125 | Cisplatin–vinorelbine–cetuximab | itt | 4.8 | 0.94 | 0.83, 1.08 | 0.39 | 11.3 | 0.87 | 0.76, 1.00 | 0.044 | No crossover planned |
| Cisplatin–vinorelbine | 4.8 | 10.1 | |||||||||||
| Mitsudomi et al., 201033 and 201234 (wjtog 3405) | pfs | 172 | Gefitinib Cisplatin–docetaxel | itt (egfr M+) | 9.2 | 0.49 | 0.34, 0.71 | <0.0001 | 36 | 1.19 | 0.77, 1.83 | 0.443 | No crossover planned; post-protocol, 91% of patients in chemotherapy arm received an egfr tki |
| 6.3 | 39 | ||||||||||||
| Reck et al., 201025 (avail) | pfs (originally os) | 692 | Cisplatin–gemcitabine–bevacizumab | itt (7.5 mg/kg bevacizumab) | 6.7 | 0.75 | 0.64, 087 | 0.0003 | 13.6 | 0.93 | 0.78, 1.11 | 0.42 | Crossover not permitted |
| Cisplatin–gemcitabine | 6.1 | 13.1 | |||||||||||
| 698 | Cisplatin–gemcitabine–bevacizumab | itt (15 mg/kg bevacizumab) | 6.5 | 0.85 | 0.73, 1.00 | 0.0456 | 13.4 | 1.03 | 0.86, 1.23 | 0.761 | |||
| Cisplatin–gemcitabine | 6.1 | 13.1 | |||||||||||
| Zhou et al., 201130 (optimal) | pfs | 154 | Erlotinib Carboplatin–gemcitabine | Received 1 dose of drug (egfr M+) | 13.1 | 0.16 | 0.10, 0.26 | <0.0001 | 22.7 | 1.065 | ns | No crossover planned; post-protocol, 71% of patients in chemotherapy arm received an egfr tki. | |
| 4.6 | 28.9 | ||||||||||||
| Han et al., 201231 (First-signal) | os | 309 | Gefitinib Cisplatin–gemcitabine | itt | 5.8 | 1.2 | 0.94, 1.5 | ns | 22.3 | 0.932 | 0.72, 1.2 | 0.604 | No crossover planned; post protocol, 75% of in patients chemotherapy arm received an egfr tki |
| 6.4 | 22.9 | ||||||||||||
| 96 | Gefitinib Cisplatin–gemcitabine | Subgroup: egfr M+ | 0.38 | 0.21, 0.68 | <0.001 | 1.043 | 0.50, 2.2 | ns | |||||
| Rosell et al., 201232 (eurtac) | pfs | 173 | Erlotinib Standard chemotherapy | itt (egfr M+) | 9.7 | 0.37 | 0.25, 0.54 | <0.0001 | 19.3 | 1.04 | 0.65, 1.68 | 0.87 | Crossover |
| 5.2 | 19.5 | ||||||||||||
| Yang et al., 201235 (lux-Lung3) | pfs | 345 | Afatinib Cisplatin–pemetrexed | itt (egfr M+) | 11.1 | 0.58 | 0.43, 0.78 | 0.0004 | No crossover planned | ||||
| 6.9 | |||||||||||||
Pts = patients; hr = hazard ratio; cl = confidence limits; ecog = Eastern Cooperative Oncology Group; itt = intention-to-treat; ns = nonsignificant; egfr = epidermal growth factor receptor; M+ = mutation positive; tki = tyrosine kinase inhibitor.
The two practice-changing bevacizumab trials, Eastern Cooperative Oncology Group 459923,24 and avail25,26, may support the Buyse group’s prediction. In the 4599 trial, the addition of bevacizumab to carboplatin and paclitaxel resulted in a significantly prolonged pfs, with a hazard ratio of 0.66, which translated into a significant os benefit. However, the addition of bevacizumab to cisplatin and gemcitabine in the avail trial resulted in a statistical improvement in pfs, with a hazard ratio of 0.75, that did not translate into an os benefit. Crossover could not explain that result, because crossover was not allowed in avail.
