Abstract
The aging population is at an increased risk of tendon injury and tendinopathy. Elucidating the molecular basis of tendon aging is crucial to understanding the age-related changes in structure and function in this vulnerable tissue. In this study, the structural and functional features of tendon aging are investigated. In addition, the roles of decorin and biglycan in the aging process were analyzed using transgenic mice at both mature and aged time points. Our hypothesis is that the increase in tendon injuries in the aging population is the result of altered structural properties that reduce the biomechanical function of the tendon and consequently increase susceptibility to injury. Decorin and biglycan are important regulators of tendon structure and therefore, we further hypothesized that decreased function in aged tendons is partly the result of altered decorin and biglycan expression. Biomechanical analyses of mature (day 150) and aged (day 570) patellar tendons revealed deteriorating viscoelastic properties with age. Histology and polarized light microscopy demonstrated decreased cellularity, alterations in tenocyte shape, and reduced collagen fiber alignment in the aged tendons. Ultrastructural analysis of fibril diameter distributions indicated an altered distribution in aged tendons with an increase of large diameter fibrils. Aged wild type tendons maintained expression of decorin which was associated with the structural and functional changes seen in aged tendons. Aged patellar tendons exhibited altered and generally inferior properties across multiple assays. However, decorin-null tendons exhibited significantly decreased effects of aging compared to the other genotypes. The amelioration of the functional deficits seen in the absence of decorin in aged tendons was associated with altered tendon fibril structure. Fibril diameter distributions in the decorin-null aged tendons were comparable to those observed in the mature wild type tendon with the absence of the subpopulation containing large diameter fibrils. Collectively, our findings provide evidence for age-dependent alterations in tendon architecture and functional activity, and further show that lack of stromal decorin attenuates these changes.
Keywords: Tendon, Aging, Biglycan, Decorin, Proteoglycan, Extracellular matrix
1. Introduction
Tendons are composed of uniaxially arranged collagen fibrils, organized as fibers (fibril bundles) that provide the tensile properties required for transmission of force from muscle to bone. Small leucine rich proteoglycans (SLRPSs) have been implicated in regulation of collagen fibril assembly and therefore tendon structure and function. As tendons age, there is an increased risk for injury (Maffulli et al., 1999), yet the molecular basis of tendon aging has not been elucidated. Understanding the roles of these molecules during the aging process is instructive to developing an understanding of the aging tendon itself. The predominant SLRPs in tendon are decorin and biglycan—class I SLRPs, with one or two chondroitin sulfate glycosaminoglycan (GAG) chains, respectively (Iozzo, 1999). Fibromodulin and lumican, class II SLRPS, also are expressed in the tendon. Fibromodulin has keratin sulfate GAG chains while lumican is present in the glycoprotein form (Jepsen et al., 2002). Researchers have not shown that GAG chains directly influence viscoelastic properties in tendons and ligaments (Lujan et al., 2007, 2009; Fessel and Snedeker, 2009, 2011), thereby suggesting that GAGs’ role is primarily organizational (Rühland et al., 2007) as opposed to mechanical. Both classes of SLRPs have been implicated in regulation of tendon structure and function (Zhang et al., 2005). However, the roles of these molecules are both tissue-specific and tendon-specific (Zhang et al., 2006; Dourte et al., 2012).
Decorin-null mice (Dcn−/−) exhibit fragile skin and abnormal fibril morphology (Danielson et al., 1997). Decorin also has been implicated as a mediator of lateral fibril growth (Birk et al., 1995; Reed and Iozzo, 2002) and shown to correlate with both size and density of collagen fibrils (Watanabe et al., 2005). Biglycan-null mice (Bgn−/−) have a known tail tendon fibril phenotype marked by greater irregularity in both size and shape (Corsi et al., 2002). In addition, these mice have diminished bone mass, that becomes increasingly pronounced with age, have been used as a model for osteoporosis (Xu et al., 1998; Young et al., 2002), and suffer ectopic ossification of their tendons (Kilts et al., 2009). A mechanism by which biglycan, with fibromodulin, affects the differentiation of tendon stem/progenitor cells (TSPCs) has been proposed to explain these effects (Bi et al., 2007). Currently, SLRPs are generally accepted to be capable of facilitating and influencing a myriad of processes, including collagen fibril growth and degradation, inflammation, and cell matrix cross-talk (Merline et al., 2009). For these reasons, Bgn−/− and Dcn−/− mice are excellent models for studying how these molecules regulate structure–function relationships in tendon aging.
The prevalence of decorin and biglycan in tendon tissue has been well documented; however, the specific functions of these molecules in the tendon extracellular matrix remain areas of active research (Robinson et al., 2004a, 2004b, 2005; Dourte et al., 2012). Biglycan has been shown to be expressed at particularly high levels during early development of tendon structure while decorin expression rises and remains relatively constant in the mature tendon (Zhang et al., 2006; Ansorge et al., 2011). Although the effects of SLRPs have been studied extensively in developing tendons, their roles in the aging process have not been examined. Because the tendon injuries are more common in people of a more advanced age (Maffulli et al., 1999), there is particular impetus to investigate the relationship of SLRPs to the aging tendon.
Previous research has described a decline in tendon mechanics with age, possibly as a result of increased levels of collagen V (Dressler et al., 2002). An increase in crosslinking of collagen with age also has been established (Bailey and Shimokomaki, 1971). In equine superficial digital flexor tendons and in mice tibialis anterior tendons, aging was shown to increase the modulus (Gillis et al., 1995; Wood et al., 2011). Although biglycan absence has been linked to accelerated decline of intervertebral disks (Furukawa et al., 2009), little is known about SLRPs’ influence on the aging process in tendon.
