Abstract
New antitumour immunotherapy strategies for stage iv metastatic melanoma include ipilimumab, a monoclonal antibody against ctla-4. Patterns of response with cancer immunotherapy differ from those with cytotoxic chemotherapy. We present two cases of long-duration immune-related responses with ipilimumab in a phase ii trial.
A 66-year-old woman with multiple lung metastases from a scalp primary melanoma received 4 doses of ipilimumab with mixed clinical response. However, after the first maintenance dose, she developed severe ileitis and colitis that responded to steroid therapy. Four months later, she had surgery and radiotherapy for a single brain metastasis. Radiologically, stable disease continued for 36 months after the last ipilimumab dose, and partial response for 5 years after ipilimumab start.
A 54-year-old man with cervical lymph node and pulmonary metastases from a scalp primary melanoma received three induction doses of ipilimumab. He developed alopecia universalis and widespread vitiligo, and he discontinued treatment because of hypophysitis. Maintenance ipilimumab was started after a 6-month drug-free interval, with no further adverse events over 15 cycles. At week 12, computed tomography imaging showed no lung metastases and partial response in a supraclavicular lymph node, which was positive on positron-emission tomography. Five years after starting ipilimumab, the supraclavicular lymph node was calcified, and the patient was off steroid therapy and asymptomatic.
The foregoing patients demonstrate long responses with ipilimumab (in association with delayed severe colitis in one case, and a constellation of immune events, including alopecia universalis in another). Re-treatment with ipilimumab may be possible even after significant immune adverse events.
Keywords: Ipilimumab, melanoma, immune response, safety
1. INTRODUCTION
The 10-year survival rate for patients with stage iv metastatic melanoma remains less than 10%1,2. New antitumour immunotherapy strategies have focused on cytotoxic T lymphocyte antigen-4 (ctla-4), a negative regulator of T-cell–mediated immune response3. Ipilimumab (Bristol–Myers Squibb/Medarex, New York, NY, U.S.A.) is a fully human monoclonal antibody directed against ctla-4, for effective antitumour immune response development4. In phase ii and iii clinical trials, use of ipilimumab has resulted in modest improvements in 2-year survival rates of between 15% and 24%5–8. Some patients have shown durable objective responses and stabilization of their disease5,7–9. However, immune adverse events (iaes) after ipilimumab treatment are common10.
We present 2 patients treated with ipilimumab, with follow-ups of 5 and 5.5 years, who experienced sustained responses after immune toxicities. One patient was re-treated with ipilimumab after initial immune adverse events. Both patients participated in a multicentre double-blind randomized phase ii trial of open-label ipilimumab, with prophylactic budesonide to ameliorate gastrointestinal side effects5. The protocol involved 4 cycles of induction therapy with intravenous ipilimumab 10 mg/kg in weeks 1, 4, 7, and 10, and daily oral medication (budesonide 9 mg or placebo) until week 12, with gradual tapering to discontinuation at week 165. Maintenance ipilimumab (10 mg/kg every 12 weeks) for eligible patients started at week 24.
2. CASE DESCRIPTIONS
2.1. Patient 1
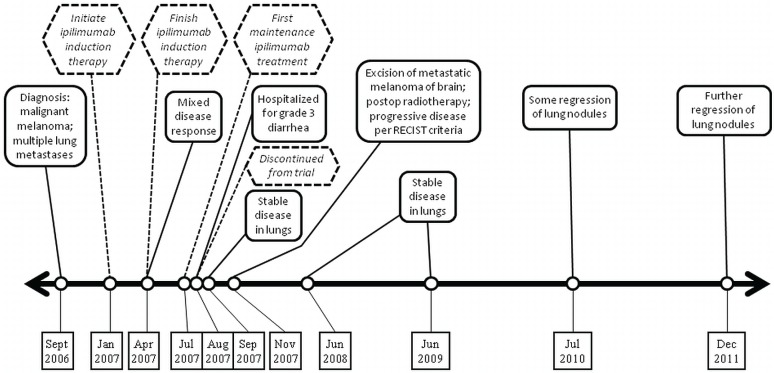
A 66-year-old woman with pT2a nodular melanoma of the scalp had multiple lung metastases on computed tomography (ct ) imaging in September 2006 (Figure 1). Biopsy confirmed metastatic melanoma. Magnetic resonance imaging of the brain was negative, lactate dehydrogenase was normal, and the woman’s performance status (ps) was 0. She received ipilimumab from January to April 2007, with only minor side effects. The right upper-lobe nodule decreased in diameter to 2.5 cm from 2.9 cm, but the left upper-lobe nodule increased to 4 cm from 3.2 cm, and a left lower-lobe nodule increased to 2.2 cm from 2 cm.
