Abstract
This study investigated whether prepubertal dietary exposure to genistein reduces mammary tumorigenesis by up-regulating Brca1 expression in mice. Heterozygous Brca1+/− mice and their wildtype (WT) littermates were fed control AIN93G diet or 500 ppm genistein supplemented AIN93G diet from postnatal day (PND) 15 to PND30, and then switched to AIN93G diet. Prepubertal dietary exposure to genistein reduced DMBA-induced mammary tumorigenesis and aggressivity of the tumors in the WT mice and up-regulated the expression of Brca1 in their mammary glands. In contrast, prepubertal genistein diet neither significantly reduced mammary tumorigenesis or tumor aggressivity, nor increased Brca1 mRNA expression in the Brca1+/− mice. These results may be related to the opposing effects of prepubertal genistein diet on the expression of Rankl and CK5/CK18 ratio (marker of luminal epithelial cell differentiation) in the mammary gland, and estrogen receptor (ER-α) and progesterone receptor (PgR) protein levels in the mammary tumor: these all were reduced in the WT mice or increased in Brca1+/− mice. Both the WT and Brca1+/− mice exhibited reduced levels of amphiregulin, CK5 and CK18, delayed ductal elongation and a reduction in terminal end bud number in the normal mammary gland, and reduced HER-2 protein levels in the mammary tumors; however, these effects were not sufficient to significantly reduce mammary tumorigenesis in Brca1+/− mice. Our results show that up-regulation of Brca1 may be required for prepubertal dietary genistein exposure to reduce later mammary tumorigenesis, perhaps because in the absence of this up-regulation, mice do not exhibit genistein-induced down-regulation of ER-α, PgR and Rankl.
Keywords: Genistein, Soy, BRCA1, Estrogen signaling, Breast cancer
INTRODUCTION
Soy consumption during childhood and adolescence has been consistently linked to a marked reduction in breast cancer risk in Asians, American Asians and Caucasians (1–5). Genistein, a soy-derived isoflavone, is thought to play a key role in the cancer-preventive activity of pubertal soy intake. In agreement with this view, pubertal genistein exposure reduces mammary cancer risk in rodent models (6). The protective effect of soy/genistein may be limited to puberty, as an adult exposure does not alter the risk in mice or rats (6). Consumption of soy limited to adult life may not affect breast cancer risk in humans either (7).
The mechanisms mediating the protective effect of pubertal genistein exposure on mammary tumorigenesis remain to be established. Genistein is a weak estrogen receptor α (ER-α) agonist, but it binds relatively strongly to ER-β (8). However, with the exception of very low doses, genistein activates both estrogen receptors equally, similarly to estradiol (9). Previous studies indicate that genistein modifies the expression of ER-α in vitro and in vivo (10–13) and increases progesterone receptor (PgR) in human breast tumors (14), human breast cancer cells in a mouse model (15) and normal mammary tissue in animal models (16). Genistein also targets many other signaling pathways, including tyrosine kinases ErbB2/HER-2 (17). It has been shown that genistein down-regulates HER-2 in human breast cancer cells (18), and following pubertal genistein exposure, in rat mammary tumors (19). Prepubertal genistein exposure also affects mammary gland morphology. It reduces the number of targets for malignant transformation (terminal end buds, TEBs), and this is proposed to explain the cancer protective effects of genistein (6).
Because genistein targets several biological pathways, it is not clear which one of them may contribute to its breast cancer risk reducing effects. Our earlier study showed that prepubertal genistein exposure, similarly to prepubertal estradiol exposure, up-regulates the tumor suppressor gene BRCA1 (breast cancer susceptibility gene 1) (12). Others have reported that genistein up-regulates BRCA1 in vitro in human breast and prostate cancer cells (20), and in vivo in the mammary gland of ovariectomized rats (21). BRCA1 expression in the mammary gland is also elevated during pregnancy when estrogen and progesterone levels are high (22), and is reduced by ovariectomy (23). BRCA1 physically interacts with ER-α and PgR to inhibit their transcriptional activity (24). The link between BRCA1 and these steroid receptors as well as the role of BRCA1 in inducing differentiation of stem/progenitor cells to luminal cells (25) may explain why this tumor suppressor reduces breast cancer risk.
To further explore the role of BRCA1 as a mechanism mediating the effects of prepubertal genistein exposure on the mammary gland, we used Brca1+/− mice. BRCA1+/− mutations are the main known cause for familial breast cancer (26), and women who have inherited a germline BRCA1 mutation have a 66–85% risk of developing breast cancer by age 70 (27). The BRCA1-induced changes were also investigated, focusing on ER-α and PgR and their target genes amphiregulin and receptor activator of nuclear factor-kappaB ligand (Rankl), respectively. Amphiregulin is an epithelial growth factor receptor (EGFR) ligand (28, 29). It is transcriptionally regulated by ER-α (30) and BRCA1 (31), and it mediates the proliferative actions of estradiol by activating the EGFR (30). Amphiregulin is required for mammary gland ductal morphogenesis at puberty (28), and its absence prevents duct-limited mammary progenitor cell proliferation, but has no effect on lobule-limited mammary progenitor cells (32). It is not known whether genistein alters amphiregulin expression. RANKL is a member of the tumor necrosis family (TNF), and mediates progesterone induced mammary cell proliferation downstream of PgR (33). Genistein down-regulates RANKL in the bone (34) and prostate cancer cells (35), but the effects on the mammary gland have not been explored.
We found that prepubertal dietary genistein exposure reduced mammary tumor incidence in the WT mice, but failed to do so in the Brca1+/− mice. In contrast to WT mice, prepubertal genistein diet did not increase Brca1 or reduce ER-α, PgR or Rankl expression in the mammary gland or tumor in these mice. However, prepubertal genistein exposure elevated CK5/CK18 ratio in the mammary gland of the Brca1+/− mice, indicative of reduced luminal differentiation (36). Our results show that up-regulation of Brca1 may be required for prepubertal dietary genistein exposure to reduce later mammary tumorigenesis.
MATERIALS AND METHODS
Animals
Brca1tm1Cxd heterozygous (+/−) frozen mouse embryos at 129Sv/C57BL/6 background were obtained from the NCI mouse repository (Bethesda, MD). Frozen embryos were implanted in ICR female mice and a colony was established at the Georgetown University’s Animal Facility. Mice were fed a semi-purified AIN93G (the American Institute of Nutrition) diet upon arrival. Animals were housed in a temperature- and humidity-controlled room under a 12-hour light-dark cycle. All animal procedures were approved by the Georgetown University Animal Care and Use Committee, and the experiments were performed following the National Institutes of Health guidelines for the proper and humane use of animals in biomedical research.
