Abstract
Purpose
Gemcitabine and capecitabine (gem-cap), gemcitabine and erlotinib (gem-e), and folfirinox (5-fluorouracil–leucovorin–irinotecan–oxaliplatin) are new treatment options for metastatic pancreatic cancer, but they are also more expensive and potentially more toxic than gemcitabine alone (gem). We conducted a cost-effectiveness analysis of these treatment options compared with gem.
Methods
A Markov model was constructed to examine costs and outcomes of gem-cap, gem-e, folfirinox, and gem in patients with metastatic pancreatic cancer from the perspective of a government health care plan. Ontario health economic and costing data (2010 Canadian dollars) were used. Efficacy data for the treatments were obtained from the published literature. Resource utilization data were derived from a chart review of consecutive metastatic patients treated for pancreatic cancer at Princess Margaret Hospital, Toronto, Ontario, 2008–2009, and supplemented with data from the literature. Utilities were obtained by surveying medical oncologists across Canada using the EQ-5D. Incremental cost-effectiveness ratios (icers) were calculated.
Results
The icers for gem-cap, gem-e, and folfirinox compared with gem were, respectively, CA$84,299, CA$153,631, and CA$133,184 per quality-adjusted life year (qaly). The model was driven mostly by drug acquisition costs. Given a willingness-to-pay (wtp) threshold greater than CA$130,000/qaly, folfirinox was most cost-effective treatment. When the wtp threshold was less than CA$80,000/qaly, gem alone was most cost-effective. The gem-e option was dominated by the other treatments.
Conclusions
The most cost-effective treatment for metastatic pancreatic cancer depends on the societal wtp threshold. If the societal wtp threshold were to be relatively high or if drug costs were to be substantially reduced, folfirinox might be cost-effective.
Keywords: Pancreatic cancer, cost-effectiveness, chemotherapy
1. INTRODUCTION
Pancreatic cancer is an aggressive disease that ranks as the 4th leading cause of cancer-related death in Canada. In phase iii clinical trials, several different chemotherapy regimens for advanced pancreatic cancer have been shown to increase survival1–5. Gemcitabine (gem) has been considered the standard treatment for pancreatic cancer for more than a decade because of a landmark clinical trial that showed superiority for gem compared with bolus 5-fluorouracil. That trial showed that gem was associated with clinical benefit, measured as a composite endpoint of pain, functional impairment, and weight loss1. In addition, gem was also associated with a modest improvement in survival.
Subsequently, a number of randomized phase iii studies compared several promising gem-based combinations with gem alone. Despite encouraging phase ii data, it remains a matter of controversy whether the addition of capecitabine to gem (gem-cap) produces a survival benefit, because two phase iii clinical trials have failed to show a significant survival benefit for the combination compared with gem alone2,3. However, a pooled analysis of the two phase iii trials and one randomized phase ii trial was able to show that gem-cap was associated with a modest, but statistically significant, survival advantage3. In addition, two different meta-analyses also concluded that gem-based chemotherapy combinations were associated with a modest but significant survival advantage compared with gem alone6,7.
With the exception of erlotinib, the addition of targeted agents to gem has failed to produce any added benefit; however, the erlotinib combination (gem-e) increased 1-year survival to 23% from the 17% seen with gem alone4,8,9. More recently, a phase iii clinical trial in metastatic pancreatic cancer compared a combination of 5-fluorouracil, folinic acid, irinotecan, and oxaliplatin (folfirinox) with gem. That study was closed prematurely because a preplanned interim analysis showed superior response rate, progression-free survival, and overall survival favouring folfirinox5. However, those benefits came at the expense of increased toxicities, including diarrhea, nausea, vomiting, and febrile neutropenia.
Although the gem-cap, gem-e, and folfirinox combinations have each been shown to improve survival compared with gem alone, they have not been directly compared with one another in terms of efficacy or cost-effectiveness. Determining whether new treatments are cost-effective is important, because health care budgets are limited, and a disproportionate rise in spending on cancer drugs has already been occurring.
The objective of the present study was to perform a cost-effectiveness analysis from the perspective of Canada’s public health care system to determine the most cost-effective systemic therapy for treating metastatic pancreatic cancer.
2. METHODS
2.1. Study Design
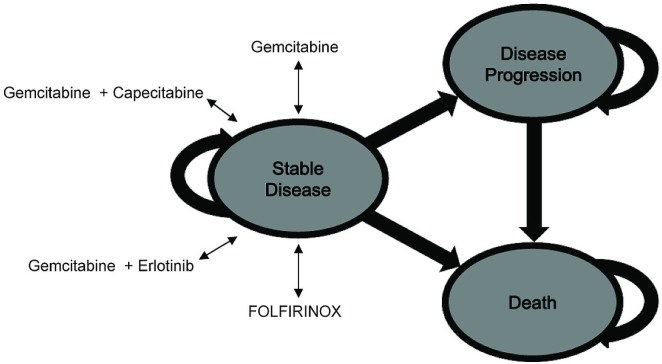
A Markov analytic decision model (Figure 1) was constructed for a hypothetical cohort of patients with metastatic pancreatic cancer undergoing chemotherapy with four different systemic therapy regimens (Appendix a). The base-case strategy was gem alone, and the experimental strategies were gem-cap, gem-e, and folfirinox. Table i describes the model parameters. The primary outcome was the incremental cost-effectiveness ratio (icer), which is measured in dollars per quality-adjusted life-year (qaly). We compared this ratio for each of the three experimental strategies with that for the gem-alone strategy.
FIGURE 1.
The Markov model.
TABLE I.