Therefore, the objective of this study was to investigate the structural and functional features of tendon aging. In addition, the roles of decorin and biglycan in the aging process were analyzed using transgenic mice as model organisms at both mature and at aged time points. We hypothesized that the increase in tendon injuries that is seen in the aging population is the result of altered structural properties which, in turn, reduce the biomechanical properties of the tendon and consequently increase susceptibility to injury. With the knowledge that decorin and biglycan are important regulators of tendon structure (and therefore function), we further hypothesized that decreased function in aged tendons is partly the result of altered decorin and biglycan expression.
2. Results
2.1. Aging affects mechanical properties of tendons
The effect of aging on tendon function was determined from comparisons of tendons from mature and aged wild type mice. An analysis of the mechanical properties of mature (150 day) and aged (570 day) patellar tendons demonstrated functional alterations associated with aging. Specifically, the data reveal that aged tendons produce significantly less stress in response to an induced strain. In addition, aged tendons are more viscous than mature tendons, i.e. more dissipative of applied force. These analyses of the mechanical properties of aged versus mature tendons demonstrate a functional deficit in the aged tendons.
The dynamic modulus, a viscoelastic property of the tendon, was significantly reduced in aged versus mature tendons across all tested strain levels and frequencies [Fig. 1A,B]. The dynamic modulus is defined as the ratio of induced stress to applied strain during low-amplitude cyclic loading. Thus, tendons with a greater dynamic modulus produce a greater stress for a given strain. Likewise, tendons with a lower dynamic modulus are more easily strained. Thus, aged tendons compared to mature tendons cannot produce the same stress when strain is applied. The decreased dynamic modulus of aged tendons therefore indicates less resistance to strain and an associated decreased ability to properly transfer force.
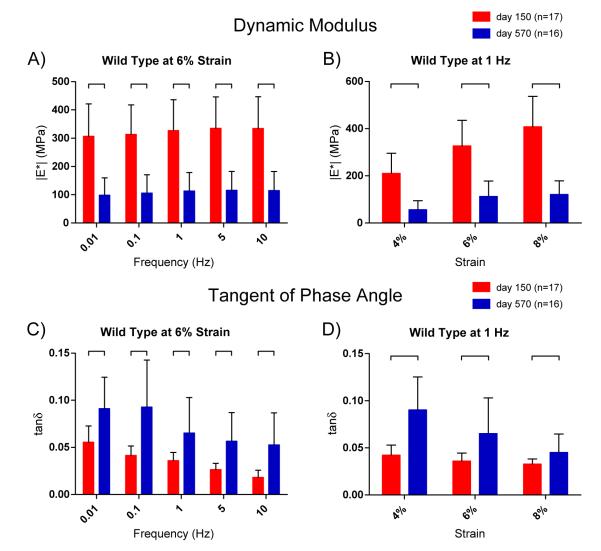
Fig. 1.
Dynamic mechanical properties of mature and aged patellar tendons. [A] The dynamic modulus (∣E*∣: the ratio of stress to strain measured in oscillatory applied displacement) at 6% strain for WT tendons at 150 and 570 days old. Aged tendons exhibited significantly decreased dynamic modulus revealing greater deformability at all frequencies. [B] Dynamic modulus at 1 Hz, shown at 4%, 6% and 8% strains. Results were consistent across all strains and frequencies (see Table 1, Supplemental Data S1). [C] Tangent of phase angle δ at 6% for WT tendons (δ: the angular gap between peaks of stress and strain sinusoids; tanδ: equal to ratio of dissipated force to stored force). Aged tendons had significantly higher phase angles demonstrating increased viscoelasticity and force dissipation. [D] Phase angle at 1 Hz, shown for 4%, 6% and 8% stains. Significance bars denote p≤0.05.
The phase angle also provides a measure of tissue viscosity, or ability to dissipate force. In a viscoelastic tissue like tendon, there is a small delay between stress and strain. This delay is called the phase angle and is measured in degrees between the peaks of the strain and stress sinusoids. The tangent of the phase angle–hereafter referred to as “tanδ”–is equal to the ratio of dissipated energy to stored energy. Tanδ was calculated for the patellar tendons of mature and aged mice and was significantly increased for aged tendons versus mature across all strains and frequencies [Fig. 1C,D]. Therefore, the aged tendons dissipated significantly more force compared to the mature tendons, which in turn compromises their ability to effectively transmit force.
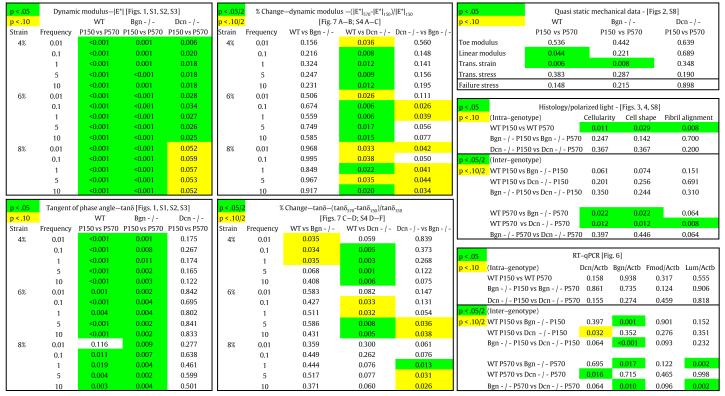
One of the ancillary goals of this study was to examine the differences observed at across the range of employed strain–frequency combinations. Our data suggests that dynamic modulus increases dramatically at greater strains, but the effect of frequency is less evident [Table 1, Fig. S1–S3]. Tanδ decreases with both strain and frequency [Table 1, Fig. S1–S3]. Finally, the effects of aging seem generally more pronounced at lower strains and higher frequencies [Table 1, Fig. S4].
Table 1.