Unfortunately, the relationship between pfs and os does not appear to be so simple. In a study reported by Scagliotti et al.27 comparing standard platinum doublet chemotherapy with platinum plus pemetrexed, no benefit in pfs was observed, but a statistically significant benefit in os was found in patients with non-squamous histology. Similarly, in the flex trial, which evaluated the addition of cetuximab to cisplatin and vinorelbine chemotherapy, no pfs benefit was observed, but an os benefit was found28.
Whether such correlations can be extrapolated to more recent trials investigating targeted therapies is also uncertain. First-line trials comparing oral tyrosine kinase inhibitors (tkis) of the epidermal growth factor receptor (egfr) with standard chemotherapy (ipass, optimal, First-signal, eurtac, wjtog 3405, and lux-Lung 3) found statistically significant improvements in pfs, with dramatic hazard ratios of 0.16–0.48, which corresponded to a 2.9- to 8.5-month pfs improvement in the population with an EGFR activating mutation (Table i), but which had no os benefit (lux-Lung 3 has not yet reported os)3,29–35. Crossover likely explains those results: 64%–91% of patients in the chemotherapy arms ultimately received an egfr tki at the time of progression.
However, median survival of patients in the aforementioned studies is obviously different from that of historical controls. In patients with an EGFR activating mutation who receive an egfr tki, the reported median survival is 17–39 months. In contrast, earlier chemotherapy trials generally reported a median survival of less than 12 months36, suggesting that an egfr tki improves os if given to a patient with an EGFR activating mutation at some point during the course of their disease.
Broglio et al.37 used a mathematical model to correlate os and pfs, also taking survival post progression (spp) into consideration. When a true treatment benefit in pfs but no treatment effect in spp was observed, the probability of also observing a statistically significant difference in os depended on the length of the median spp and the magnitude of the observed pfs difference. The authors found that a p-value improvement of 0.001 in pfs resulted in a greater than 90% probability for a statistically significant improvement in os if the median spp was 2 months, but less than 20% if the median spp was as high as 24 months. Thus, os appears to be the most reasonable primary endpoint when the median spp is short, but it is too high a bar when the median spp is greater than 12 months. The latter case may apply for the egfr tkis in patients with EGFR activating mutations. As previously mentioned, the os in this group is 17–39 months, and the pfs, 9.2–13.1 months. One explanation may be effective second- and third-line treatment options in this patient population.
3.2. PFS As a Surrogate for the OS Endpoint Beyond First-Line Treatment
Beyond first-line treatment in advanced nsclc, os has remained the primary endpoint in most of the practice-changing trials, with pfs as a secondary endpoint (Table ii)38–43. Clinical trials of second-line treatment using chemotherapy agents such as docetaxel and pemetrexed reported both pfs and os benefits (tax 317 reported time to progression only)38–40. Similarly, in br.21, the use of erlotinib in the second- or third-line setting (compared with best supportive care) led to improvements in both pfs [hazard ratio (hr): 0.61] and os (hr: 0.7)18. However, more recent trials have failed to show such a correlation. In lux-Lung 1, a study of the egfr tki afatinib compared with best supportive care, an impressive improvement in pfs with afatinib was reported (hr: 0.38). That improvement corresponded to a 2.2-month absolute pfs benefit, and yet the os, the primary endpoint of the trial, was not significantly different42. Crossover was not allowed in the trial. Furthermore, in a phase iii trial of vandetanib (an inhibitor of the vascular endothelial growth factor receptor and egfr) compared with placebo, a significant improvement in pfs (hr: 0.63) again was not associated with an os benefit. However, the actual difference in pfs benefit was only 0.1 months, and the study drug failed to show in an improvement in time to deterioration as a marker for qol, suggesting that the pfs benefit was not clinically meaningful43. In contrast, br.21, a study of erlotinib, the absolute pfs benefit was only 0.4 months (albeit statistically significant), but it was associated with a statistically significant 2-month os benefit18. The differences in pfs in these various trials may have been confounded by heterogeneity in the definition of pfs and the timing of the assessment for disease progression.