Genotyping
Wild-type (WT) and heterozygous Brca1 (+/−) mice were genotyped using DNA extracted from the tail clips using the NaOH method. PCR amplification was performed using forward and reverse primers to obtain a 450 and 550 bp bands for wild type and KO alleles, respectively. Primer sequences were: forward primer (Brca1004) : 5′-CTGGGTAGTTTGTAAGCATGC3′ and reverse primer (Brca1005) : 5′-CAATAAACTGCTGGTCTCAGG-3′ and (Brca1007) : 5′-ATCGCCTTCTATCGCCTTCTTGACGAGTTC-3′. After PCR amplification, products were analyzed by agarose gel electrophoresis. The B004/B005 primer combinations generates a 450 bp product that corresponds to the wildtype Brca1 while the B004/B007 primer combination generates a 550 bp product that corresponds to the Neo cassette.
Pre-pubertal dietary exposures
On postnatal day 15 (PND15), WT and Brca1+/− female mice were divided into two dietary groups (n= 30 mice per genotype/group): AIN93G diet (control) or genistein (500 ppm) supplemented AIN93G diet obtained from Harlan-Teklad (Madison, WI). The mice were kept on these diets until PND30, and all mice were then switched to the non-supplemented AIN93G diet.
Effects on mammary tumorigenesis
Brca1+/− mice do not develop spontaneous mammary tumors, and therefore mammary tumors were induced by administration of a subcutaneous injection of 15 mg in 100 μl of Medroxyprogesterone Acetate (MPA, DepoProvera, Pfizer) to mice at 6 weeks of age, followed by administration of 1 mg 7,12-dimethylbenz(a)anthracene (DMBA) (Sigma, St. Louis, MO) weekly for four weeks (weeks 7, 8, 9, 10). The carcinogen was dissolved in corn oil and administered by oral gavage in a volume of 0.1 ml. This study included 22 WT mice fed the control diet, 21 WT mice fed prepubertally genistein diet, 25 Brca1+/− mice fed the control diet, and 26 Brca1+/− mice fed genistein diet. Animals were examined for mammary tumors by palpation once per week. The end-points for data analysis were (i) latency to tumor appearance, (ii) the number of animals with tumors (tumor incidence), and (iii) the number of tumors per animal (tumor multiplicity). During the follow-up, animals in which tumor burden approximated 10% of total body weight were euthanized, as required by the ethical guidelines of our institution. All surviving animals were sacrificed 20 weeks after the last does of DMBA administration.
Hyperplastic alveolar nodules
Mammary gland wholemounts were obtained from mice 20 weeks after DMBA exposure, processed as described above. Whole mounts were evaluated under the microscope to determine the number of hyperplastic alveolar nodules in each gland.
Histopathological evaluation
Mammary tumors were classified according to its histopathology by a veterinarian pathologist, through ARUP veterinarian services, Salt Lake City, Utah.
Effects on mammary gland morphology and cytokeratin (CK) 5 and CK18 mRNA expression
To assess changes in mammary gland morphology, wholemounts of the 4th abdominal glands obtained on PND50 were processed as previously described (37). The total number of TEBs was counted. Identification of TEBs was based on the guidelines established by Russo and Russo (38). Ductal elongation (epithelial outgrowth) was measured as the growth, in centimeters, from the end of the lymph node to the end of the epithelial tree. All the analyses were done blindly using an Olympus dissecting microscope.
We also determined whether the mRNA expression of CK5 (committed progenitor cells) or CK18 (luminal cells) (36), or CK5/CK18 ratio were different between the WT and Brca1+/− mice, and whether prepubertal genistein exposure affected the expression of these cytokeratins.
Mammary gland and tumor ER-α and HER-2 protein levels
Protein levels of ER-α and HER-2, were assessed by Western-blot in the mammary glands and tumors. Briefly, mammary glands and tumors were homogenized, centrifuged and the protein extract collected from the supernatant. 50 μg of protein extract was loaded onto a NuPAGE 12% Bis-Tris gel (Invitrogen Life Technologies, Carlsbad, CA), and gels were run at 150 V. Membranes were then washed with TBST and blocked in 5% milk in TBST for 30 min at room temperature. After blocking, membranes were incubated with antibodies against ER-α (1:100 dilution, Vector Laboratories, Burlingame, CA) and HER-2 (1:1000 dilution, Lab Vision Corp, Fremont, CA), overnight at 4°C. Next, membranes were incubated with secondary anti-rabbit IgG or mouse IgG horseradish peroxidase antibodies (1:5000 dilution, Amersham Pharmacia Biotech, Piscataway, NJ (anti-mouse) and developed using Super Signal (Pierce, Rockford, IL). Fold differences were calculated by normalization against beta-actin.
Mammary gland and tumor PgR protein levels
Using immunohistochemistry, PgR expression was assessed in mammary glands and tumors collected at the end of the follow-up period. Briefly, tissues were fixed in 10% buffered formalin, embedded in paraffin and sectioned (5μm). Sections were deparaffinized in xylene, hydrated through graded alcohols and incubated with H2O2 to block endogenous peroxidases. Antigen retrieval was performed in a Target Retrieval solution (pH 9, Dako S2368) in a pressure cooker for 20 min + 2 h cool down. Tissue sections were incubated overnight with the primary antibody against PgR at a 1:400 dilution (polyclonal rabbit anti-human PgR, A0098, Dako CA). After several washes, sections were treated with secondary antibody (anti-rabbit) and developed using the Dako EnVision Dual Link System – HRP, DAB+ (K4065) as instructed by the manufacture. The PgR status of each sample was evaluated according to a modified version of the scoring system proposed by Allred et al. (39). The total score for each sample was calculted as the sum of the estimated proportion of positive-staining cells (0–7) plus the estimated intensity of positive-staining cells (0–4).
Mammary gland amphiregulin, Rankl, CK5 and CK18 mRNA levels
Frozen tissue samples were weighed and homogenized using a PowerGen 35 hand held homogenizer (Fisher Scientific, Pittsburgh, PA) with RNAse free disposable OMNI-Tips (Fisher Scientific). RNA extraction was then performed according to the Qiagen’s RNeasy Lipid Tissue kit (Qiagen Inc., Valencia, CA) instructions. The quantity and quality of RNA were measured by comparing the optical density ratios (OD260/OD280) obtained using a Nanodrop (ND-1000) Spectrophotomer (Thermo Scientific, Wilmington, DE). RNA samples were stored at −80°C until use.
cDNA synthesis and quantitative real-time PCR (qRT-PCR) analysis
Two hundred ng of total RNA per sample was used as a template for random primed cDNA synthesis with a recombinant Moloney murine leukemia virus reverse transcriptase (TaqMan MultiScribe Reverse Transcriptase and RT-PCR Reagents, AppliedBiosystems, Roche, New Jersey, USA), according to manufacturer’s instructions. An RT enzyme minus control reaction was also included. The cDNA samples were then used as templates for quantitative real time PCR analysis with previously described specific primers for the target genes Brca1 (40), amphiregulin (30), Rankl (41), and CK5 and CK18 (42) using QuantiTect SYBR green PCR kit (Qiagen Inc., Valencia, CA) and an ABI Prism 7900 Sequence Detection System. Each sample was run in triplicate, and qPCR run was repeated 1–2 times. Absolute gene expression levels were determined using Applied Biosystems’ SDS2.3 software and the standard curve method. Concentration of each sample was normalized to the reference gene 18S rRNA (42).