FIGURE 1.
Timeline for case 1, a 66-year-old woman.
The patient began maintenance ipilimumab in July 2007 and developed grade 3 diarrhea requiring hospitalization in August 2007. Colonoscopy and ileoscopy revealed severe terminal ileitis and right-sided colitis, confirmed on biopsy. She was treated with intravenous methylprednisolone and recovered well. She was withdrawn from the trial because of grade 3 toxicity.
In September 2007, ct imaging showed stable disease in the lungs. In November 2007, ct imaging of the brain showed an abnormality, confirmed by magnetic resonance imaging as a 1.2-cm dural-based lesion around the left temporal lobe, with extensive edema consistent with metastasis. Craniotomy and excision of the metastatic melanoma in November were followed by postoperative whole-brain radio-therapy in December 2007.
The patient has since been under ct surveillance (Figure 1). In June 2008, lung nodules were stable; in July 2010, some regression of the lung nodules was evident. In December 2011, the largest pulmonary nodule was 1.6 cm in diameter. Despite the partial response in the lungs, the response category is “progressive disease” by the Response Evaluation Criteria in Solid Tumors because of development of a brain metastasis11,12.
2.2. Patient 2
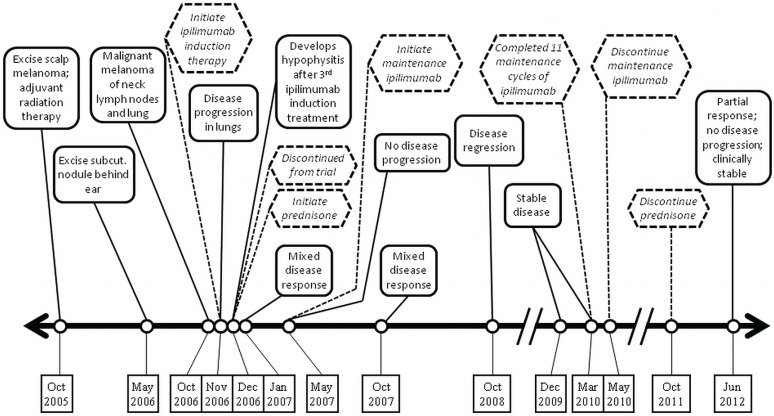
A 54-year-old man diagnosed with pT3a pN2a scalp melanoma in October 2005 was treated by wide excision of the melanoma, sentinel node biopsy, completion right cervical lymphadenectomy, and adjuvant radiation therapy (Figure 2). He developed a subcutaneous nodule behind the right ear in May 2006. Excision biopsy and subsequent wide excision were performed. In October 2006, he presented with nodules in the left supraclavicular lymph node and the right neck skin. Biopsies of both lesions were positive for metastatic melanoma. On ct imaging, multiple new pulmonary metastases were evident, the largest being 1.5 cm in diameter. The patient’s lactate dehydrogenase was normal, and his ps was 0.
FIGURE 2.
Timeline for case 2, a 54-year-old man.
The patient started ipilimumab and had significant headache after each of 3 doses. He developed alopecia universalis and widespread vitiligo, and he discontinued treatment because of hypophysitis, confirmed by magnetic resonance imaging. He was referred to endocrinology and treated for pituitary insufficiency with replacement thyroxine and a short course of prednisone 60 mg, tapering to a maintenance dose of 5 mg. His headache ceased 1 day later. Plasma testosterone was normalized in 4 weeks.
In January 2007, ct imaging showed moderate regression in many pulmonary metastases, but persistent right cervical adenopathy. Lymph node biopsy showed persistent metastatic melanoma with necrosis. He began maintenance ipilimumab in May 2007 while still on low-dose prednisone. Positron-emission tomography imaging in August 2007 showed a 2.4-cm right supraclavicular node and a 1-cm node in the aortopulmonary window.