TABLE II.
Selected trials beyond first-line treatment
| Reference (study name) | Primary endpoint | Pts (n) | Arms | Group |
Progression-free survival (pfs)
|
Overall survival (os)
|
Post-protocol treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | hr | 95% cl | p Value | Months | hr | 95% cl | p Value | ||||||
| Fossella et al., 200039 (tax 320) | os | 373 | Docetaxel | itt | 26 Weeks: 17% | 0.031 | 1 Year: 32% | 0.025 | No crossover planned | ||||
| Vinorelbine or ifosfamide | 26 Weeks: 8% | 1 Year: 19% | |||||||||||
| Shepherd et al., 200038 (tax 317) | os | 204 | Docetaxel | itt | 7.0 | 0.48 | 0.047 | No crossover planned | |||||
| Best supportive care | 4.6 | ||||||||||||
| Hanna et al., 200440 (jmei) | os | 571 | Pemetrexed | itt | 2.9 | 0.97 | 0.82,1.16 | ns | 8.3 | 0.99 | 0.82, 1.2 | 0.226 | No crossover planned |
| Docetaxel | 2.9 | 7.9 | |||||||||||
| Subgroup: non-squamous | 0.82 | 0.66, 1.02 | 0.76 | 0.78 | 0.61, 1.00 | 0.047 | |||||||
| Shepherd et al. 2005 (br.21)18 | os | 731 | Erlotinib | itt | 2.2 | 0.61 | 0.51,0.74 | <0.001 | 6.7 | 0.7 | 0.58, 0.85 | <0.001 | No crossover planned |
| Placebo | 1.8 | 4.7 | |||||||||||
| Thatcher et al., 200541 (isel) | os | 1692 | Gefitinib | itt | 5.6 | 0.89 | 0.77,1.02 | 0.087 | No crossover planned | ||||
| Placebo | 5.1 | ||||||||||||
| 767 | Subgroup: adenocarcinoma | 6.3 | 0.84 | 0.68, 1.03 | 0.089 | ||||||||
| 5.4 | |||||||||||||
| Miller et al., 201142 (lux-Lung1) | os | 585 | Afatinib | itt | 3.3 | 0.38 | 0.31, 0.48 | <0.0001 | 10.8 | 1.08 | 0.86, 1.35 | 0.74 | No crossover planned |
| Placebo | 1.1 | 12.0 | |||||||||||
| Lee et al., 201243 (zephyr) | os | 924 | Vandetanib | itt | 1.9 | 0.63 | 0.54, 0.74 | <0.001 | 8.5 | 0.95 | 0.81,1.11 | 0.527 | No crossover planned |
| Placebo | 1.8 | 7.8 | |||||||||||
Pts = patients; hr = hazard ratio; cl = confidence limits; itt = intention-to-treat; ns = nonsignificant.
The relationship of pfs to os therefore remains unclear in this setting. With limited effective subsequent lines of treatment in this patient population, and a short median os (4–11 months)38–43, the argument for a dilution effect on os by subsequent therapies and longer follow-up times is weak, and a surrogate for os beyond first-line treatment may be unnecessary.
3.3. PFS As a Surrogate for the OS Endpoint in Maintenance Treatment for NSCLC
Soon et al.44 reviewed 3027 patients from 13 randomized conventional-chemotherapy maintenance clinical trials excluding pemetrexed. Compared with the control arm, extended treatment with single-agent chemotherapy or a switch to a different agent improved pfs (hr: 0.75), with a modest improvement in os (hr: 0.92), although impairment of health-related qol was a tradeoff. The caveat in this study was that published results were used and not individual patient data; thus, the benefit may have been overestimated.