Model parameters
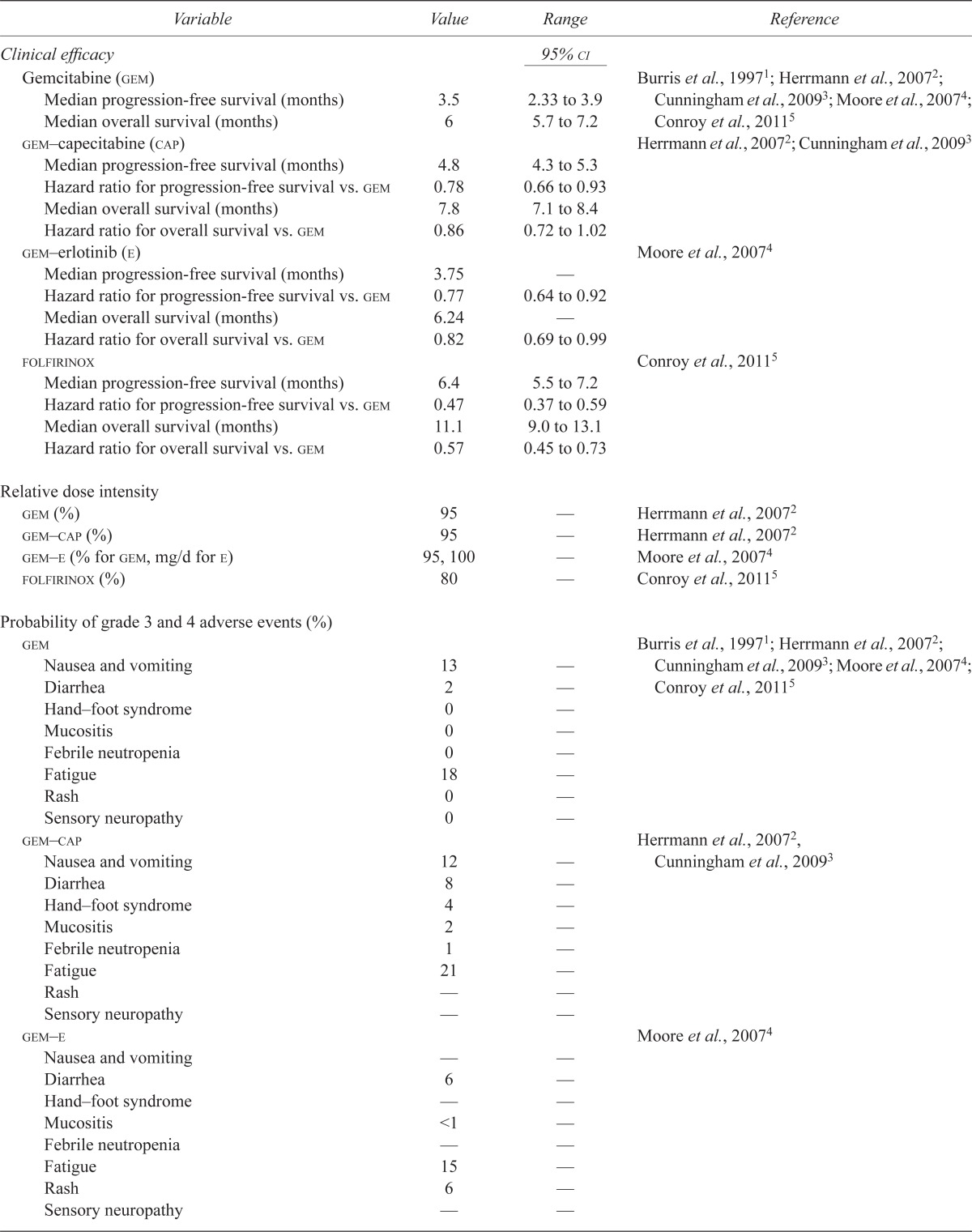
| Variable | Value | Range | Reference |
|---|---|---|---|
| Clinical efficacy |
95%
ci
|
||
| Gemcitabine (gem) | Burris et al., 19971; Herrmann et al., 20072; Cunningham et al., 20093; Moore et al., 20074; Conroy et al., 20115 | ||
| Median progression-free survival (months) | 3.5 | 2.33 to 3.9 | |
| Median overall survival (months) | 6 | 5.7 to 7.2 | |
| gem–capecitabine (cap) | Herrmann et al., 20072; Cunningham et al., 20093 | ||
| Median progression-free survival (months) | 4.8 | 4.3 to 5.3 | |
| Hazard ratio for progression-free survival vs. gem | 0.78 | 0.66 to 0.93 | |
| Median overall survival (months) | 7.8 | 7.1 to 8.4 | |
| Hazard ratio for overall survival vs. gem | 0.86 | 0.72 to 1.02 | |
| gem–erlotinib (e) | Moore et al., 20074 | ||
| Median progression-free survival (months) | 3.75 | — | |
| Hazard ratio for progression-free survival vs. gem | 0.77 | 0.64 to 0.92 | |
| Median overall survival (months) | 6.24 | — | |
| Hazard ratio for overall survival vs. gem | 0.82 | 0.69 to 0.99 | |
| folfirinox | Conroy et al., 20115 | ||
| Median progression-free survival (months) | 6.4 | 5.5 to 7.2 | |
| Hazard ratio for progression-free survival vs. gem | 0.47 | 0.37 to 0.59 | |
| Median overall survival (months) | 11.1 | 9.0 to 13.1 | |
| Hazard ratio for overall survival vs. gem | 0.57 | 0.45 to 0.73 | |
| Relative dose intensity | |||
| gem (%) | 95 | — | Herrmann et al., 20072 |
| gem–cap (%) | 95 | — | Herrmann et al., 20072 |
| gem–e (% for gem, mg/d for e) | 95, 100 | — | Moore et al., 20074 |
| folfirinox (%) | 80 | — | Conroy et al., 20115 |
| Probability of grade 3 and 4 adverse events (%) | |||
| gem | Burris et al., 19971; Herrmann et al., 20072; Cunningham et al., 20093; Moore et al., 20074; Conroy et al., 20115 | ||
| Nausea and vomiting | 13 | — | |
| Diarrhea | 2 | — | |
| Hand–foot syndrome | 0 | — | |
| Mucositis | 0 | — | |
| Febrile neutropenia | 0 | — | |
| Fatigue | 18 | — | |
| Rash | 0 | — | |
| Sensory neuropathy | 0 | — | |
| gem–cap | Herrmann et al., 20072, Cunningham et al., 20093 | ||
| Nausea and vomiting | 12 | — | |
| Diarrhea | 8 | — | |
| Hand–foot syndrome | 4 | — | |
| Mucositis | 2 | — | |
| Febrile neutropenia | 1 | — | |
| Fatigue | 21 | — | |
| Rash | — | — | |
| Sensory neuropathy | — | — | |
| gem–e | Moore et al., 20074 | ||
| Nausea and vomiting | — | — | |
| Diarrhea | 6 | — | |
| Hand–foot syndrome | — | — | |
| Mucositis | <1 | — | |
| Febrile neutropenia | — | — | |
| Fatigue | 15 | — | |
| Rash | 6 | — | |
| Sensory neuropathy | — | — | |
| folfirinox | Conroy et al., 20115 | ||
| Nausea and vomiting | 14 | — | |
| Diarrhea | 13 | — | |
| Hand–foot syndrome | — | — | |
| Mucositis | — | — | |
| Febrile neutropenia | 5 | — | |
| Fatigue | 23 | — | |
| Rash | — | — | |
| Sensory neuropathy | 9 | — | |
| Cost of managing patients with metastatic pancreatic cancer receiving first-line chemotherapya ($/month) | 885 | — | Chart review at Princess Margaret Hospital in present study |
| Cost of patient receiving palliative care ($/month) | 6,633 | — | Guerriere et al., 201010 |