In October 2007, 11 months after induction treatment, ct imaging showed an overall mixed response: unchanged in the neck, progression of the aortopulmonary window node, and complete resolution of several lung nodules observed in November 2006. Compared with imaging in January 2007, lymph nodes were stable and notably smaller than in November 2006. The patient completed 11 maintenance cycles of ipilimumab with good tolerance, but elected to discontinue treatment in May 2010 (Figure 2). He discontinued prednisone in October 2011. At his last follow-up in June 2012, 5.5 years after ipilimumab start, the patient was clinically stable with a partial response overall.
3. DISCUSSION
The 2 patients treated described here had sustained responses to ipilimumab at 5 and 5.5 years’ follow-up, after immune toxicities. Two large phase iii trials of ipilimumab in metastatic melanoma demonstrated an overall survival benefit, with 2-year survival rates of 23.5% and 28.5%8,13. Patients entered into both trials had good ps and an anticipated life expectancy of more than 3 and more than 4 months, suggesting that low-bulk disease favours immunotherapy. Both of our cases had low-volume metastatic disease and good ps and demonstrated a long duration of response.
Patient 1 had a mixed response at 12 weeks, progressive disease marked by a brain metastasis at 8 months, and eventual partial remission. Patient 2 also had an initial mixed response and eventual stable disease. Other case reports of atypical response patterns after ipilimumab treatment have documented a mixed response, with some lesions disappearing, others stabilizing, and others progressing14–16. These unusual patterns may reflect the different mechanism of action of an immune antitumour response and the complex interactions that may lead to apparent “dormancy” of metastases15,16. Metastatic cell heterogeneity, and differences in the phenotypes of the primary tumour and metastatic cells, may also play a role.
In phase iii trials, some patients experienced sustained remission with ipilimumab despite low overall response rates of 15%13 and 9%8 by the Response Evaluation Criteria in Solid Tumors11,12, which do not accurately reflect disease outcomes with ipilimumab. New immune-related response criteria have been proposed to better describe the observed responses: regression of metastases without new lesions; stable disease, sometimes followed by gradual regression; response after initial increase in metastasis diameter; and response in the context of new lesions17. In practice, this approach means that ipilimumab can be continued, even if there is evidence of progression radiologically.
Responses to ipilimumab are typically slow in evolution, as in the patients presented here. Ipilimumab-treated patients do not begin to exhibit superior progression-free survival until 2 months8 or more than 3 months13 after induction. Ipilimumab has been approved for second-line treatment of metastatic melanoma in Canada and the United States, recommended at a 3 mg/kg dose13 in ps 0–1 patients, with an option to use “re-induction” therapy for stable disease or in improved patients, provided that at least 3 months has elapsed since completion of the initial therapy18.
Ipilimumab predominantly has iaes, as reported for three phase ii clinical trials10. Major sites and prevalence of serious (grade 3 or 4) iaes are the gastrointestinal tract (12%), liver (7%), skin (3%), and endocrine system (3%)19. Average timelines for iaes are 2–3 weeks for dermatologic events, 6–7 weeks for gastrointestinal and hepatic events, and 9 weeks for endocrine events. Patient 1 had severe colitis at 27 weeks, the latest incidence reported to date20. In the trial that included both patients reported here, prophylactic budesonide did not reduce the rate of serious gastrointestinal iaes5. Early intervention with corticosteroid therapy controls most immune colitis19. Management guidelines for iaes have been mandated21. Treatment-related mortality dropped from 2.4% in the first phase iii trial13 to 0% in the second8, suggesting that experience and vigilance can reduce mortality from iaes. Recently presented data22 and a literature review19 indicate that re-treatment or maintenance ipilimumab in a patient with a previous iae less than grade 3 may be both considered and effective, as in patient 2.
Hypophysitis occurs in up to 6% of ipilimumab patients19. Patient 2 was treated with prednisone 5 mg for more than 3 years. Prednisone did not abrogate the benefit of ipilimumab in his disease. In an analysis of steroid therapy use in all patients with iaes in a first-line ipilimumab trial, 70% recovered completely, but no patients with grade 2 endocrinopathy (n = 3) experienced resolution23. No attenuation of ipilimumab efficacy was evident with corticosteroid use.