All calculated p-values.
|
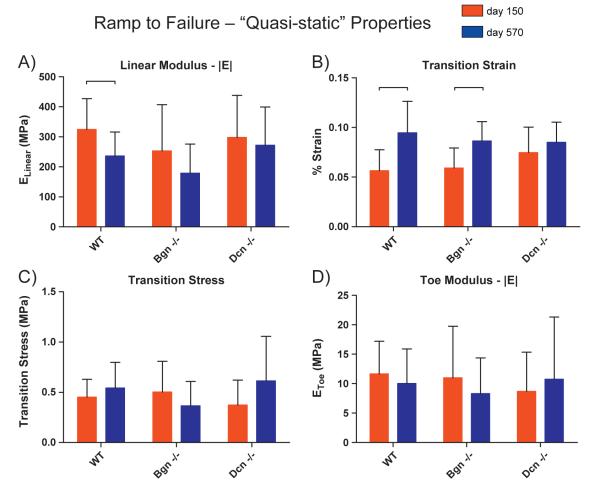
Quasi-static (as opposed to dynamic) properties were also evaluated and further demonstrated the deteriorating properties of the aging tendon. The last step of our mechanical testing protocol consisted of a slow ramp to failure where stress and strain were continually measured. The stress–strain curve was then approximated by a bilinear fit [Supplemental Fig. S1] providing “toe” and “linear” regions, the slopes of which are the toe linear moduli. After the “transition” strain, the incremental production of stress increases much more rapidly. The aged tendons underwent this transition significantly later [Fig. 2B] and exhibited a significantly lower linear modulus [Fig. 2A] than the mature tendons. Both parameters further reveal a decreased ability of aged tendons to produce stress when strained.
Fig. 2.
Quasi-static mechanical properties of mature and aged patellar tendons across genotypes. [A] A decrease in linear modulus was detected for WT only (p=0.04), revealing that WT tendons become more easily deformable with age. [B] WT and Bgn−/− had significant increases with age in transition strain, which is indicative that with age, these genotypes’ tendon properties are changing in ways not observed in Dcn−/−. [C] No significant differences were observed for transition stress. [D] No significant differences by age were observed for toe modulus for any genotype. Significance bars denote p≤0.05.
2.2. Aging affects tendon structure
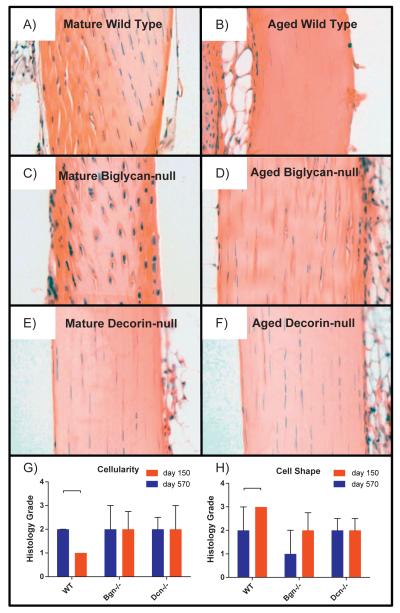
The mechanical data indicate a functional deficit in aged tendons. Our hypothesis predicts a structural basis for altered function. Therefore, changes in overall tendon structure, fiber orientation and fibril structure were analyzed in mature and aged patellar tendons. The gross structures of mature and aged tendons were analyzed in hematoxylin and eosin stained longitudinal sections [Fig. 3A,B]. The overall structures were comparable with no obvious differences in tendon architecture or in cellular composition. However, there was a significant decrease in cellularity (number of tenocytes) observed in aged versus mature tendons [Fig. 3G]. The decreased cellularity was associated with an elongation of tenocytes in aged compared to mature tendons [Fig. 3H].
Fig. 3.
Comparisons of morphological features of tendons across age and genotype. WT tendons exhibited significant decreased cellularity (number of cells) with age [G] and significantly elongated (more spindle like) cell shape [H]. Mean with interquartile range. Significance bars denote p≤0.05. Note: Grading performed on original images [Supplemental Data S9]. For publication, filters applied to images [A–F]: auto-tone, auto-contrast, and auto-color (all three to each individually). Contrast decreased and brightness increased (to all at once). Cropped.
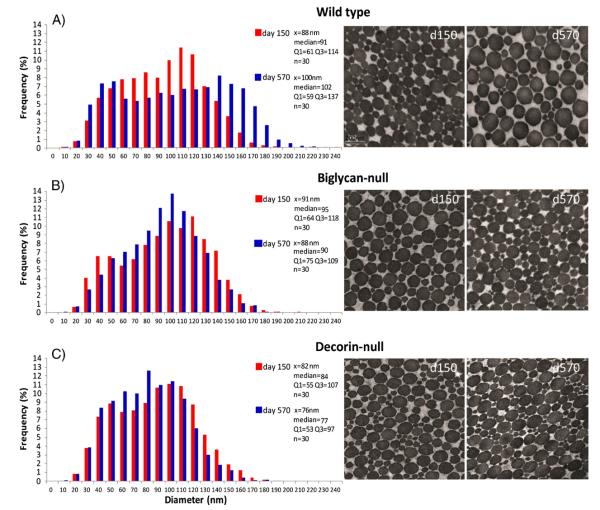
Collagen fibrils organized as fibers (fibril bundles) influence the mechanical properties of tendons. Collagen fiber alignment in mature versus aged tendons was analyzed using polarized light microscopy. Tendons showed significantly decreased collagen fiber alignment in the aged compared to mature tendons [Fig. 4A]. In addition, the structure of the fibrils making up the fibers was analyzed using transmission electron microscopy in mature and aged patellar tendons [Fig. 5A]. The aged fibril structure was distinctly different from the mature. Specifically, the fibril distribution is substantially broader in the aged tendons. This was associated with a shift to a distinct bimodal distribution in the aged tendons characterized by a subpopulation of large diameter fibrils centered around 140 nm.