| Cost of chemotherapy at full dose (CA$) | Sunnybrook Health Sciences Centre pharmacy | ||
| gem (per cycle) | 329 | — | |
| gem–cap (per cycle) | 653 | — | |
| gem–e (per cycle) | 2,691 | — | |
| folfirinox (per cycle) | 1775 | — | |
| Cost of chemotherapy administration ($/h) | 178 | — | Sunnybrook Health Sciences Centre |
|
|
|||
| Cost of treatment of grade 3 and 4 adverse events ($)b | (±50%) | ||
|
|
|||
| Nausea and vomiting | 4607 | 2,304–6,911 | Ontario Case Costing Initiative11 |
| Diarrhea | 4468 | 2,234–6,702 | Ontario Case Costing Initiative11 |
| Stomatitis | 6644 | 3,322–9,966 | Ontario Case Costing Initiative11 |
| Febrile neutropenia | 6557 | 3,279–9,836 | Lathia et al., 201012 |
| Fatigue | 5270 | 2,635–7,905 | Ontario Case Costing Initiative11 |
| Rash | 295 | 148–442 | Clinical experiencec |
| Hand–foot syndrome | 14 | 7–21 | Clinical experienced |
| Neuropathy | 0 | 0 | Clinical experiencee |
|
|
|||
| Utility states | Standard deviation | Survey of oncologists in present study | |
|
|
|||
| Stable disease | 0.720 | 0.185 | |
| Supportive care | 0.136 | 0.184 | |
| Grade 3/4 nausea and vomiting | 0.526 | 0.235 | |
| Grade 3/4 diarrhea | 0.508 | 0.207 | |
| Grade 3/4 stomatitis | 0.279 | 0.231 | |
| Grade 3/4 febrile neutropenia | 0.589 | 0.171 | |
| Grade 3/4 fatigue | 0.247 | 0.239 | |
| Grade 3/4 rash | 0.626 | 0.166 | |
| Grade 3/4 hand-foot syndrome | 0.409 | 0.210 | |
| Grade 3/4 neuropathy | 0.494 | 0.177 |
Excluding costs of chemotherapy and management of adverse events.
We made the assumption that one third of the patients with grade 3 and 4 nausea and vomiting, diarrhea, stomatitis and fatigue were hospitalized.
Cost includes oral doxycycline and a dermatology consult.
Cost includes cream recommended for hand–foot syndrome.
No cost, because usual treatment consists of dose reductions or stopping the chemotherapy potentially responsible for neuropathy.
The economic analysis was conducted from the perspective of the Ministry of Health and Long-Term Care (mohltc) of Ontario, Canada. Costs and benefits were discounted at 3% annually. The time horizon chosen for this analysis was 2 years, because most patients with metastatic pancreatic cancer included in the phase iii trials had died by that time point, regardless of the first-line chemotherapy regimen received. The cycle length was 1 month. The Tree-Age Pro software application (TreeAge Software, Williamstown, MA, U.S.A.) was used to develop the Markov model in the present study.
2.2. Efficacy
Data on overall and progression-free survival were obtained from the completed phase iii clinical trials of metastatic pancreatic cancer for each of the four chemotherapy regimens. The efficacy data for gem were derived from the trial by Burris et al.1 and the subsequent phase iii trials that used gem as a standard comparator. The efficacy data for gem-cap were derived from the phase iii trials by Herrmann et al.2 and Cunningham et al.3. The efficacy data for the combination of gem-e were obtained from the ncic pa.3 clinical trial results published by Moore et al.4. The efficacy data for folfirinox were derived from the prodige 4/accord 11 trial5.