Statistical analyses
Data obtained on (i) mammary gland morphology (total number of TEBs and ductal elongation), (ii) mRNA levels, (iii) mammary gland and tumor HER-2 and ER-α protein levels, (iv) PgR staining and (v) number of hyperplastic alveolar nodules were analyzed by two-way ANOVA using genotype (WT or knockout) and treatment (control or genistein) as variables. Kaplan-Meier curves were used to compare differences in tumor incidence, followed by the log-rank test. Normalized quantitative RT-PCR results were analyzed with Kruskal-Wallis one-way ANOVA on Ranks and one-way ANOVA with Holm-Sidak method for pairwise multiple comparison procedures. All tests were performed using the SPSS SigmaStat software, and differences were considered significant if the p-value was less than 0.05. All probabilities are two-tailed.
RESULTS
Mammary tumorigenesis
Prepubertal genistein exposure significantly reduced mammary tumor incidence in the WT mice (p=0.029) (Figure 1A). Among the Brca1+/− mice, however, the difference in mammary tumor incidence failed to reach statistical significance (p=0.26) (Figure 1B). The number of HANs (Figure 1C), tumor multiplicity (the total number of tumors per animal; ranged between 1.2 and 1.6 in the WT and Brca1+/− mice) or tumor latency (time to tumor appearance; ranged between 10.1–11.9 weeks) were not affected by prepubertal genistein diet in either the WT or Brca1+/− mice (data not shown).
Figure 1. Mammary tumorigenesis and Brca1 expression.
Effect of prepubertal dietary exposure to 500 ppm genistein on (A) tumor incidence in wildtype mice (n=21–22 mice/group) and (B) in Brca1+/− mice (n=25–26 mice/group), (C) hyperplastic alveolar nodules (HANs) in these mice, and (D) Brca1 mRNA levels (n=4–6 mice per group) on postnatal day 50. Means ± SEM of Brca1 mRNA are shown; bars with different letters are significantly different from each other, p<0.05. Prepubertal genistein exposure significantly reduced mammary tumorigenesis in WT mice (p<0.029), but not in Brca1+/− mice (p<0.26).
There was no evidence that the incidence of mammary tumors was higher in the Brca1+/− mice; in fact, a slightly lower number of these mice (14/25, 56%) developed mammary tumors, compared to WT mice (15/22, 68%). The number of pre-malignant lesions; i.e., hyperplastic alveolar nodules (HANs), was significantly higher in the Brca1+/− mice than in the WT mice (p=0.047) (Figure 1C). These findings suggest that the Brca1+/− mice have a higher potential to develop mammary tumors than the WT mice do, but most of these HANs do not progress to malignancy in the Brca1+/− mice.
Histopathological analysis indicated that most mammary tumors were malignant carcinomas (Table 1) in all four groups. The incidence of benign adenomas was 10% and 8% in the WT and Brca1+/− mice fed the control diet, respectively, and 20% in both groups which were fed genistein containing diet before puberty. The malignant tumors consisted of adenocarcinomas and other carcinomas. These malignant carcinomas were divided into low grade and high grade tumors, and the ratio of low to high grade tumors was similar in the WT and Brca1+/− mice (Table 1). In the WT mice, the incidence of high grade malignant tumors dropped from 60% in the control diet group to 20% in the genistein group (p<0.001), but only from 67% to 50% in the Brca1+/− mice (non-significant). The tumor mitotic index, another indicator of tumor growth and aggressiveness, was reduced by prepubertal exposure to genistein in the WT mice (p<0.011), but genistein had no effect on the Brca1+/− mice (p=0.93) (Table 1).
Table 1a.
Mammary tumor multiplicity and latency in DMBA-exposed wildtype (WT) and Brca1 +/− mice fed a control or genistein supplemented AIN93G diet during prepuberty. Mean±SEM are shown.
| Group | Number of tumors | Tumor multiplicity (number of tumors/tumor bearing mice) | Tumor latency (weeks) |
|---|---|---|---|
| WT control | 10 | 1.2 ± 0.4 | 10.1 ± 0.7 |
| WT genistein | 5 | 1.6 ± 0.3 | 11.6 ± 1.2 |
| Brca1+/− control | 12 | 1.2 ± 0.3 | 11.9 ± 1.0 |
| Brca1+/− genistein | 10 | 1.2 ± 0.2 | 10.8 ± 1.1 |
Mammary gland Brca1 mRNA levels
The expression levels of Brca1 mRNA in the mammary gland were determined on PND50 (Figure 1D). As expected, RT-qPCR showed lower levels of Brca1 mRNA in Brca1+/− mice compared to WT mice (p<0.001), regardless of the diet. Higher levels of Brca1 mRNA were observed in the mammary glands of WT mice fed genistein containing diet compared to those fed AIN93G control diet (p<0.042). No effects by genistein diet on Brca1 levels were observed in Brca1+/− mice (Figure 2).
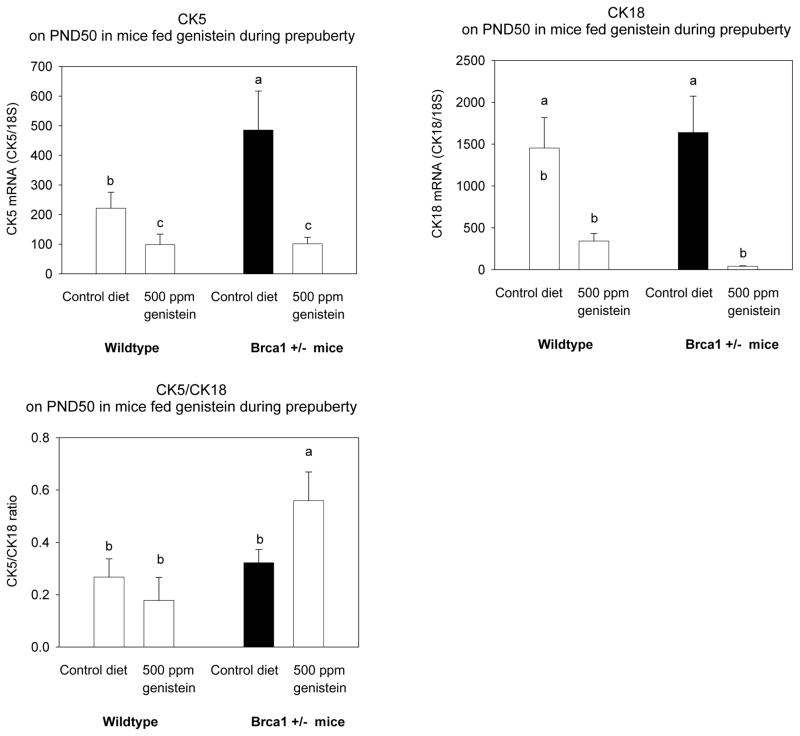
Figure 2. CK5 and CK18 expression, and CK5/CK18 ratio.