Fig. 4.
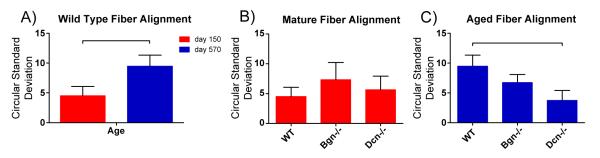
Collagen fiber alignment by age and genotype. Mean with standard deviation. Circular standard deviation is a measure of un-alignment. Lower values reflect more aligned fibrils. [A] WT tendon collagen is significantly less aligned with age. [B] There are no significant differences in alignment across genotypes in mature tendons. [C] Dcn−/− at day 570 is significantly more aligned than WT at day 570—which may explain the reduced decrease in mechanical properties with age. Significance bars denote p≤0.05.
Fig. 5.
Collagen fibril diameter measurements by age and genotype. Transmission electronmicroscopymeasured distribution of fibril diameters at P150 and P570 for [A]WT, [B] Bgn−/− and [C] Dcn−/−. With aging, WT undergoes a shift to a bimodal distribution with an increase in larger fibrils. Bgn−/− converges toward a single mean. Dcn−/− experiences the least change with age.
Aged tendons exhibited decreased collagen alignment and possessed more large-diameter collagen fibrils as compared to mature tendons. In addition, aged tendons exhibited fewer, more elongated cells. Overall, these data suggest that alterations in fibril structure and alignment are the structural basis of the mechanical differences observed between mature and aged tendons.
2.3. Decorin is associated with changes in fibril structure and fiber alignment in aged tendons
SLRPs have been implicated in the regulation of collagen fibril and tendon matrix assembly and the expression of decorin and biglycan in tendons is well established. Thus, our hypothesis is that alterations in these SLRPs are responsible for the age related changes in tendon structure and therefore function. To address the roles of decorin and closely related biglycan, two well characterized mouse models deficient in decorin or biglycan expression were utilized. Patellar tendons from mature (day 150) and aged (day 570) decorin-null and biglycan-null mice were analyzed and compared with the wild type data.
Histological analysis did not reveal differences between the wild type and null genotypes in overall architecture or cellular composition [Fig. 3]. However, unlike the wild type tendons, no significant differences in cellularity [Fig. 3A] or cell shape [Fig. 3B] were detected between mature and aged decorin- and biglycan-null tendons. Thus in the absence of decorin expression, tenocyte number and shape were unaltered in decorin-null aged tendons.
The relationship between fiber alignment and decorin and biglycan expression was analyzed using mature and aged tendons from the mutant genotypes. The significant decrease in fiber alignment observed in the mature versus aged wild type tendons was not present in either decorin- or biglycan-null tendons. In fact, fiber alignment was significantly greater in the aged tendons of decorin-null mice than in wild type [Fig. 4C]. These data suggest an association between decorin expression and decreased fiber organization in aged tendons.
The structure of the fibrils was analyzed in mature and aged patellar tendons from biglycan- and decorin-null tendons [Fig. 5B,C] and compared to wild type tendons. The collagen fibril diameter distribution in biglycan- and decorin-null tendons did not demonstrate the changes normally associated with aging. At day 570, both mutant genotypes have fibril diameter distributions comparable to the mature wild type tendons. These distributions did not broaden and develop the subpopulation of larger diameter fibrils observed in the day 570 wild type mice; rather a mature distribution was maintained comparable to that in day 150 wild type mice. Overall, the mature and aged distributions for these mutant genotypes were similar and comparable to the wild type mature distribution [Fig. 5].
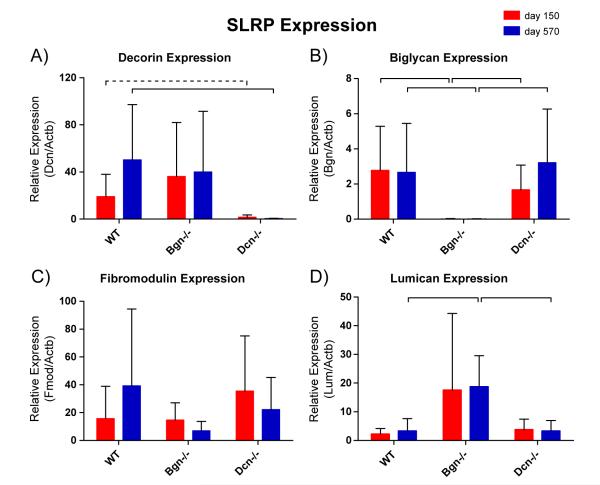
We analyzed the expression of SLRPs in the wild type and mutant genotype to address possible compensation. In wild type tendons, decorin expression was increased in aged tendons, but the increase did not reach statistical significance [Fig. 6A]. However, the relative decorin/beta-actin transcript ratio averaged 50.3 in aged tendons and 18.5 in mature tendons (p=0.16), Therefore, upregulation of decorin with age should not be categorically ruled out. No significant differences in expression by age were detected for biglycan, decorin, lumican, or fibromodulin [Fig. 6A–D]. In addition, the data confirmed the lack of decorin and biglycan expression in respective transgenic animal models, as expected [Fig. 6A,B]. The only suggestion of compensation was the significantly increased expression of lumican in the aged biglycan-null tendons.
Fig. 6.
RT-qPCR for SLRP expression. Biglycan [A] and decorin [B] are effectively suppressed in respective knockouts. The apparent increase in decorin expression with age for WT did not reach statistical significance. [C] Fibromodulin is evidently neither up- nor down-regulated by either genotype or age. [D] Lumican expression is significantly greater in the Bgn−/− genotype at P570. Significance bars denote p ≤0.05; dashed bars denote p≤0.1.