2.3. Utilities
Utilities were obtained from a survey of academic medical oncologists across Canada who are considered experts in the treatment of non-colorectal gastrointestinal malignancies. Paper surveys were distributed to 60 medical oncologists by postal mail. The surveys contained various clinical scenarios involving metastatic pancreatic cancer patients undergoing chemotherapy with or without side effects (Appendix b). The medical oncologists were asked to rate the health utilities of the patients in the scenarios using the EQ-5D measure13. Questions from the EQ-5D covered mobility, self-care, usual activities (for example, work, study, housework, and family or leisure activities), pain and discomfort, and anxiety and depression. An overall utility score was calculated based on the survey responses. The qalys for each disease state were calculated based on the utility scores and the frequency of grades 3 and 4 adverse events for each chemotherapy regimen.
2.4. Resource Utilization
Resource utilization was determined by a systematic chart review of metastatic pancreatic cancer patients treated at Princess Margaret Hospital, Toronto, Ontario. Consecutive patients with metastatic pancreatic cancer who started treatment with gem, gem-cap, gem-e, or folfirinox between January 2008 and December 2009 were identified through the Princess Margaret Hospital Cancer Registry. The electronic medical record for each of those patients was reviewed, and data for the first-line chemotherapy regimen, number of cycles administered, outpatient visits, emergency department visits, laboratory investigations, diagnostic imaging, procedures, and hospitalizations were recorded.
2.5. Adverse Events
The frequencies of grades 3 and 4 adverse events—obtained from the literature1–5 and summarized in Table i—were used to calculate the costs and health utilities associated with adverse events. The adverse events most commonly associated with the four treatment regimens were included in the model: specifically, nausea and vomiting, diarrhea, hand–foot syndrome, mucositis, febrile neutropenia, fatigue, rash, and sensory neuropathy.
2.6. Costs
Table ii lists costs and cost sources. The costs are presented in 2010 Canadian dollars. Total costs were obtained by multiplying the resource utilization determined during the systematic chart review by the unit cost of each item.
TABLE II.
Results of costs and effectiveness
| Result | Treatment | |||
|---|---|---|---|---|
| gem | gem–cap | gem–e | folfirinox | |
| Cost (CA$) | 29,423 | 33,572 | 41,239 | 58,243 |
| Life expectancy (years) | 0.677 | 0.762 | 0.790 | 1.005 |
| qalys | 0.487 | 0.536 | 0.564 | 0.703 |
| icer compared with gem alone ($/qaly) | — | 84,299 | 153,631 | 133,184 |
gem = gemcitabine; cap = capecitabine; e = erlotinib; folfirinox = 5-fluorouracil–leucovorin–irinotecan–oxaliplatin; qalys = quality-adjusted life years; icer = incremental cost-effectiveness ratio.
The acquisition costs per cycle of the four systemic therapy regimens were obtained from Sunnybrook’s Odette Cancer Centre pharmacy. The cost of administering each treatment accounted for the following factors: preparation of the regimen, chemotherapy chair time, hourly wage for the pharmacist, hourly wage for the chemotherapy nurse, and overhead costs at Sunnybrook Health Sciences Centre, Toronto, Ontario. The cost of each outpatient physician assessment and of each diagnostic imaging investigation and medical procedure performed on patients by a physician was based on the 2010 Schedule of Benefits published by the Ontario mohltc14.
The cost of each adverse event requiring hospitalization was determined from the Ontario Case Costing Initiative11. During this process, a list of adverse events was provided to the Ontario Case Costing Initiative to determine the costs of hospital admissions in a population with a diagnosis of pancreatic cancer. The results were provided both as aggregate costs (number of cases, indirect and direct costs, average total cost, length of stay) and as disaggregate costs, which provided a further cost breakdown for each event by functional centre. Laboratory test costs were obtained from the 2010 Schedule of Laboratory Fees published by the Ontario mohltc15. Indirect costs or opportunity costs were not included in the analysis.
2.7. Sensitivity Analyses
One-way sensitivity analyses were performed to determine the robustness of the model to changes in key parameters. Probabilistic sensitivity analysis was also performed to assess the cost-effectiveness of the regimens over a range of willingness-to-pay (wtp) thresholds.
3. RESULTS
Utilities were derived from a total of 33 responses to the practitioner survey, corresponding to a response rate of 55%. Most respondents worked at an academic cancer centre (73%) and were “very” or “somewhat” familiar with economic analyses (88%). The resource utilization data were based on a systematic chart review of 42 consecutive patients who started systemic treatment for metastatic pancreatic cancer at Princess Margaret Hospital between January 2008 and December 2009. Table i shows the results of the utilities.
Table ii summarizes the total cost associated with administering 1 month of each chemotherapy regimen, the expected survival, and the number of qalys (based on utility scores). The most expensive regimen was folfirinox, whose total cost was $58,243 per month of treatment. In contrast, gem alone was the least expensive at $29,423. However, the efficacy of folfirinox was also the highest, being associated with a life expectancy of 1 year and a qaly of 0.703 compared with a life expectancy of just 8 months and a qaly of 0.487 with gem. The icers for gem-cap, gem-e, and folfirinox compared with gem alone were, respectively, $84,299, $153,631, and $133,184 per qaly.