Effect of prepubertal dietary exposure to 500 ppm genistein on CK5, CK18 expression and CK5/CK18 ratio in 50-day-old wildtype and Brca1+/− mice. Mean ± SEM of 4–6 mice/group are shown; bars marked with different letters are significantly different from each other: p<0.05. Prepubertal genistein exposure tended to have a different effect on CK5/CK18 ratio in the wildtype and Brca1+/− mice (p<0.079 for interaction).
Mammary gland morphology
Mammary gland morphology, and the expression of CK5 and CK18 were assessed at PND50. Progenitor cells, including committed myoepithelial and some ductal luminal progenitor cells, express CK5, and the expression is lost when the cells differentiate to mature myoepithelial and luminal cells (36). Differentiated luminal cells express CK18. Thus, mammary glands expressing high CK5 and low CK18 are more undifferentiated than glands which express low CK5/CK18 ratio.
CK5 levels were significantly higher in the Brca1+/− mice fed the control diet than in the WT controls (p<0.016) (Figure 2A), but CK18 levels were not altered (Figure 2B). Regardless of the genotype, the mice fed genistein during prepuberty exhibited significantly reduced levels of both cytokeratines, compared to the mice fed the control diet (CK5: p<0.002; CK18: p<0.003). The ratio between CK5 and CK18 was higher in the Brca1+/− mice than in the WT mice (p<0.022). The difference was particularly apparent in mice fed genistein during pre-puberty, because genistein reduced it in the WT mice, and significantly increased the ratio in the Brca1+/− mice (p<0.04) (p for interaction = 0.079) (Figure 2C).
The reductions in both CK5 and CK18 by genistein reflected changes in the mammary gland morphology. As seen in Figure 3A, mice fed genistein during prepuberty exhibited significantly smaller mammary epithelium than mice fed the control diet. No significant differences in the number of TEBs or ductal elongation were seen between the WT and Brca1+/− mice, when determined at PND50. However, prepubertal genistein exposure reduced the number of TEBs (p<0.001) and inhibited ductal elongation (p=0.007) in both the WT and Brca1+/− mice (Figure 3B).
Figure 3. Mammary gland morphology.
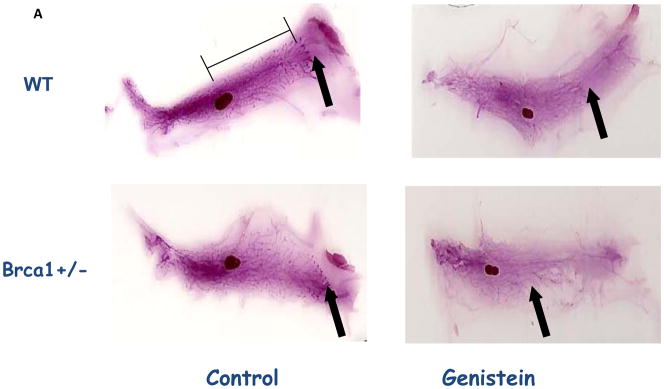
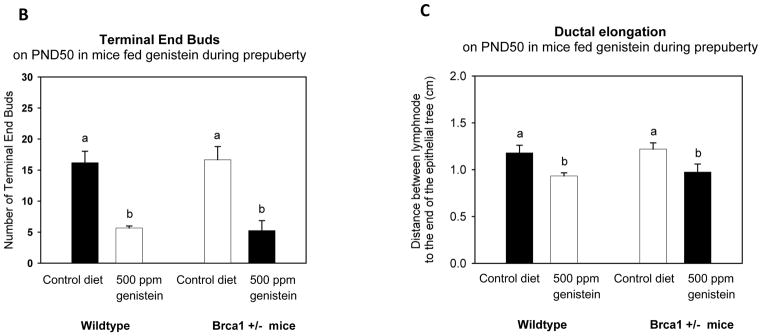
(A) Histological depiction of the 4th abdominal mammary gland whole-mounts (arrow indicates where the epithelial tree ends); (B) number of terminal end buds (TEBs); and (C) ductal elongation (distance, in cm, from the end of the lymph node to the end of the epithelial tree) on PND50 in wildtype and Brca1+/− mice fed 500 ppm genistein during pre-puberty. Mean ± SEM of 4–6 mice/group are shown; bars marked with different letters are significantly different from each other: p<0.05.
Mammary gland and mammary tumor protein expression levels of ER-α, ER-β, PgR and HER-2, and their down-stream targets amphiregulin and RANKL
Because genistein acts by binding to ER-α and ER-β (8, 9), and up-regulation of PgR is indicative of ER-α activation, we determined the expression of these receptors. We also tested pathways downstream of ER-α and PgR; i.e., the expression of amphiregulin and RANKL. In addition, genistein is a tyrosine kinase inhibitor (17), and therefore HER-2 levels were assessed. ER-α and HER-2 protein levels were detected by Western blot, ER-β, amphiregulin and Rankl mRNA levels by RT-qPCR, and PgR by RT-qPCR and immunohistochemistry.
Changes in mammary glands
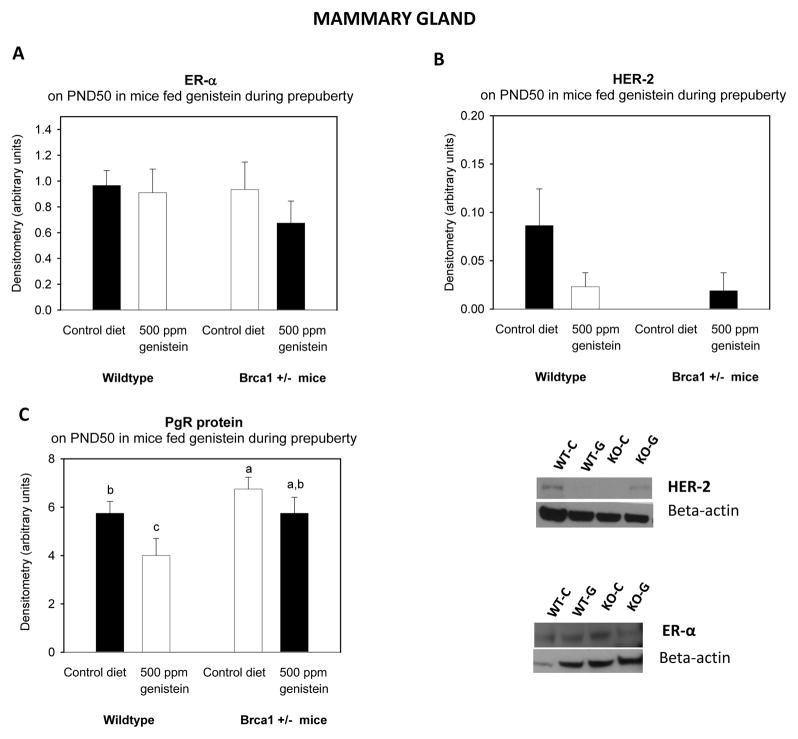
The assays revealed that mammary glands and tumors were positive for ER-α, ER-β, PgR and HER-2 expression. Protein levels of ER-α (Figure 4A, mammary gland) and HER-2 (Figure 4B) in mammary gland tissues were not different between the genotypes or dietary groups. PgR protein levels were higher in the mammary glands of Brca1+/− mice than in the WT mice (p<0.033) (Figure 4C). Genistein decreased the PgR protein levels in the WT mice (p<0.030). ER-β protein levels were not different among the groups (data not shown).