Taking these findings together, the patellar tendons of decorin-null mice do not undergo the changes in fibril structure and fiber alignment normally associated with aging. This suggests that continued decorin expression is involved with tendon aging.
2.4. Decorin is involved in the alteration of viscoelastic mechanics in aged tendons
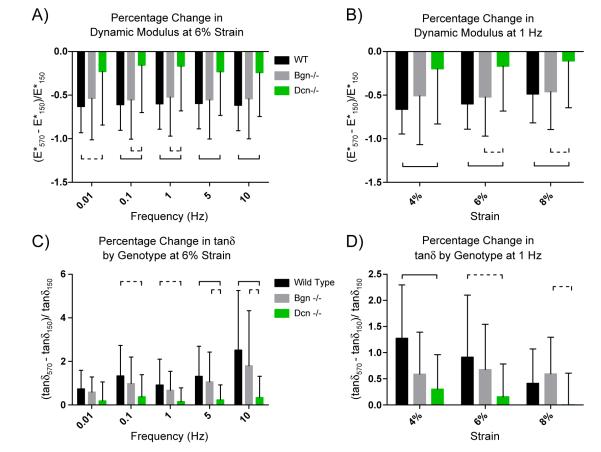
The absence of decorin expression in the null tendons ameliorated the mechanical deficiencies seen in the aged tendon. Presumably because the fibril structure and fibril alignment does not change with age as it does in wild type tendons, the decline in mechanical properties associated with aging was far less substantial for the decorin-null tendons. While the trends observed in the wild type tendons were seen again in the knockout mice, the decrease in dynamic modulus with age was significantly smaller for decorin-null than it was in wild type. Furthermore, the difference in tanδ with age in the decorin-null was not significant at any strain or frequency [Supplemental Fig. S3]. Of the three genotypes, Dcn−/− had the smallest percentage change in dynamic modulus and tanδ at every strain–frequency combination [Fig. 7; Table 1 for statistics]. These data indicate that the absence of decorin prevents the normal tendon aging process which in turn suggests that decorin expression is a major contributing factor in tendon aging.
Fig. 7.
Percentage change relationships of biomechanical data suggest decorin expression is a major factor in tendon aging. [A] Within-genotype percentage change with age for dynamic modulus at 6% strain. The percentage change for Dcn−/− is significantly smaller than it is for WT. This means that the increase in deformability (decrease in modulus) that is associated with age, is much less pronounced for Dcn−/− tendons. [B] Within-genotype percentage change with age for dynamic modulus at 1 Hz. The percentage decline is smaller for Dcn−/− than for wild type or Bgn−/− [C] Within-genotype percentage change with age for tangent of phase angle at 6% strain. The percentage change for Dcn−/− is significantly smaller than it is for WT or Bgn−/−. Thus, the increase in viscosity associated with age is less pronounced in the Dcn−/− tendons. [D] Within-genotype percentage change with age for tangent of phase angle at 1 Hz. Dcn−/− shows the least percentage change. Significance bars denote p≤0.05; dashed bars denote p≤0.1. See SD 4 for 4% and 8% strains; Table 1 for complete statistics.
3. Discussion
The importance of biglycan and decorin to tendon development and function has been known for some time. However, this is the first study to characterize the roles of decorin and biglycan in tendon aging. A multidisciplinary approach was utilized to define the relationship between structural and functional changes in the aged tendon. Biomechanical and structural analyses defined changes in the mechanical properties of aged versus mature wild type patellar tendons. These changes correlated with changes in tendon structure including tenocyte number, fiber alignment and fibril structure. Using mouse models null for decorin or biglycan, a role for decorin in the age related changes in both mechanical properties and structure was demonstrated.
With age, tendons become less stiff and are less able to transmit forces. This is illustrated by the decrease in dynamic modulus and increase in phase angle observed in aged tendons regardless of genotype. This allows us to characterize aged murine patellar tendons as more easily strained and as more viscous (or dissipative). The tendons of both modified genotypes exhibited differences from wild type tendons. The biglycan-null and decorin-null tendons exhibited markedly different mechanics from one another that were not reflected in structural parameters. It is possible that properties not directly measured in this study (e.g. cross-linking) could contribute to age-related mechanical changes and explain this apparent discrepancy. A key finding in this work is that structural and functional changes associated with tendon aging were markedly less pronounced in decorin-null tendons, indicating that decorin expression is associated with tendon aging.
Overall, this study demonstrates that the aging process in decorin-null tendons is distinct from that of wild type and biglycan-null tendons. These findings are totally unexpected given the major role that decorin plays in maintaining proper dermal and tendon function in both embryonic and adult life. Collectively, our data indicate that decorin expression within tendons has negative effects on the overall aging processes of these structures. Whether this is due to a mechanical or a signaling property of this SLRP is the focus of future studies. Nonetheless, the absence of decorin prevents the natural decline in mechanical properties and the increased propensity to injury, the two hallmarks of aging in tendons.
Decorin was consistently expressed in the aged wild type tendons. The increased fibril diameters seen in aged wild type tendons, but not in aged decorin-null tendons indicate that a continuous presence of decorin is involved in the lateral growth of fibrils associated with tendon aging. Early work suggested that during development decorin inhibits lateral fibril growth (Scott and Orford, 1981). However, decorin is associated with increased collagen fibril diameter in vitro (Kuc and Scott, 1997; Rühland et al., 2007). In addition, decorin is associated with increased fibril diameters in cartilage (Hagg, et al., 1998). It has been argued that decorin’s role is better described as mediation—which is highly variable and dependant on local factors (Reed and Iozzo, 2002). Decorin is important in the regulation of tissue-specific fibril structure. We speculate that during aging continued decorin–fibril interactions result in facilitated lateral growth of mature fibrils resulting in altered structural and functional properties in aged tendons. This is similar to the known regulatory role of fibromodulin in lateral fibril assembly and growth (Ezura, et al., 2000). Additionally, the changes associated with patellar tendinopathy have been attributed to metabolic turnover instead of expression (Samiric et al., 2009) and unlike aggrecan and versican, decorin and biglycan are catabolized slowly, with a half-life over twenty days (Samiric et al., 2004). The presence or absence of decorin may modulate this process acting to regulate turnover via interaction with catabolic enzymes therefore regulating accessibility.