Fidias et al.45 evaluated docetaxel given immediately after platinum-based chemotherapy against delayed docetaxel upon progression. The significant pfs that was observed did not translate into an os benefit (os being the primary endpoint of the trial), nor was qol improved. More recent practice-changing clinical trials supporting maintenance systemic treatments after a platinum doublet have used pfs as the primary endpoint (Table iii)46–49. Pemetrexed has been studied in the jmen and paramount trials, and erlotinib in the saturn trial, as maintenance after platinum doublet, and both a pfs and an os benefit have been reported, suggesting a correlation46–49.
TABLE III.
Selected maintenance trials
| Reference (study name) | Primary endpoint | Pts (n) | Arms | Group | Progression-free survival (pfs) | Overall survival (os) | Post-protocol treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Months | hr | 95% cl | p Value | Months | hr | 95% cl | p Value | ||||||
| Ciuleanu et al., 200946 (jmen) | pfs | 663 | Platinum doublet →pemetrexed maintenance | itt | 4.3 | 0.50 | 0.42, 0.61 | <0.0001 | 13.4 | 0.79 | 0.65, 0.95 | 0.012 | No crossover planned; post-protocol, 18% of patients in placebo arm received pemetrexed |
| Platinum doublet | 2.6 | 10.6 | |||||||||||
| Platinum doublet pemetrexed → maintenance | Subgroup: non-squamous | 4.5 | 0.44 | 0.36, 0.55 | <0.0001 | 15.5 | 0.70 | 0.56, 0.88 | 0.002 | ||||
| Platinum doublet | 2.6 | 10.3 | |||||||||||
| Fidias et al., 200945 | os | 566 | Immediate docetaxel | itt | 5.7 | 0.0001 | 12.3 | 0.08 | Crossover not applicable | ||||
| Delayed docetaxel | 2.7 | 9.7 | |||||||||||
| Cappuzzo et al., 201047 (saturn) | pfs | 889 | Platinum doublet →erlotinib maintenance | itt | 12.3 Weeks | 0.71 | 0.62, 0.82 | 0.0001 | 12.0 | 0.81 | 0.70, 0.95 | 0.009 | No crossover planned; post protocol, 21% of patients in placebo arm received an egfr tki |
| Platinum doublet | 11.1 Weeks | 11.0 | ` | ||||||||||
| Platinum doublet →erlotinib maintenance | Subgroup: egfr M+ | 0.1 | 0.04, 0.25 | 0.0001 | 0.83 | 0.34, 2.02 | 0.681 | No crossover planned; post-protocol, 67% of patients in placebo arm received an egfr tki | |||||
| Platinum doublet | |||||||||||||
| Platinum doublet →erlotinib maintenance | Subgroup: egfr M– | 0.78 | 0.63, 0.96 | 0.0185 | 0.77 | 0.61, 0.97 | 0.024 | ||||||
| Platinum doublet | |||||||||||||
| Paz–Ares et al., 201248,49 (paramount) | pfs | 539 | Cisplatin–emetrexed →pemetrexed maintenance | itt | 4.1 | 0.62 | 0.49, 0.78 | 0.0001 | 13.9 | 0.78 | 0.0195 | No crossover planned; post-protocol, 4% of patients in placebo arm received pemetrexed | |
| Cisplatin–pemetrexed | 2.8 | 11.0 | |||||||||||
Pts = patients; hr = hazard ratio; cl = confidence limits; itt = intention-to-treat; egfr = epidermal growth factor receptor; M+ = mutation positive; tki = tyrosine kinase inhibitor; M– = mutation negative.