Figure 4.
Figure 4a. Mammary gland and tumor protein levels of ER-α, PgR and HER-2.
Protein levels of (A) ER-α, (B) HER-2 and (C) PgR determined on PND50. All values are expressed as the mean ± SEM of 4–6 mice/group. ER-α and HER-2 levels were determined by western blot, and PR levels were determined by IHC. Bars marked with different letters are significantly different from each other: p<0.05.
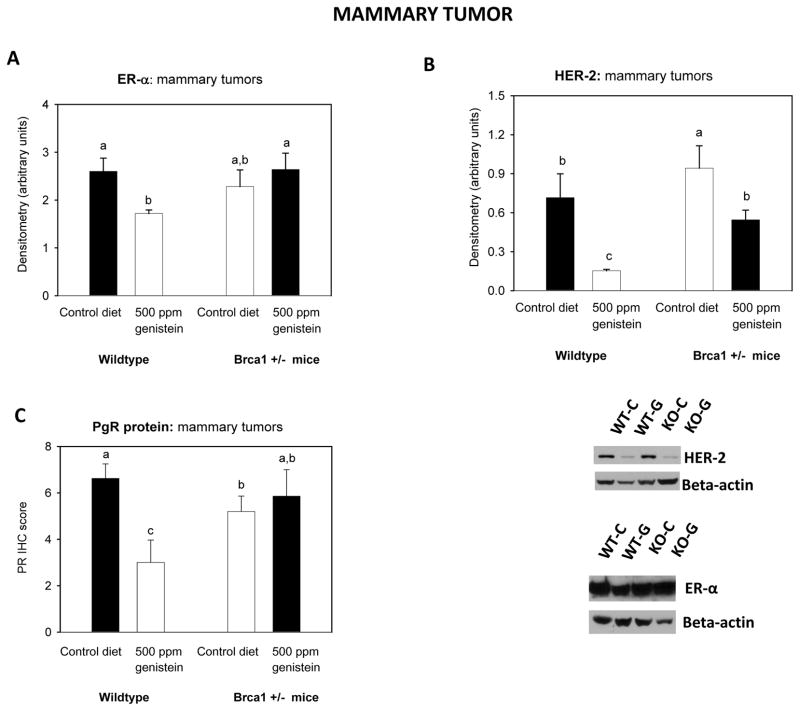
Figure 4b. Mammary tumor protein levels of ER-α, PR and HER-2.
Protein levels of (A) ERα, (B) HER-2 and (C) PgR were determined in the DMBA-induced adenocarcinomas. All values are expressed as the mean ± SEM of 5–7 mice/group. Bars marked with different letters are significantly different from each other: p<0.05. Genistein had opposing effect on ER-α (p<0.04 for interaction) and PgR expression (p< 0.034 for interaction) in wildtype and Brca1+/− mice
Changes in mammary tumors
Assessment of expression of the different receptors in the mammary tumors revealed that ER-α protein levels were not different in the mammary tumors between WT and Brca1+/− mice fed the control diet. The expression of ER-α was reduced in the tumors by prepubertal genistein exposure in the WT but not in the Brca1+/− mice (p for interaction <0.04) (Figure 4A, mammary tumor).
In contrast to normal mammary gland tissue, the levels of PgR were significantly lower in the mammary tumors of the Brca1+/− mice than in the WT mice (p=0.007) (Figure 4C). Further, although the WT mice exposed to genistein at pre-puberty exhibited a significant reduction in PgR expression, no reduction was seen in the in mammary tumors of the Brca1+/− mice (p for interaction <0.034).
Mammary tumors of Brca1+/−− mice expressed higher levels of HER-2 than the tumors in the WT mice, regardless of diet (p<0.03) (Figure 4B). Prepubertal exposure to genistein diet decreased the expression levels of HER-2 in mammary tumors of WT and Brca1+/− mice, compared to their respective controls (p<0.002).
Amphiregulin and Rankl expression in the mammary gland
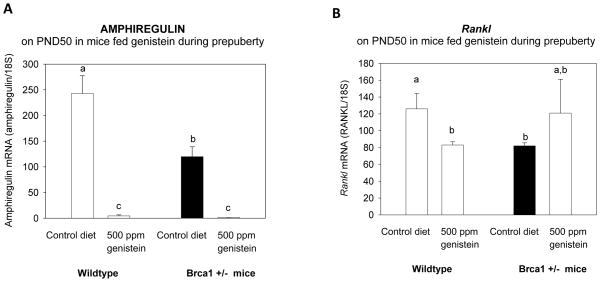
Although mammary gland ER-α levels were similar in the WT and Brca1+/− mice, amphiregulin mRNA levels were lower in the mammary glands of Brca1+/− mice than WT mice (p<0.011) (Figure 5A). Prepubertal genistein exposure significantly reduced the levels in both groups (p<0.001), and consequently no differences in amphiregulin levels between the two genotypes were seen.
Figure 5. Expression of amphiregulin and Rankl in the mammary gland.
mRNA levels of (A) amphiregulin, and (B) Rankl in the mammary gland on PND50, determined using qRT-PCR. All values are expressed as the mean ± SEM of 4–6 mice/group. Bars marked with different letters are significantly different from each other: p<0.05. Genistein reduced amphiregulin expression in both the wildtype and Brca1+/− mice, but had opposing effect on Rankl expression in the two genotypes (p<0.026 for interaction).
PgR’s down-stream target Rankl was significantly higher in the control diet fed WT mice than in the Brca1+/− mice on PND50 (p<0.018), but the difference was lost in mice fed genistein during prepuberty (Figure 5B). This is because among the WT mice genistein reduced Rankl, but among the Brca1+/− mice genistein exposure increased Rankl mRNA (p<0.026 for interaction).
DISCUSSION
Effect of prepubertal genistein diet on mammary tumorigenesis
We found that in the WT mice prepubertal dietary exposure to 500 ppm genistein significantly reduced carcinogen-induced mammary tumor incidence, in accordance with data previously reported in rats (6). Since the dose we used generates blood genistein levels comparable to those seen in the Asians (43), our study adds to the convincing evidence obtained in human studies that childhood soy intake protects against the development of breast cancer later in life (1–5).
However, no significant reduction in mammary tumorigenesis was seen in the Brca1+/− mice fed the genistein diet before puberty onset. Further, in contrast to the WT mice, these mice did not exhibit an increase in Brca1 expression, possibly because the remaining Brca1 allele was already maximally expressed in the heterozygous Brca1+/− mice. The increase in Brca1 expression in the WT mice is in agreement with the earlier findings obtained in both in vitro and in vivo studies indicating that genistein up-regulates BRCA1 (12, 20, 21). Our results thus suggest that the genistein-induced up-regulation of Brca1 may be required for prepubertal genistein exposure to reduce breast cancer risk.