Decorin serves an important role in regulating fibril development, growth, fusion, and orientation during tendon development. However, the regulatory role of decorin during advanced aging has not been previously described. Our data suggest that in aging tendons, continued expression of decorin is associated with the presence of a subpopulation of larger diameter fibrils. Fibril alignment is known to lead to enhanced mechanical properties (Lake et al., 2009). Since fibrils with a wide distribution of sizes pack poorly, this latter effect is likely to reduce fibril area fraction. Furthermore, the larger fibrils associated with aging tendon may be more susceptible to damage. Therefore, we propose that decreased mechanical properties (lower ∣E*∣ and higher tanδ) in aging wild-type mice are a result of decreased alignment, reduced fibril area fraction, and increased fragility. Thus, age-related alterations in the mechanical properties of tendon are at least partially the result of deleterious effects induced by decorin’s continued influence on lateral fibril growth and structure during the aging process. Additionally, because aged decorin-null tendons had a higher dynamic modulus than aged wild type tendons, our results support the notion that decorin does not play a direct role in fibril to fibril force transmission.
Our dynamic data revealed marked differences with age that were generally mirrored in the static data; however, the differences in the quasi-static data were less pronounced. Static measures of linear modulus may not reflect typical in vivo loading of the tendon. Alternatively, dynamic oscillation at physiological strains may more accurately mimic normal repetitive activities, such as walking or running for the patellar tendon. The observation that aged tendons are most unlike mature tendons at lower strains and higher frequencies may suggest that aged tendons differ more dramatically from mature tendons in activities marked by repetition or speed than they differ in activities marked by large loads or strains. The study of these dynamic properties is relatively new and is not to be confused with often reported quasi-static measures such as stiffness, which may not be as sensitive.
This study provides new insights into the roles of biglycan and decorin, especially as they relate to the aging process. However, this study is not without limitations. We were not able to isolate temporal changes in roles of SLRPs. Additionally, in our model, it is not possible to completely delineate the influence of SLRPs’ multiple effects—for example, during development and in mature tendon. Future research will separate the effects of cumulative SLRP deficiency and the present-time roles of these molecules. Additionally, we are working to further elucidate the roles of these molecules in other tendons, during development and in the response to injury.
4. Experimental procedures
4.1. Animals and sample collection
A total of 102 C57BL/6 wild-type (WT), decorin transgenic null (Dcn−/−), and biglycan transgenic null (Bgn−/−) female mice were used in this study. Animals were sacrificed at either mature (150 days old) or aged (570 day old) time points. WT mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and transgenic mice were bred by the authors at the University of South Florida and shipped to the University of Pennsylvania prior to sacrifice. Animal use was approved by The University of Pennsylvania and University of South Florida’s Institutional Animal Care and Use Committees.
Immediately after euthanizing the mice, hind limbs were removed, and a randomly selected limb was wrapped in phosphate buffered saline (PBS) soaked gauze, then frozen for later biomechanical testing. The skin from the contralateral limb was removed, and further dissected under magnification. The patellar tendon was longitudinally bisected and then prepared for a randomly selected two of three assays: transmission electron microscopy, PCR, and histology. TEM samples were immediately placed in fixative (described in Section 4.5). PCR samples were flash frozen in liquid nitrogen and then stored in at −80 °C freezer. Histological samples retained the limb’s musculoskeletal architecture and were immediately fixed in formalin.
4.2. Biomechanical testing
Patella–patellar tendon–tibia complexes were carefully isolated by dissection under magnification and tendons were stamped into a “dog-bone shape.” Before and after stamping, tendon cross-sectional area was measured with a custom, laser-based device. The distal half of the tibia was potted in polymethyl methacrylate (PMMA) and the greater tuberosity was secured with a staple. Tendons were speckled with Verheoff’s stain for optical tracking. The pot and the patella were gripped with custom fixtures and loaded into a materials testing system (Model 5848, Instron; Norwood, MA) with a 10 N load cell and submerged in a 37 °C PBS bath. Samples were tested using a modified version of an established protocol (Lujan et al., 2009; Dourte et al., 2012). This protocol consisted of preloading and preconditioning followed by dynamic testing (including stress relaxation and sinusoidal frequency sweeps) at 4%, 6%, and 8% strains. Each frequency sweep included 10-cycle oscillations at an amplitude of 0.125% strain and frequencies 0.01, 0.1, 1, 5 and 10 Hz. Because tendon is a non-linear viscoelastic material, its mechanical properties depend on both strain and frequency. Therefore our tests were designed to investigate the potential effects of these different strains and frequencies. After a return to gauge length, a ramp to failure at 0.1%/s was performed to measure quasi-static properties using optical tracking, enabled by a digital camera that was used to capture two images per second throughout the test.
4.3. Histology/polarized light microscopy
Fixed samples were treated with Immunocal (Decal Chemical Corporation, Tallman, NY) to decalcify bone, embedded in wax, sectioned into 7 μm slices, stained with hematoxylin and eosin, and imaged at 200× magnification with traditional and polarized light. Traditional light images were evaluated by three blinded graders for cell shape and cellularity. Polarized light images were quantitatively evaluated with custom software for collagen fiber alignment.