Skin toxicity is the earliest type of iae observed with ipilimumab. Diffuse maculopapular rash with associated pruritus is typically observed, but it is severe only in 4% of patients. Vitiligo occurs in up to 11% of patients7. Patient 2 experienced both vitiligo and alopecia universalis. The latter has been reported only once previously with ipilimumab therapy24.
4. SUMMARY
Ipilimumab is about to enter general oncology practice in Canada. This agent differs in response pattern, toxicity pathogenesis, and management from the usual chemotherapy agents. The patients reported here had mixed radiologic responses, significant immune-mediated toxicities, and most importantly, long survival. Adherence to management algorithms can control adverse events, avoid clinical deterioration, and permit ongoing therapy, if required.
5. ACKNOWLEDGMENTS
The authors thank Dagmar Gross for assistance with preparation of this manuscript.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest and no sources of funding to declare.
7. REFERENCES
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 ajcc melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garbe C, Eigentler TK. Diagnosis and treatment of cutaneous melanoma: state of the art 2006. Melanoma Res. 2007;17:117–27. doi: 10.1097/CMR.0b013e328042bb36. [DOI] [PubMed] [Google Scholar]
- 3.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (ctla-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614–27. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 5.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled phase ii study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage iii or iv melanoma. Clin Cancer Res. 2009;15:5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 6.O’Day SJ, Maio M, Chiarion–Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase ii study. Ann Oncol. 2010;21:1712–17. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 7.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 9.Farolfi A, Ridolfi L, Guidoboni M, et al. Ipilimumab in advanced melanoma: reports of long-lasting responses. Melanoma Res. 2012;22:263–70. doi: 10.1097/CMR.0b013e328353e65c. [DOI] [PubMed] [Google Scholar]
- 10.Lutzky J, Wolchock J, Hamid O, et al. Association between immune-related adverse events (iraes) and disease control or overall survival in patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase ii clinical trials [abstract 9034] J Clin Oncol. 2009;27 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=34815; cited January 12, 2013] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised recist guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [Erratum in: N Engl J Med 2010;363:1290] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Ledezma B, Binder S, Hamid O. Atypical clinical response patterns to ipilimumab. Clin J Oncol Nurs. 2011;15:393–403. doi: 10.1188/11.CJON.393-403. [DOI] [PubMed] [Google Scholar]
- 16.Wilgenhof S, Pierret L, Corthals J, et al. Restoration of tumor equilibrium after immunotherapy for advanced melanoma: three illustrative cases. Melanoma Res. 2011;21:152–9. doi: 10.1097/CMR.0b013e328343ece0. [DOI] [PubMed] [Google Scholar]
- 17.Wolchok J, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immunerelated response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 18.Pan-Canadian Oncology Drug Review (pcodr) pCODR Expert Review Committee (pERC) Final Recommendation for Ipilimumab (Yervoy) for Advanced Melanoma. Toronto, ON: PCODR; 2012. [Google Scholar]
- 19.Weber JS, Kahler KC, Hauschild A. Management of immunerelated adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 20.Yervoy (Ipilimumab) [label submission] Princeton, NJ: Bristol–Myers Squibb; 2011. Bristol–Myers Squibb. Highlights of prescribing information. [Google Scholar]
- 21.Yervoy (Ipilimumab) Immune-Related Adverse Reaction Management Guide. Princeton, NJ: Bristol–Myers Squibb; 2011. Bristol–Myers Squibb. [Google Scholar]
- 22.Hodi FS, O’Day S, McDermott DF, et al. Re-induction with ipilimumab, gp100 peptide vaccine, or a combination of both from a phase iii, randomized, double-blind, multicenter study of previously treated patients with unresectable stage iii or iv melanoma [abstract 8509] J Clin Oncol. 2010;28 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=74&abstractID=47740; cited January 12, 2013] [Google Scholar]
- 23.Baurain JF, Smylie M, Ascienrto PA, et al. Outcomes of ipilimumab treatment-related adverse events in patients with metastatic melanoma (mm) who received systemic corticosteroids in a phase iii trial [abstract 8539] J Clin Oncol. 2012;30 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=114&abstractID=93252; cited January 12, 2013] [Google Scholar]
- 24.Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage iv melanoma treated with anticytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166–72. doi: 10.1001/archderm.142.2.166. [DOI] [PubMed] [Google Scholar]