Mammary tumorigenesis in the Brca1+/− mice: role of PgR signaling
Palpable mammary tumor incidence was not different between the genotypes, although Brca1+/− mice developed more premalignant HAN lesions than WT mice did. Since Brca1+/− mice expressed higher levels of PgR than those of the WT mice; a finding which is consistent with the data by Poole et al (44) in conditional Brca1−/− /p53−/− mice, these genetically modified mice should be at least as sensitive as the wildtype mice to the MPA exposure, which is required in both wildtype and various genetically modified mouse models for DMBA to induce mammary tumors (45). This was confirmed by determining that MPA similarly increased Rankl expression and TEB number in the WT and Brca1+/− mice (data not shown).
Differences in the response to prepubertal genistein diet between the genotypes
Prepubertal genistein diet affected some end-points in the mammary glands and tumors differentially, and some similarly in the WT and Brca1+/− mice. This diet had an opposite effect on the following end-points in the two genotypes: Rankl expression and CK5/CK18 ratio in the mammary gland, PgR expression in the mammary gland and tumor, ER-α expression in the tumor, and histopathology of the tumor. In the WT mice, prepubertal genistein exposure reduced the expression of Rankl in the mammary gland and PgR both in the mammary gland and tumor. The effect on the PgR is in agreement with the study by Pei et al (46) showing that prepubertal genistein exposure reduced PgR expression in the (rat) mammary gland. Thus, consumption of genistein during prepuberty may induce persistent down-regulation of PgR. Down-regulation of Rankl by genistein is consistent with previous data in bone and prostate (34, 35). Since reduced expression of PgR and Rankl may be associated with a reduction in the mammary progenitor cell proliferation (47), one of the mechanisms by which prepubertal genistein exposure reduces breast cancer risk may be through increased mammary luminal cell differentiation. Changes in these three end-points could have occurred through up-regulation of BRCA1, which is known to suppress progesterone signaling (24) and induce luminal specific differentiation of mammary progenitor cells (25).
Prepubertal genistein diet affected Rankl and CK5/CK18 ratio differently in the Brca1+/− mice. Genistein treated Brca1+/− mice expressed more Rankl, and higher CK5/CK18 ratio than the control diet fed mice. Since high CK5/CK18 ratio is indicative of increased presence of undifferentiated luminal cells (36) as is high Rankl expression (47), prepubertal genistein exposure may have led to reduced luminal cell differentiation in the Brca1+/− mice.
Prepubertal genistein diet reduced mammary tumor grade and tumor mitotic index, both of which are indicators of aggressiveness of a tumor, in the wildtype but not Brca1+/− mice. In addition, tumors in the prepubertally genistein fed wildtype mice expressed significantly lower levels of the ER-α, PgR and HER-2 than tumors in the control diet fed wildtype mice. Again, mammary tumors in Brca1+/− mice failed to show similar changes, with the exception of down-regulation of HER-2 in the genistein fed mice. These findings indicate that genistein failed to reduce aggressiveness of the mammary tumors in the mice lacking one Brca1 allele.
Similarities in the response to prepubertal genistein diet in the two genotypes
Genistein had some effects on the Brca1+/− mice which were similar to those seen in the WT mice; i.e., it reduced the number of targets for malignant transformation (TEBs), inhibited ductal elongation, down-regulated amphiregulin expression in the mammary gland and reduced HER-2 expression in the tumors. Some of these changes have been previously reported to occur by genistein (6, 17, 48), but we are not aware of any studies showing that genistein delays ductal elongation or inhibits amphiregulin expression.
Activation of ER-α increases amphiregulin expression which then binds to the epidermal growth factor receptor (EGFR/ErbB1/HER-1) and induces cell proliferation (30). ErbB2/HER-2 does not have any known ligands, but its activity and possibly expression can be modified through hetero-dimerization with amphiregulin bound EGFR (49, 50). Amphiregulin expression was lower in the control diet fed Brca1+/− mice than in the WT controls suggesting that ER-α signaling may be reduced in the Brca1+/− mice, although no differences in the ER-α expression in the normal mammary gland were seen between the genotypes. Our results also indicate that both in the Brca1+/− and WT mice, prepubertal genistein diet induced a persistent reduction in mammary amphiregulin levels. Lamber et al. have reported that BRCA1 suppresses amphiregulin (31), and therefore the reduction in the WT mice may be due to genistein-induced up-regulation of Brca1. However, since amphiregulin was reduced also in the genistein exposed Brca1+/− mice, but genistein failed to up-regulate their Brca1 expression, other mechanisms than genistein-induced up-regulation of Brca1 suppressed their amphiregulin expression.
Amphiregulin was recently found to mediate self-renewal of stem/progenitor cells in the mammary duct (32); its inhibition by siRNA reduced mammosphere formation by preventing the expansion of ductal progenitor cells. Reduction in amphiregulin expression by prepubertal genistein diet could thus explain the significant delay in ductal elongation observed here in the postpubertal mammary gland in both WT and Brca1+/− mice. Since amphiregulin also is expressed in the TEBs (51), genistein-induced reduction in TEB number could perhaps be explained by a reduction in amphiregulin expression. Mammary gland morphology on PND50 in the control diet fed WT and Brca1+/− mice was similar; i.e., loss of one Brca1 allele did not affect gross mammary gland morphology. In humans, mammographic density also is similar in germline BRCA1 mutation carriers and controls (52, 53). Animal studies have generated conflicting data and reported that conditional or mammary specific BRCA1 mutations impair mammary ductal development (16, 54–56) or induce ductal elongation (57).
Conclusions
In this study we showed that a pre-pubertal dietary exposure to genistein in mice reduces the risk of developing mammary tumors later in life and up-regulates Brca1 mRNA expression in an adult mammary gland. We also showed that the protective effect was not seen in mice with a germline Brca1 mutation, and neither was the remaining Brca1 allele up-regulated by genistein in these mice. WT mice fed genistein before puberty onset exhibited reduced expression of PgR and Rankl; changes in the opposite direction were seen in the Brca1+/− mice, and they also exhibited increased CK5/CK18 ratio. These findings are indicative of reduced progenitor cell proliferation and increased luminal differentiation in the genistein exposed WT mice, but reduced differentiation in the genistein exposed Brca1+/− mice (36, 47).
Genistein down-regulated amphiregulin expression and inhibited ductal elongation and reduced TEBs in the mammary glands of both the WT and Brca1+/− mice, and also reduced the expression of Her-2 in the mammary tumors, suggesting that prepubertal dietary genistein exposure induces some breast cancer risk protective effects in the mammary gland, regardless of the level of Brca1 expression. However, these effects do not offer full protection to Brca1+/− mice, indicating that up-regulation of Brca1 is required for prepubertal genistein to prevent cancer.
Table 1b.