4.4. RT-qPCR
Expression levels of biglycan, decorin, lumican, and fibromodulin were quantified using a real-time quantitative polymerase chain reaction assay (qPCR). Total RNA was isolated from individual samples by mechanically homogenizing tissues with a Tissue-Tearor (Model 398, Biospec Products, Inc., Bartlesville, OK) in QIAzol reagent (Qiagen, Valencia, CA) and using the RNeasy Lipid Kit (Qiagen) as described by the manufacturers. The mRNA of 25 ng of total RNA was reverse transcribed into cDNA and amplified using the WT-Ovation RNA Amplification System (NuGEN, San Carlos, CA). The resulting Single Primer Isothermal Amplification (SPIA) product was purified with a QIAquick PCR Purification Kit and the amplified cDNA was eluted with 30 μl. For qPCR analysis, 1 μl of amplified cDNA template was added to a reaction volume of 20 μl per well in an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) with a Fast SYBR Green Master Mix (Applied Biosystems). Mouse specific primers for β-actin (Actb: F— AGATGACCCAGATCATGTTTGAGA; R— CACAGCCTGGATGGCTACGT), decorin (Dcn: F— GCTGCGGAAATCC GACTTC; R— TTGCCGCCCAGTTCTATGAC), biglycan (Bgn: F— CCTTCCG CTGCGTTACTGA; R— GCAACCACTGCCTCTACTTCTTATAA), fibromodulin (Fmod: F— GAAGGGTTGTTACGCAAATGG; R— AGATCACCCCCTAGT CTGGGTTA) and lumican (Lum: F— TCCACTTCCAAAGTCCCTGCAAGA; R— AAGCCGAGACAGCATCCTCTTTGA) were used. The amplified cDNA for each individual patellar tendon sample was analyzed in triplicate with a single negative RT control (0.83 ng total RNA per well) for each sample and each gene. Gene specific efficiencies were calculated using LinRegPCR v7.5 software for each qPCR plate and the relative quantity of mRNA for each gene of interest was computed using the comparative efficiency (ΔΔCT) method relative to Actb (Ramakers et al., 2003; Schefe et al., 2006). Outliers were removed if their measured expression was greater than two standard deviations from the mean. Statistical analysis was done by t-test.
4.5. Transmission electron microscopy
Tendon bisections were fixed in “Karnovsky’s Fixative,” a specialized solution containing 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate and 8 mM CaCl2, adjusted to pH 7.4 with NaOH. After 15 min, samples were placed on a rocker in a 4 °C cold-room for at least 2 h, then transferred to “transport buffer,” a glucose enriched version of the above, and cold-shipped to USF. Tendons were then removed from transport buffer, rinsed with cacodylate buffer and post-fixed for 1 h with 1% osmium tetroxide, dehydrated in an ethanol series followed by 100% propylene oxide, infiltrated and embedded over a 3 day period in a mixture of Embed 812, nadic methyl anhydride, dodecenylsuccinic anhydride and DMP-30 (EM Sciences, Fort Washington, PA) and polymerized over-night at 60 °C. Ultra-thin, approximately 90 nm, cross-sections were prepared using a Leica ultramicrotome and post-stained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. The sections were examined and imaged at 80 kV using a JEOL 1400 transmission electron microscope (JEOL Ltd., Tokyo, Japan) equipped with a Gatan Orius widefield side mount CC Digital camera (Gatan Inc., Pleasanton, CA).
Tendon diameter analysis was obtained from pooled data from one tendon from each of five mice of the same genotype and age. Six digital images from each tendon were taken from non-overlapping areas at ×60,000. Images were randomized and masked before fibril diameters were measured using a RM Biometrics-Bioquant Image Analysis System (Nashville, TN). A region of interest (ROI) of appropriate size was determined within the image so that a minimum of 80 fibrils were measured from each image. All fibrils in the region of interest were measured and multiple regions of interest were used if necessary to collect at least 80 fibril diameter measurements per image. Fibril diameters were measured along the minor axis of the fibril cross-section. Tendon diameter measurements were pooled into groups by age and genotype.
4.6. Data analysis
Using load and displacement data recorded by the Instron, cross-sectional area, and length (obtained from calibrated images), the dynamic modulus, ∣E*∣ (the ratio of stress amplitude to strain amplitude), was calculated for each tendon at each strain and frequency. The phase angle, δ, between the stress and strain curves was also calculated. Tanδ gives the ratio of viscous dissipation to elastic storage.
To make comparisons across genotypes, examining the magnitude of changes associated with aging, established bootstrapping techniques (Hall, 1992) were employed—using software to create 1000 random pairings and then outputting the median values. This allows for statistical comparison when matched pair sample values are not possible. From this ratio, 1 is subtracted to arrive at a percentage change associated with aging.
From the optical data, calculated with a bilinear fit custom program, toe and linear moduli, as well as transition stress and strain were obtained for each sample.
For dynamic and static comparisons across age within each genotype, as well as for percentage change across genotype, two-tailed Student’s t-tests assuming equal variance were used to evaluate significant differences at each strain–frequency combination. PCR statistics were likewise calculated via two-tailed Student’s t-tests. For histological statistics, one-tailed Mann–Whitney tests were used for cellularity and cell shape, while two-tailed Mann–Whitney tests were used for circular standard deviation. For all mechanical, PCR, and histologic comparisons, significance was set at p<0.05 and a trend was defined as p<0.1 for comparisons across age within genotype; for comparisons across genotypes, where each group was statistically tested in two comparisons, at p<0.05/2 and p<0.1/2 [Table 1].
Supplementary Material
Acknowledgments
This study was funded by NIH grant 5R01AR055543. The authors would like to thank LeAnn Dourte for helpful discussions.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.matbio.2012.11.005.