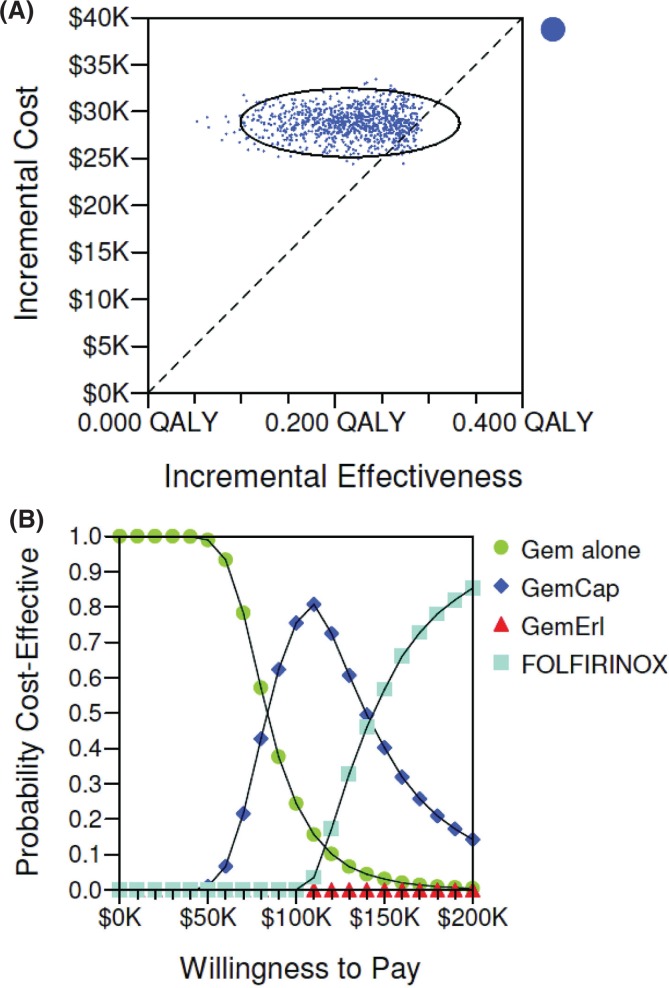
Table iii shows the results of the one-way sensitivity analyses. The icers were robust to most variables, being sensitive mostly to the drug acquisition cost. Compared pairwise with gem alone, folfirinox might be considered cost-effective if the wtp threshold were $100,000/qaly and the cost of drugs were lower by about 40%. Probabilistic sensitivity analysis revealed that the probability of folfirinox being cost-effective at a wtp threshold of $100,000/qaly was less than 5% [Figure 2(A)].
TABLE III.
One-way sensitivity analysis
| Variable | Base case | Alternatives | icer gem-cap vs. gem ($/qaly) | icer gem-e vs. gem ($/qaly) | icer folfirinox vs. gem ($/qaly) |
|---|---|---|---|---|---|
| Discounting | |||||
| 3% | 84,299 | 153,631 | 133,184 | ||
| 5% | 84,674 | 154,506 | 133,800 | ||
| 0% | 83,770 | 152,323 | 132,258 | ||
| Relative dose intensity gem | |||||
| 100% | 84,299 | 153,631 | 133,184 | ||
| 90% | 87,604 | 155,754 | 133,939 | ||
| Relative dose intensity folfirinox | |||||
| 80% | — | — | 133,184 | ||
| 90% | — | — | 148,634 | ||
| 70% | — | — | 117,732 | ||
| Drug cost of folfirinox (per cycle) | |||||
| 1,775 | — | — | 133184 | ||
| +50% | — | — | 194991 | ||
| −50% | — | — | 71376 | ||
| Drug cost of gem–e (per cycle) | |||||
| 2,691 | — | 153631 | — | ||
| +50% | — | 231725 | — | ||
| −50% | — | 75543 | — | ||
| Drug cost of gem–cap (per cycle) | |||||
| 653 | 84,299 | — | — | ||
| +50% | 137,980 | — | — | ||
| −50% | 30,604 | — | — | ||
icer = incremental cost-effectiveness ratio; gem = gemcitabine; cap = capecitabine; e = erlotinib; folfirinox = 5-fluorouracil–leucovorin–irinotecan–oxaliplatin; qalys = quality-adjusted life years.
FIGURE 2.
Probabilistic sensitivity analysis. (A) Incremental cost-effectiveness scatterplot (n = 10,000). The slope of the broken line represents a willingness-to-pay (wtp) threshold of $100,000 per quality-adjusted life-year (qaly). Each point is a simulated result of the probability sensitivity analysis. Points below the line are considered cost-effective with a wtp threshold of $100,000 per qaly. Only 5% of the simulations fell below that line. The oval denotes a region where 95% of the simulations occur. (B) Cost-effectiveness acceptability curve. This plot shows the probability of various metastatic pancreatic cancer treatments being cost-effective at various wtp thresholds. Gem = gemcitabine; Cap = capecitabine; Erl = erlotinib.
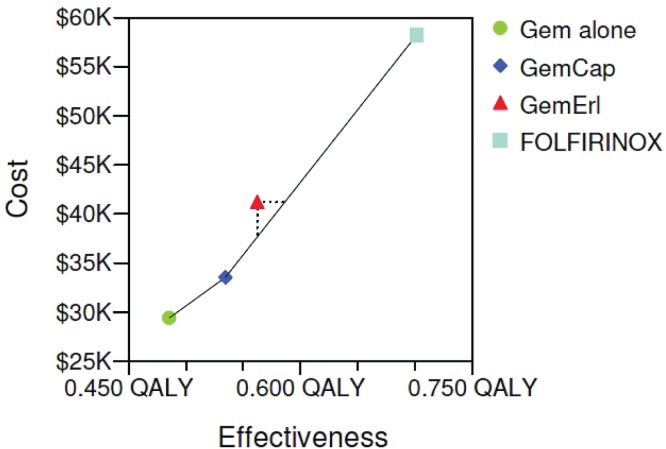
Figure 2(B) shows the cost-effectiveness acceptability curve. The gem-alone regimen appeared to be most cost-effective when the wtp threshold was less than $80,000/qaly. The gem-cap regimen was the most cost-effective when the wtp threshold was between $80,000/qaly and $130,000/qaly. The folfirinox regimen appeared to be most cost-effective when the wtp threshold was above $130,000/qaly. The gem-e regimen was dominated by the other strategies by extended dominance and was not cost-effective at any wtp threshold (Figure 3).