Mammary tumor histopathology in DMBA-exposed wildtype (WT) and Brca1+/− mice fed a control or genistein supplemented AIN93G diet during prepuberty
| Benign | Malignant | Tumor grade | Mitotic Index | |||
|---|---|---|---|---|---|---|
| Group | Adenoma | Adeno-carcinoma | Carcinoma (Other) | Low | High | (mean±SEM) |
| WT control | 1 (10%) | 6 (60%) | 3(30%) | 3(33%) | 6(67%)a | 5.0±0.8a |
| WT genistein | 1 (20%) | 1 (20%) | 3 (60%) | 3(75%) | 1(25%)b | 1.4±1.1b |
| Brca1+/− control | 1 (8%) | 9 (75%) | 2 (17%) | 3(27%) | 8(63%)a | 2.1±0.7b |
| Brca1+/− genistein | 2 (20%) | 4 (40%) | 4 (40%) | 3(37%) | 5(63%)a | 2.0±0.8b |
Values within a column which are marked with a different letter are significantly different from each other, p<0.05.
Acknowledgments
The work was supported by the National Cancer Institute (U54 CA100970) and American Cancer Society (post-doctoral fellowship 116602-PF-09-018-01-CNE).
Thank Salim Shah for advising in establishing Brca1+/− mouse colony, LCCC Animal Shared Resource for help in feeding mice and in tumorigenesis experiments and Histopathology Shared Resource for tissue processing.
Footnotes
No conflicts of interest.
Reference List
- 1.Korde LA, Wu AH, Fear T. Childhood soy intake and breast cancer risk in Asian-American women. 2008. Submitted. [DOI] [PubMed] [Google Scholar]
- 2.Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, Ruan Z, Gao YT, Zheng W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women, Cancer Epidemiol. Biomarkers Prev. 2001;10:483–488. [PubMed] [Google Scholar]
- 3.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 4.Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada) Cancer Causes Control. 2006;17:1253–1261. doi: 10.1007/s10552-006-0062-2. [DOI] [PubMed] [Google Scholar]
- 5.Lee SA, Shu XO, Li H, Yang G, Cai H, Wen W, Ji BT, Gao J, Gao YT, Zheng W. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women’s Health Study. Am J Clin Nutr. 2009;89:1920–1926. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warri A, Saarinen NM, Makela SI, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer. 2008 doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 8.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der BB, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 9.Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen Receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol. 2008;22:1032–1043. doi: 10.1210/me.2007-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Yang S, McKimmey C, Liu B, Edgerton SM, Bales W, Archer LT, Thor AD. Genistein induces enhanced growth promotion in ER-positive/erbB-2-overexpressing breast cancers by ER-erbB-2 cross talk and p27/kip1 downregulation. Carcinogenesis. 2010;31:695–702. doi: 10.1093/carcin/bgq007. [DOI] [PubMed] [Google Scholar]
- 11.Di X, Yu L, Moore AB, Castro L, Zheng X, Hermon T, Dixon D. A low concentration of genistein induces estrogen receptor-alpha and insulin-like growth factor-I receptor interactions and proliferation in uterine leiomyoma cells. Hum Reprod. 2008;23:1873–1883. doi: 10.1093/humrep/den087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabanes A, Wang M, Olivo S, de Assis S, Gustafsson JA, Khan G, Hilakivi-Clarke L. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25:741–748. doi: 10.1093/carcin/bgh065. [DOI] [PubMed] [Google Scholar]
- 13.Su Y, Eason RR, Geng Y, Till SR, Badger TM, Simmen RC. In utero exposure to maternal diets containing soy protein isolate, but not genistein alone, protects young adult rat offspring from NMU-induced mammary tumorigenesis. Carcinogenesis. 2007;28:1046–1051. doi: 10.1093/carcin/bgl240. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Liu X, Holman CD. Effect of dietary intake of isoflavones on the estrogen and progesterone receptor status of breast cancer. Nutr Cancer. 2010;62:765–773. doi: 10.1080/01635581003605979. [DOI] [PubMed] [Google Scholar]
- 15.Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;16:219–226. doi: 10.1007/s10787-008-8020-0. [DOI] [PubMed] [Google Scholar]
- 16.Hertrampf T, Schmidt S, Seibel J, Laudenbach-Leschowsky U, Degen GH, Diel P. Effects of genistein on the mammary gland proliferation of adult ovariectomised Wistar rats. Planta Med. 2006;72:304–310. doi: 10.1055/s-2005-916229. [DOI] [PubMed] [Google Scholar]
- 17.Peterson G. Evaluation of the biochemical targets of genistein in tumor cells. J Nutr. 1995;125:784S–789S. doi: 10.1093/jn/125.suppl_3.784S. [DOI] [PubMed] [Google Scholar]
- 18.Sakla MS, Shenouda NS, Ansell PJ, MacDonald RS, Lubahn DB. Genistein affects HER2 protein concentration, activation, and promoter regulation in BT-474 human breast cancer cells. Endocrine. 2007;32:69–78. doi: 10.1007/s12020-007-9006-1. [DOI] [PubMed] [Google Scholar]
- 19.Peng JH, Zhu JD, Mi MT, Li FJ, Cai L, Dong JZ, Zhang HX, Zhao Y, Xue RL. Prepubertal genistein exposure affects erbB2/Akt signal and reduces rat mammary tumorigenesis. Eur J Cancer Prev. 2010;19:110–119. doi: 10.1097/CEJ.0b013e3283362a3e. [DOI] [PubMed] [Google Scholar]
- 20.Fan S, Meng Q, Auborn K, Carter T, Rosen EM. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer. 2006;94:407–426. doi: 10.1038/sj.bjc.6602935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vissac-Sabatier C, Coxam V, Dechelotte P, Picherit C, Horcajada MN, Davicco MJ, Lebecque P, Bignon YJ, Bernard-Gallon D. Phytoestrogen-rich diets modulate expression of Brca1 and Brca2 tumor suppressor genes in mammary glands of female Wistar rats. Cancer Res. 2003;63:6607–6612. [PubMed] [Google Scholar]
- 22.Mueller CR, Roskelley CD. Regulation of BRCA1 expression and its relationship to sporadic breast cancer. Breast Cancer Res. 2003;5:45–52. doi: 10.1186/bcr557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marquis ST, Rajan JV, Wynshaw-Boris A, Xu J, Yin GY, Abel KJ, Weber BL, Chodosh LA. The developmental pattern of BRCA1 expression implies a role in differentiation of the breast and other tissues. Nat Genet. 1995;11:17–26. doi: 10.1038/ng0995-17. [DOI] [PubMed] [Google Scholar]
- 24.Katiyar P, Ma Y, Fan S, Pestell RG, Furth PA, Rosen EM. Regulation of progesterone receptor signaling by BRCA1 in mammary cancer. Nucl Recept Signal. 2006;4:e006. doi: 10.1621/nrs.04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 27.Antoniou AC, Pharoah PD, Easton DF, Evans DG. BRCA1 and BRCA2 cancer risks. J Clin Oncol. 2006;24:3312–3313. doi: 10.1200/JCO.2006.06.7934. [DOI] [PubMed] [Google Scholar]
- 28.McBryan J, Howlin J, Napoletano S, Martin F. Amphiregulin: role in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:159–169. doi: 10.1007/s10911-008-9075-7. [DOI] [PubMed] [Google Scholar]