References
- Ansorge HL, Adams S, Birk DE, Soslowsky LJ. Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann. Biomed. Eng. 2011;39(7):1904–1913. doi: 10.1007/s10439-011-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AJ, Shimokomaki MS. Age related changes in the reducible cross-links of collagen. FEBS Lett. 1971;16(2):86–88. doi: 10.1016/0014-5793(71)80338-1. [DOI] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev. Dyn. 1995;202(3):229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers–Danlos-like changes in bone and other connective tissues. J. Bone Miner. Res. 2002;17(7):1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997 Feb 10;136(3):729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourte LM, Pathmanathan L, Jawad AF, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin on the mechanical, compositional, and structural properties of the mouse patellar tendon. J. Biomech. 2012;134(3):031005. doi: 10.1115/1.4006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. J. Orthop. Res. 2002;20(6):1315–1322. doi: 10.1016/S0736-0266(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000;151(4):779–788. doi: 10.1083/jcb.151.4.779. (13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel G, Snedeker JG. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 2009;28(8):503–510. doi: 10.1016/j.matbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Fessel G, Snedeker JG. Equivalent stiffness after glycosaminoglycan depletion in tendon—an ultra-structural finite element model and corresponding experiments. J. Theor. Biol. 2011;268(1):77–83. doi: 10.1016/j.jtbi.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Ito K, Nuka S, Hashimoto J, Takei H, Takahara M, Ogino T, Young MF, Shinomura T. Absence of biglycan accelerates the degenerative process in mouse intervertebral disc. Spine. 2009;34(25):E911–E917. doi: 10.1097/BRS.0b013e3181b7c7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis C, Sharkey N, Stover SM, Pool RR, Meagher DM, Willits N. Effect of maturation and aging on material and ultrasonographic properties of equine superficial digital flexor tendon. Am. J. Vet. Res. 1995;56(10):1345–1350. [PubMed] [Google Scholar]
- Hagg R, Bruckner P, Hedbom E. Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX. J. Cell Biol. 1998;142(1):285–294. doi: 10.1083/jcb.142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. Springer Series in Statistics. Springer; New York: 1992. The bootstrap and Edgeworth expansion. [Google Scholar]
- Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J. Biol. Chem. 1999;274(27):18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, Oldberg A, Birk DE, Chakravarti S. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J. Biol. Chem. 2002;277(38):35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- Kilts T, Ameye L, Syed-Picard F, Ono M, Berendsen AD, Oldberg A, Heegaard AM, Bi Y, Young MF. Potential roles for the small leucine-rich proteoglycans biglycan and fibromodulin in ectopic ossification of tendon induced by exercise and in modulating rotarod performance. Scand. J. Med. Sci. Sports. 2009;19(4):536–546. doi: 10.1111/j.1600-0838.2009.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuc IM, Scott PG. Increased diameters of collagen fibrils precipitated in vitro in the presence of decorin from various connective tissues. Connect. Tissue Res. 1997;36(4):287–296. doi: 10.3109/03008209709160228. [DOI] [PubMed] [Google Scholar]
- Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J. Orthop. Res. 2009;27(12):1596–1602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan TJ, Underwood CJ, Henninger HB, Thompson BM, Weiss JA. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J. Orthop. Res. 2007;25(7):894–903. doi: 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- Lujan TJ, Underwood CJ, Jacobs NT, Weiss JA. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J. Appl. Physiol. 2009;106(2):423–431. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffulli N, Waterston SW, Squair J, Reaper J, Douglas AS. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin. J. Sport Med. 1999;9(3):157–160. doi: 10.1097/00042752-199907000-00007. [DOI] [PubMed] [Google Scholar]
- Merline R, Schaefer RM, Schaefer L. The matricellular functions of small leucine-rich proteoglycans (SLRPs) J. Cell Commun. Signal. 2009;3(3–4):323–335. doi: 10.1007/s12079-009-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative realtime polymerase chain reaction (PCR) data. Neurosci. Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2002;19(4–5):249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Lin TW, Jawad AF, Iozzo RV, Soslowsky LJ. Investigating tendon fascicle structure–function relationships in a transgenic-age mouse model using multiple regression models. Ann. Biomed. Eng. 2004a;32(7):924–931. doi: 10.1023/b:abme.0000032455.78459.56. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J. Biomech. Eng. 2004b;126(2):252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J. Biomech. Eng. 2005;127(1):181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- Rühland C, Schönherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274(16):4246–4255. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- Samiric T, Ilic MZ, Handley CJ. Large aggregating and small leucine-rich proteoglycans are degraded by different pathways and at different rates in tendon. Eur. J. Biochem. 2004;271(17):3612–3620. doi: 10.1111/j.0014-2956.2004.04307.x. [DOI] [PubMed] [Google Scholar]
- Samiric T, Parkinson J, Ilic MZ, Cook J, Feller JA, Handley CJ. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. 2009;28(4):230–236. doi: 10.1016/j.matbio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel ‘gene expression’s Ct difference’ formula. J. Mol. Med. 2006;84(11):901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- Scott JE, Orford CR. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem. J. 1981;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Hosaka Y, Yamamoto E, Ueda H, Sugawara K, Takahashi H, Takehana K. Control of the collagen fibril diameter in the equine superficial digital flexor tendon in horses by decorin. J. Vet. Med. Sci. 2005;67(9):855–860. doi: 10.1292/jvms.67.855. [DOI] [PubMed] [Google Scholar]
- Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J. Appl. Physiol. 2011;111:999–1006. doi: 10.1152/japplphysiol.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosislike phenotype in mice. Nat. Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- Young MF, Bi Y, Ameye L, Chen XD. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj. J. 2002;19(4–5):257–262. doi: 10.1023/A:1025336114352. [DOI] [PubMed] [Google Scholar]
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal Interact. 2005;5(1):5–21. [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell Biochem. 2006;98(6):1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.