FIGURE 3.
Cost-effectiveness plane. The red triangle point that represents gemcitabine plus erlotinib (GemErl) lies to the right of the line joining the blue diamond point [gemcitabine plus capecitabine (GemCap)] and the light blue square point (folfirinox), meaning that GemErl was dominated by extended dominance. qaly = quality-adjusted life-year.
4. DISCUSSION
This study is the first to examine the cost-effectiveness of all clinically effective and relevant treatments in first-line metastatic pancreatic cancer simultaneously. We found that the icers for gem-cap, gem-e, and folfirinox were relatively high compared with that for gem alone. The major driver of the cost-effectiveness of the new treatments was the drug cost. Our results show that folfirinox can be considered the most cost-effective treatment only if the societal wtp threshold is relatively high or if the drug acquisition cost is lowered compared with the present-day cost.
There is no universal consensus of what constitutes an acceptable societal wtp. Laupacis et al.16 suggested that interventions with an icer of less than $20,000/qaly are cost-effective and those with an icer of more than $100,000/qaly are not cost-effective; however, those figures are based on 1992 dollars. A few influential and widely cited articles use $50,000/qaly as the threshold17,18. In a recent survey of oncologists in both Canada and the United States, 49%–56% of respondents thought that a reasonable cost-effectiveness ratio was $50,000 to $100,000 per life-year; only 30%–33% of respondents believed that a cost-effectiveness ratio exceeding $100,000 per life-year was reasonable19. The World Health Organization recommended 3 times the gross domestic product per capita of the country as an acceptable wtp threshold20. In Canada in 2011, that wtp threshold would be approximately $151,000/qaly21. Based on that threshold, folfirinox might be considered cost-effective compared with gem alone.
A recently reported study suggested that the icer for folfirinox compared with gem alone is $54,196/qaly22. That result contrasts with our finding of a higher icer of $133,184/qaly for folfirinox compared with gem. This apparent discrepancy can likely be explained by the absence of a maximum number of cycles of folfirinox in our study, which is clinically more realistic and congruent with the prodige 4/accord 11 trial, in which the number of folfirinox cycles administered ranged from 1 to 475. The use of a hard cap on the number of folfirinox cycles coupled with the assumption of the same clinical benefit seen in the clinical trial would lead to a substantially lower drug cost and a more favourable icer. Also, our study obtained resource utilization data from a detailed chart review of consecutive patients and used a provincial administrative database to estimate most of the costs for managing side effects. The results of our study therefore provide a substantially more realistic estimate of the true icer when folfirinox is used in clinical practice. Another notable difference between the two studies is that our study was not funded by a pharmaceutical company. A recent study found that economic analyses funded by pharmaceutical companies were more likely to report favourable cost estimates23.
Our results suggest that gem-cap should be considered the most cost-effective treatment if the wtp threshold is $100,000/qaly. The survival benefit of adding cap to gem is controversial, because two phase iii clinical trials have failed to show a significant survival benefit for this combination compared with gem alone2,3. However, the pooled analysis by Cunningham et al.3 did conclude that the gem-cap combination is associated with a statistically significant survival benefit, albeit modest when compared with the folfirinox data.
One notable strength of the present study compared with other economic analyses of systemic treatment for metastatic pancreatic cancer is that all the chemotherapy regimens that are regarded as reasonable treatment options for this disease were considered. Our analysis was able to determine the most cost-effective regimen at various wtp thresholds, allowing for comparisons of the cost-effectiveness of all treatments simultaneously. For example, an earlier study suggested that the icer for gem-e compared with gem alone could be in the range $430,000/qaly to $510,000/qaly. That finding implies that, if the societal wtp threshold were higher than $510,000/qaly, gem-e would be cost-effective. However, when we examined the cost-effectiveness of all treatments simultaneously rather than just focusing on pairwise comparisons, gem-e failed to be cost-effective regardless of the societal wtp, because gem-e was dominated by other strategies by extended dominance. Essentially, the other strategies or some combination of the other strategies is always more effective and less expensive than gem-e.
We felt that our icer of $153,631/qaly for gem-e compared with gem alone was more realistic than an icer of $430,000/qaly to $510,000/qaly reported in a previous study24 because we avoided using the median incremental overall survival when modelling the incremental effectiveness of gem-e compared with gem alone. Instead of the median incremental overall survival, we used hazard ratios to estimate the mean incremental effectiveness through Markov modeling. Given that the survival curves for gem-e and gem alone came closest at the median survival mark in the pivotal clinical trial by Moore et al.4, using only the median incremental overall survival would lead to an underestimation of the true benefit of gem-e compared with gem alone and would result in an overestimation of the icer.
One limitation of our study is that cost structures may be different in other countries, which may limit the generalizability of our findings. Given that our model was robust to most of the cost variables except the drug cost, the applicability of our results to other countries depends mostly on the price of drugs in the other jurisdictions relative to the price in Ontario, Canada. For example, oxaliplatin is a generic drug in France, and therefore its price is substantially lower than it is in other countries in which it is still under patent. As a result, folfirinox would be considerably more cost-effective in France than in Canada.