- 29.Kenney NJ, Smith GH, Rosenberg K, Cutler ML, Dickson RB. Induction of ductal morphogenesis and lobular hyperplasia by amphiregulin in the mouse mammary gland. Cell Growth Differ. 1996;7:1769–1781. [PubMed] [Google Scholar]
- 30.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci US A. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamber EP, Horwitz AA, Parvin JD. BRCA1 represses amphiregulin gene expression. Cancer Res. 2010;70:996–1005. doi: 10.1158/0008-5472.CAN-09-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Booth BW, Boulanger CA, Anderson LH, Jimenez-Rojo L, Brisken C, Smith GH. Amphiregulin mediates self-renewal in an immortal mammary epithelial cell line with stem cell characteristics. Exp Cell Res. 2010;316:422–432. doi: 10.1016/j.yexcr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA. 2010;107:2989–2994. doi: 10.1073/pnas.0915148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Li Q, Wan HY, Helferich WG, Wong MS. Genistein and a soy extract differentially affect three-dimensional bone parameters and bone-specific gene expression in ovariectomized mice. J Nutr. 2009;139:2230–2236. doi: 10.3945/jn.109.108399. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Kucuk O, Hussain M, Abrams J, Cher ML, Sarkar FH. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006;66:4816–4825. doi: 10.1158/0008-5472.CAN-05-3752. [DOI] [PubMed] [Google Scholar]
- 36.Boecker W, Moll R, Dervan P, Buerger H, Poremba C, Diallo RI, Herbst H, Schmidt A, Lerch MM, Buchwalow IB. Usual ductal hyperplasia of the breast is a committed stem (progenitor) cell lesion distinct from atypical ductal hyperplasia and ductal carcinoma in situ. J Pathol. 2002;198:458–467. doi: 10.1002/path.1241. [DOI] [PubMed] [Google Scholar]
- 37.Hilakivi-Clarke L, Cho E, Raygada M, Kenney N. Alterations in mammary gland development following neonatal exposure to estradiol, transforming growth factor alpha, and estrogen receptor antagonist ICI 182, 780. J Cell Physiol. 1997;170:279–289. doi: 10.1002/(SICI)1097-4652(199703)170:3<279::AID-JCP9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 38.Russo IH, Russo J. Mammary gland neoplasia in long-term rodent studies. Environ Health Perspect. 1996;104:938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 40.Tan-Wong SM, French JD, Proudfoot NJ, Brown MA. Dynamic interactions between the promoter and terminator regions of the mammalian BRCA1 gene. Proc Natl Acad Sci US A. 2008;105:5160–5165. doi: 10.1073/pnas.0801048105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nannuru KC, Futakuchi M, Sadanandam A, Wilson TJ, Varney ML, Myers KJ, Li X, Marcusson EG, Singh RK. Enhanced expression and shedding of receptor activator of NF-kappaB ligand during tumor-bone interaction potentiates mammary tumor-induced osteolysis. Clin Exp Metastasis. 2009;26:797–808. doi: 10.1007/s10585-009-9279-2. [DOI] [PubMed] [Google Scholar]
- 42.Wang XY, Yin Y, Yuan H, Sakamaki T, Okano H, Glazer RI. Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and Notch pathways. Mol Cell Biol. 2008 doi: 10.1128/MCB.00040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allred CD, Allred KF, Ju YH, Goeppinger TS, Doerge DR, Helferich WG. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis. 2004;25:1649–1657. doi: 10.1093/carcin/bgh178. [DOI] [PubMed] [Google Scholar]
- 44.Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 2006;314:1467–1470. doi: 10.1126/science.1130471. [DOI] [PubMed] [Google Scholar]
- 45.Yin Y, Russell RG, Dettin LE, Bai R, Wei ZL, Kozikowski AP, Kopelovich L, Glazer RI. Peroxisome proliferator-activated receptor delta and gamma agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res. 2005;65:3950–3957. doi: 10.1158/0008-5472.CAN-04-3990. [DOI] [PubMed] [Google Scholar]
- 46.Pei RJ, Sato M, Yuri T, Danbara N, Nikaido Y, Tsubura A. Effect of prenatal and prepubertal genistein exposure on N-methyl-N-nitrosourea-induced mammary tumorigenesis in female Sprague-Dawley rats. In Vivo. 2003;17:349–357. [PubMed] [Google Scholar]
- 47.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 48.Wietrzyk J, Mazurkiewicz M, Madej J, Dzimira S, Grynkiewicz G, Radzikowski C, Opolski A. Genistein alone or combined with cyclophosphamide may stimulate 16/C transplantable mouse mammary cancer growth. Med Sci Monit. 2004;10:BR414–BR419. [PubMed] [Google Scholar]
- 49.Sternlicht MD, Sunnarborg SW. The ADAM17-amphiregulin-EGFR axis in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2008;13:181–194. doi: 10.1007/s10911-008-9084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 51.Kenney NJ, Huang RP, Johnson GR, Wu JX, Okamura D, Matheny W, Kordon E, Gullick WJ, Plowman G, Smith GH. Detection and location of amphiregulin and Cripto-1 expression in the developing postnatal mouse mammary gland. Mol Reprod Dev. 1995;41:277–286. doi: 10.1002/mrd.1080410302. [DOI] [PubMed] [Google Scholar]
- 52.Gierach GL, Loud JT, Chow CK, Prindiville SA, Eng-Wong J, Soballe PW, Giambartolomei C, Mai PL, Galbo CE, Nichols K, Calzone KA, Vachon C, Gail MH, Greene MH. Mammographic density does not differ between unaffected BRCA1/2 mutation carriers and women at low-to-average risk of breast cancer. Breast Cancer Res Treat. 2010;123:245–255. doi: 10.1007/s10549-010-0749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell G, Antoniou AC, Warren R, Peock S, Brown J, Davies R, Mattison J, Cook M, Warsi I, Evans DG, Eccles D, Douglas F, Paterson J, Hodgson S, Izatt L, Cole T, Burgess L, Eeles R, Easton DF. Mammographic density and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Res. 2006;66:1866–1872. doi: 10.1158/0008-5472.CAN-05-3368. [DOI] [PubMed] [Google Scholar]
- 54.Triplett AA, Montagna C, Wagner KU. A mammary-specific, long-range deletion on mouse chromosome 11 accelerates Brca1-associated mammary tumorigenesis. Neoplasia. 2008;10:1325–1334. doi: 10.1593/neo.08524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 56.Hoshino A, Yee CJ, Campbell M, Woltjer RL, Townsend RL, van der MR, Shyr Y, Holt JT, Moses HL, Jensen RA. Effects of BRCA1 transgene expression on murine mammary gland development and mutagen-induced mammary neoplasia. Int J Biol Sci. 2007;3:281–291. doi: 10.7150/ijbs.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones LP, Tilli MT, Assefnia S, Torre K, Halama ED, Parrish A, Rosen EM, Furth PA. Activation of estrogen signaling pathways collaborates with loss of Brca1 to promote development of ERalpha-negative and ERalpha-positive mammary preneoplasia and cancer. Oncogene. 2007 doi: 10.1038/sj.onc.1210674. [DOI] [PMC free article] [PubMed] [Google Scholar]