Docetaxel, pemetrexed, and erlotinib are all agents that have shown a pfs and os benefit in the second-line setting, and thus it is not surprising that, compared with best supportive care, giving active drug earlier delays progression as has been reported18,38–40. However, to be clinically meaningful, exposing patients to systemic therapies earlier should be associated with either an os benefit or an improvement in qol. A large proportion of the patients in the control arms of these studies did not receive active drug. Of patients in the delayed arm in the Fidias trial, 37% never received docetaxel; of patients in the control arm of the jmen trial, 82% did not receive pemetrexed; and of patients in the control arm of saturn, 79% did not receive an egfr tki. It can therefore be speculated that the os benefit seen in the jmen and saturn trials may in large part derive from the difference in the patients receiving active drug and not from prevention of disease progression. In fact, in the docetaxel maintenance study, Fidias et al.45 reported that patients in the delayed arm who received docetaxel had a median os identical to that of patients in the immediate-docetaxel arm.
The same theory cannot be applied to the paramount clinical trial. Although only 4% of patients in the control arm received pemetrexed as a post-study treatment, there is no evidence to support secondline pemetrexed after progression on cisplatin and pemetrexed in the first-line setting.
4. DISCUSSION
Unlike disease sites such as colorectal cancer, renal cell carcinoma, and ovarian carcinoma, for which pfs has been suggested to be an acceptable surrogate for the os endpoint in antineoplastic clinical trials50, in advanced nsclc, pfs and os have not been shown to consistently correlate. In the studies already discussed, there are examples of improvement in pfs without an os benefit, and an os benefit without a pfs benefit, suggesting that factors other than preventing disease progression may be important in improving os in advanced nsclc. In fact, a randomized controlled clinical trial of early initiation of palliative care in patients with advanced nsclc resulted in significant improvements in qol and mood, which were coupled with a 2-month statistically significant os benefit, even though the study arm had less aggressive treatment51. Another hypothesis is that the antineoplastic agents may change the biology of the tumour after progression, as was seen upon withdrawal of egfr tkis before initiation of an alternative treatment that has been associated with accelerated disease progression52. Or it just may be that the surrogate threshold level suggested by Buyse et al. has not been met by the pfs, and thus no os benefit is seen21,22.
5. SUMMARY
The goals of any new cancer treatment are to allow the patient to live longer and to live better. Thus, clinical trials in nsclc have two important endpoints: overall survival and the quality of that survival. All other endpoints should be considered intermediate, becoming surrogates to those important two endpoints only if formally validated. Uncertainty remains about whether an improvement in pfs represents a clinical benefit in patients with nsclc in the same way that prolongation of survival or an improvement in symptoms and qol does. Also, the relationship between pfs and os has not yet been established in advanced nsclc. Buyse et al.21,22 is the only published attempt to identify a surrogate threshold effect of pfs to os. It may apply to cytotoxic agents in the first-line setting, but it cannot be generalized to targeted agents or to treatment beyond the first line. As a primary endpoint, pfs may be acceptable in phase ii trials to identify active new agents (as suggested by Mandrekar et al.20) or in scenarios in which crossover occurs and the os seen in the study population is dramatically different from that in historical controls (as was seen in the first-line egfr tki trials).
As other groups have concluded, before pfs can be used as a surrogate for the os endpoint in advanced nsclc, it must be validated as a surrogate endpoint, and the scenarios in which the correlation applies must be determined53. Until such surrogacy has been established, os should remain the primary endpoint of clinical trials in advanced nsclc.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to disclose. Both authors contributed equally to this manuscript.