Using cross-trial comparisons of results was also a potential limitation of our study. However, the patients included in the phase iii trials of gem, gem-cap, and gem-e were comparable in age, sex, performance status, and stage of pancreatic cancer. In the folfirinox phase iii clinical trial, those parameters are also similar, but the trial included only metastatic pancreatic cancer patients with an Eastern Cooperative Oncology Group performance status of 0 or 1. Despite those differences, a comparison of the overall survival results for patients in the gem arm of each trial shows a narrow range of 5.6–7.2 months1–5.
Utilities were obtained by using the EQ-5D to survey medical oncologists instead of surveying patients or the public directly. Surveying health care workers to obtain health utilities is regarded as a reasonable surrogate for a direct measurement of health utilities25. We used both one-way deterministic sensitivity analyses and probabilistic sensitivity analysis to examine the uncertainty with respect to utilities, and we found that our results were robust to utility variables.
Another potential limitation is that our study took the perspective of the Ontario mohltc as a single payer instead of a societal perspective. As a result, the indirect costs related to any loss of productivity incurred on patients or caregivers as a result of the toxicities of the various treatments were not captured in our study.
5. CONCLUSIONS
The most cost-effective treatment in metastatic pancreatic cancer depends on the societal wtp threshold. In Canada, folfirinox may be cost-effective if the societal wtp threshold is relatively high. When funded by public programs, new anticancer therapies should ideally be priced in proportion to their clinical benefit. That approach will help to ensure that the therapies are cost-effective, maximizing societal benefits and maintaining the sustainability of the country’s health care system.
6. ACKNOWLEDGMENTS
This work was presented in part at the 47th Annual Meeting of the American Society of Clinical Oncology; Chicago, Illinois, U.S.A.; June 4, 2011.
APPENDIX A: CHEMOTHERAPY REGIMENS
| Regimen | Agent | Starting dose | Schedule per cycle |
|---|---|---|---|
| Gemcitabine alone (gem) | gem | 1000 mg/m2 IV | Once weekly for 7 of 8 weeks for the first cycle, then 3 of 4 weeks |
| Gemcitabine–capecitabine (gem–cap) | gem | 1000 mg/m2 IV | Once weekly 3 of every 4 weeks |
| cap | 1660 mg/m2 daily PO | In divided doses twice daily for 3 of every 4 weeks | |
| Gemcitabine–erlotinib (gem–e) | gem | 1000 mg/m2 IV | Once weekly for 7 of 8 weeks for the first cycle, then 3 of 4 weeks |
| e | 150 mg PO | Once daily for the duration of each cycle | |
| folfirinox | Oxaliplatin | 85 mg/m2 IV | Once every 2 weeks |
| Irinotecan | 180 mg/m2 IV | Once every 2 weeks | |
| 5-Fluorouracil | 400 mg/m2 IV bolus, then 2400 mg/m2 IV continuous infusion over 46 h | Once every 2 weeks | |
| Folinic acid | 400 mg/m2 IV | Once every 2 weeks |
iv = intravenously; po = orally.
APPENDIX B: QUALITY OF LIFE IN ADVANCED PANCREATIC CANCER TREATED WITH CHEMOTHERAPY SURVEY
We are conducting a survey that uses expert opinion to explore the quality of life (qol) as estimated by a utility score in patients with advanced pancreatic cancer. Please read the following scenarios and scale qol according to the statements below each scenario.
- Demographics
Age: ❐ <30 ❐ 30–39 ❐ 40–49 ❐ 50–59 ❐ 60–69 ❐ 70+ Gender: ❐ Female ❐ Male Specialty: ❐ Medical oncology ❐ Other: __________________________________________ Years in practice: ❐ 0–4 ❐ 5–9 ❐ 10–14 ❐ 15–20 ❐ 20–24 ❐ 25–29 ❐ 30–34 ❐ 35+ -
A 60-year-old man presents with painless jaundice and was found on computed tomography (ct) imaging to have a pancreatic mass and multiple liver masses. A stent was placed in the common bile duct and a biopsy of the pancreatic mass confirmed pancreatic adenocarcinoma. The patient is treated with chemotherapy without any significant sideeffects.
Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
-
The patient is treated with chemotherapy and develops nausea and vomiting. He vomited 7 times and required intravenous (IV) hydration and IV antiemetics in the emergency department.
Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
-
The patient is treated with chemotherapy and develops severe diarrhea with 7 bowel movements per day and eventually requires hospitalization for IV fluids. He is discharged after 2 days.
Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
-
The patient is treated with gemcitabine and capecitabine chemotherapy. He develops hand–foot syndrome with erythema and peeling of his hands and feet. He has significant pain preventing him from buttoning his shirts and walking long distances.
Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
- The patient is treated with gemcitabine and capecitabine and develops severe mucositis. He is unable to eat or drink adequately due to the pain despite using mucositis mouthwash. He is admitted to hospital for IV hydration.

Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
- The patient is treated with chemotherapy and develops febrile neutropenia. He is admitted to hospital and treated with IV antibiotics. He is discharged after 5 days. Please take into account the potential side effects of antibiotics, including allergy, rash, and diarrhea.
Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
-
The patient is treated with chemotherapy and develops severe fatigue. He is not able to do his daily activities and spends more than half the day either in bed or sitting on his couch at home.
Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
-
The patient is treated with gemcitabine and erlotinib and develops an acneiform rash over his chest and back. It is pruritic and painful. Erlotinib is held, and he is given oral doxycycline and topical clindamycin.
Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
- The patient is treated with folfirinox and develops a sensory neuropathy, with numbness and tingling in his hands and feet. He has difficulty buttoning his shirts and finds it more difficult to walk.
Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
-
The patient is treated with chemotherapy, but after 3 cycles his restaging ct scans show that he has progressive disease. He remains asymptomatic and is treated with a different chemotherapy regimen. He tolerates treatment well without any significant sideeffects.

Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
-
The patient is treated with chemotherapy, but after 3 cycles his restaging ct scans show that he has progressive disease. He now has severe epigastric pain requiring morphine to control the pain. He is severely fatigued and has a diminished appetite. Along with the oncologist, he has decided that he does not want any further treatment, only supportive care.
Mobility: ❐ No problems walking ❐ Some problems walking ❐ Confined to bed Self-care: ❐ No problems with self-care ❐ Some problems washing or dressing himself ❐ Unable to wash or dress himself Usual activities: (e.g. work, study, housework, family or leisure activities) ❐ No problems with performing usual activities ❐ Some problems performing usual activities ❐ Unable to perform usual activities Pain/discomfort: ❐ No pain or discomfort ❐ Moderate pain or discomfort ❐ Extreme pain or discomfort Anxiety/depression: ❐ Not anxious or depressed ❐ Moderately anxious or depressed ❐ Extremely anxious or depressed Please estimate the patient’s health state on the scale below:
7. CONFLICT OF INTEREST DISCLOSURES
VCT has received honoraria from Sanofi–Aventis Canada Inc. YJK has received honoraria and research funding from Sanofi–Aventis Canada Inc. All other authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase iii trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–17. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Chau I, Stocken DD, et al. Phase iii randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–18. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase iii trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, et al. folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25:2607–15. doi: 10.1200/JCO.2006.09.2551. [DOI] [PubMed] [Google Scholar]
- 7.Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase iii trial of the Cancer and Leukemia Group B (calgb 80303) J Clin Oncol. 2010;28:3617–22. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philip PA, Benedetti J, Corless CL, et al. Phase iii study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group–directed Intergroup Trial S0205. J Clin Oncol. 2010;28:3605–10. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerriere DN, Zagorski B, Fassbender K, Masucci L, Librach L, Coyte PC. Cost variations in ambulatory and home-based palliative care. Palliat Med. 2010;24:523–32. doi: 10.1177/0269216310364877. [DOI] [PubMed] [Google Scholar]
- 11.Ontario Case Costing Initiative (occi) OCCI Costing Analysis Tool [Web resource] Toronto, ON: OCCI; n.d. [Available at: http://www.occp.com/mainPage.htm; cited December 15, 2011] [Google Scholar]
- 12.Lathia N, Mittmann N, DeAngelis C, et al. Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer. 2010;116:742–8. doi: 10.1002/cncr.24773. [DOI] [PubMed] [Google Scholar]
- 13.Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25:365–84. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 14.Ontario Ministry of Health and Long-Term Care (mohltc) Schedule of Benefits for Physician Services Under the Health Insurance Act [Web page] Toronto, ON: MOHLTC; n.d. [Available at: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/physserv_mn.html; cited November 10, 2010] [Google Scholar]
- 15.Ontario Ministry of Health and Long-Term Care (mohltc) Ontario Health Insurance (OHIP) Schedule of Benefits and Fees: Schedule of Benefits for Laboratory Services [Web page] Toronto, ON: MOHLTC; n.d. [Available at: http://www.health.gov.on.ca/english/providers/program/ohip/sob/lab/lab_mn.html; cited November 10, 2010] [Google Scholar]
- 16.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–81. [PMC free article] [PubMed] [Google Scholar]
- 17.Kuntz KM, Tsevat J, Goldman L, Weinstein MC. Cost-effectiveness of routine coronary angiogram after acute myocardial infarction. Circulation. 1996;94:957–65. doi: 10.1161/01.CIR.94.5.957. [DOI] [PubMed] [Google Scholar]
- 18.Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med. 1998;13:716–17. doi: 10.1046/j.1525-1497.1998.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry SR, Bell CM, Ubel PA, et al. Continental divide? The attitudes of U.S. and Canadian oncologists on the costs, cost-effectiveness, and health policies associated with new cancer drugs. J Clin Oncol. 2010;28:4149–53. doi: 10.1200/JCO.2010.29.1625. [DOI] [PubMed] [Google Scholar]
- 20.Tan–Torres Edejer T, Baltussen R, Adam T, et al., editors. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva, Switzerland: World Health Organization; 2003. [Available at: http://www.who.int/choice/publications/p_2003_generalised_cea.pdf; cited August 5, 2012] [Google Scholar]
- 21.The World Bank . Data: GDP per capita (current US$) [Web page] Washington, DC: The World Bank; n.d. [Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD; cited February 12, 2013] [Google Scholar]
- 22.Attard CL, Brown S, Alloul K, Moore MJ, on behalf of the Cornerstone Research Group Inc Cost-effectiveness of folfirinox for first-line treatment of metastatic pancreatic cancer [abstract 199] J Clin Oncol. 2012. p. 30. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=115&abstractID=88317; cited January 8, 2013] [DOI] [PMC free article] [PubMed]
- 23.Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30:1316–20. doi: 10.1200/JCO.2011.38.6078. [DOI] [PubMed] [Google Scholar]
- 24.Miksad RA, Schnipper L, Goldstein M. Does a statistically significant survival benefit of erlotinib plus gemcitabine for advanced pancreatic cancer translate into clinical significance and value? J Clin Oncol. 2007;25:4506–7. doi: 10.1200/JCO.2007.13.0401. [DOI] [PubMed] [Google Scholar]
- 25.Suarez–Almazor ME, Conner–Spady B. Rating of arthritis health states by patients, physicians, and the general public. Implications for cost-utility analyses. J Rheumatol. 2001;28:648–56. [PubMed] [Google Scholar]