7. REFERENCES
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Rapp E, Pater JL, Willan A, et al. Chemotherapy can prolong survival in patients with advanced non-small-cell lung cancer—report of a Canadian multicenter randomized trial. J Clin Oncol. 1988;6:633–41. doi: 10.1200/JCO.1988.6.4.633. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.United States, Department of Health and Human Services, Food and Drug Administration (fda) Guidance for Industry: Clinical Trial Endpoints for the Approval of Non-Small Cell Lung Cancer Drugs and Biologics. Bethesda, MD: FDA; 2011. [Available online at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM259421.pdf; cited February 5, 2013] [Google Scholar]
- 5.Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012;30:1030–3. doi: 10.1200/JCO.2011.38.7571. [DOI] [PubMed] [Google Scholar]
- 6.Shih W. Problems in dealing with missing data and informative censoring in clinical trials. Curr Control Trials Cardiovasc Med. 2002;3:4. doi: 10.1186/1468-6708-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miksad RA, Zietemann V, Gothe R, et al. Progression-free survival as a surrogate endpoint in advanced breast cancer. Int J Technol Assess Health Care. 2008;24:371–83. doi: 10.1017/S0266462308080495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherrill B, Amonkar M, Wu Y, et al. Relationship between effects on time-to-disease progression and overall survival in studies of metastatic breast cancer. Br J Cancer. 2008;99:1572–8. doi: 10.1038/sj.bjc.6604759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kay A, Higgins J, Day AG, Meyer RM, Booth CM. Randomized controlled trials (rcts) in the era of molecular oncology. Methodology, biomarkers, and endpoints. Ann Oncol. 2012;23:1646–51. doi: 10.1093/annonc/mdr492. [DOI] [PubMed] [Google Scholar]
- 10.Sacher AG, Leighl NB. Shifting patterns in the interpretation of phase iii clinical trial outcomes in the treatment of advanced nsclc [abstract 6105] J Clin Oncol. 2011;29 doi: 10.1200/JCO.2013.52.7804. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=102&abstractID=82903; cited February 15, 2013] [DOI] [PubMed] [Google Scholar]
- 11.Sridhara R, Johnson JR, Justice R, Keegan P, Chakravarty A, Pazdur R. Review of oncology and hematology drug product approvals at the U.S. Food and Drug Administration between July 2005 and December 2007. J Natl Cancer Inst. 2010;102:230–43. doi: 10.1093/jnci/djp515. [DOI] [PubMed] [Google Scholar]
- 12.Buyse M, Burzykowski T, Michiels S, Carroll K. Individual- and trial-level surrogacy in colorectal cancer. Stat Methods Med Res. 2008;17:467–75. doi: 10.1177/0962280207081864. [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–40. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P. Measuring the clinical response: what does it mean? Eur J Cancer. 2002;38:1817–23. doi: 10.1016/S0959-8049(02)00182-X. [DOI] [PubMed] [Google Scholar]
- 16.Gatzemeier U, von Pawel J, Gottfried M, et al. Phase iii comparative study of high-dose cisplatin versus a combination of paclitaxel and cisplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000;18:3390–9. doi: 10.1200/JCO.2000.18.19.3390. [DOI] [PubMed] [Google Scholar]
- 17.Gandara DR, Crowley J, Livingston RB, et al. Evaluation of cisplatin intensity in metastatic non-small-cell lung cancer: a phase iii study of the Southwest Oncology Group. J Clin Oncol. 1993;11:873–8. doi: 10.1200/JCO.1993.11.5.873. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 19.Lara PN, Jr, Redman MW, Kelly K, et al. on behalf of the Southwest Oncology Group Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group Randomized Trials. J Clin Oncol. 2008;26:463–7. doi: 10.1200/JCO.2007.13.0344. [DOI] [PubMed] [Google Scholar]
- 20.Mandrekar SJ, Qi Y, Hillman SL, et al. Endpoints in phase ii trials for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:3–9. doi: 10.1097/JTO.0b013e3181c0a313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buyse ME, Squifflet P, Laporte S, et al. Prediction of survival benefits from progression-free survival in patients with advanced non small cell lung cancer: evidence from a pooled analysis of 2,838 patients randomized in 7 trials [abstract 8019] J Clin Oncol. 2008;26 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=32835; cited January 9, 2013] [Google Scholar]
- 22.Douillard JY, Laporte S, Fossella F, et al. Comparison of docetaxel- and vinca alkaloid–based chemotherapy in the first-line treatment of advanced non-small cell lung cancer: a meta-analysis of seven randomized clinical trials. J Thorac Oncol. 2007;9:939–46. doi: 10.1097/JTO.0b013e318153fa2b. [DOI] [PubMed] [Google Scholar]
- 23.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 24.Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1416–23. doi: 10.1097/JTO.0b013e3181da36f4. [DOI] [PubMed] [Google Scholar]
- 25.Reck M, von Pawel J, Zatloukal P, et al. Phase iii trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for non squamous non-small cell lung cancer: avail. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 26.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomized phase iii trial (avail) Ann Oncol. 2010;21:1804–9. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scagliotti GV, Parikh P, von Pawel J, et al. Phase iii study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 28.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (flex): an open-label randomised phase iii trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 29.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase iii, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (ipass) J Clin Oncol. 2011;29:2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 30.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (optimal, ctong-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 31.Han JY, Park K, Kim SW, et al. First-signal: first-line single-agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–8. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 32.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (eurtac): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 33.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (wjtog 3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 34.Misudomi T, Morita S, Tatabe Y, et al. Updated overall survival results of wjtog 3405, a randomized phase iii trial comparing gefitinib (g) with cisplatin plus docetaxel (cd) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (egfr) [abstract 7521] J Clin Oncol. 2012;30 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=114&abstractID=93530; cited January 9, 2013] [Google Scholar]
- 35.Yang JC, Schuler MH, Yamamoto N, et al. lux-Lung 3: a randomized, open-label, phase iii study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations [abstract 7500] J Clin Oncol. 2012;30 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=114&abstractID=91942; cited January 9, 2013] [Google Scholar]
- 36.Schiller JH, Harrington D, Belani CP, et al. on behalf of the Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–9. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 37.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression free survival. J Natl Cancer Inst. 2009;101:1642–9. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non–small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 39.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase iii trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The tax 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 40.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase iii trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 41.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 42.Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (lux-Lung 1): a phase 2b/3 randomized trial. Lancet Oncol. 2012;13:528–38. doi: 10.1016/S1470-2045(12)70087-6. [Erratum in: Lancet Oncol 2012;13:e186] [DOI] [PubMed] [Google Scholar]
- 43.Lee JS, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase iii trial (zephyr) J Clin Oncol. 2012;30:1114–21. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 44.Soon YY, Stockler MR, Askie LM, Boyer MJ. Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27:3277–83. doi: 10.1200/JCO.2008.19.4522. [DOI] [PubMed] [Google Scholar]
- 45.Fidias PM, Dakhil SR, Lyss AP, et al. Phase iii study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small cell lung cancer. J Clin Oncol. 2009;27:591–8. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 46.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 47.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 48.Paz–Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (paramount): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–55. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 49.Paz–Ares L, De Marinis F, Dediu M, et al. paramount: final overall survival (os) results of the phase iii study of maintenance pemetrexed (pem) plus best supportive care (bsc) versus placebo (plb) plus bsc immediately following induction treatment with pem plus cisplatin (cis) for advanced nonsquamous (ns) non-small cell lung cancer (nsclc) [abstract 7507] J Clin Oncol. 2012;30 doi: 10.1200/JCO.2011.39.7646. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=114&abstractID=94836; cited January 9, 2013] [DOI] [Google Scholar]
- 50.Maroun JA. The significance of progression-free survival as an endpoint evaluating the therapeutic value of antineoplastic agents. Curr Oncol. 2011;18(suppl 2):S3–4. doi: 10.3747/co.v18iS2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small cell lung cancer. N Engl J Med. 2010;363:733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 52.Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011;17:6298–303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pilz LR, Manegold C, Schmid–Bindert G. Statistical considerations and endpoints for clinical lung cancer studies: can progression free survival (pfs) substitute overall survival (os) as a valid endpoint in clinical trials for advanced non-small-cell lung cancer? Transl Lung Cancer Res. 2012;1:26–35. doi: 10.3978/j.issn.2218-6751.2011.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]


