Abstract
Objective
Determine whether adults with hepatitis C, regardless of substance use disorder, are more likely to discount delayed rewards than adults without hepatitis C, and explore the relationship between delay discounting and neuropsychological functioning.
Methods
Procedures included clinical interviews, neuropsychological testing, and a delay discounting task.
Results
Regardless of substance abuse history, adults with hepatitis C were significantly more likely to choose smaller immediate rewards over larger delayed rewards. Delay discounting correlated with performance on executive functioning tasks.
Conclusions
Increased discounting is associated with broad executive dysfunction, suggesting that HCV associated executive dysfunction may lead to altered decision making style.
Keywords: hepatitis C, neuropsychology, impulsive behavior, substance-related disorders, delay discounting
Introduction
Hepatitis C Associated Executive Dysfunction
Chronic hepatitis C (HCV) infects approximately 2.2% of adults worldwide (“Global burden of disease (GBD) for hepatitis C”, 2004), 1.8% of adults in the United States (Seeff & Hoofnagle, 2003), and 5.4% of veterans seeking healthcare through facilities in the Veterans Healthcare Administration (VA) (Dominitz et al., 2004). Recent studies have documented a wide range of cognitive problems, which occur in approximately one-third of HCV+ adults with and without cirrhosis, including problems with verbal learning, aspects of attention/working memory, and several executive functions (e.g., mental flexibility and reasoning) (Huckans et al., 2009; Perry, Hilsabeck, & Hassenein, 2008 for a review). Not surprisingly, HCV associated cognitive impairments are predictive of important functional outcomes. For example, HCV associated impairments in speed of information processing are predictive of declines in the independent performance of instrumental activities of daily living (IADLs), while HCV associated impairments in fine-motor coordination predict declines in both IADLs and physical activities of daily living (PADLs) (Vigil et al., 2008).
Executive dysfunction, in particular, has been consistently demonstrated among persons with HCV. Our group (Huckans et al., 2009) found that 22.2% of HCV+ adults without advanced liver disease evidenced impairment on an executive domain consisting of tasks of reasoning, abstraction, and mental flexibility [Delis-Kaplin Executive Functioning System (D-KEFS) Sorting and Proverbs (Delis et al., 2001) and Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) Matrix Reasoning (Wechsler, 1997)], compared with only 5.4% of HCV-controls. Similarly, Bieliauskas and colleagues (Bieliauskas et al., 2006) found that 27% of HCV+ adults with advanced fibrosis were impaired on a task of mental flexibility [Wisconsin Card Sorting Task (WCST) (Heaton, 1981)]. Weissenborn and colleagues (Weissenborn et al., 2004) demonstrated that both mildly and moderately fatigued HCV+ adults were impaired relative to non-infected controls on a verbal reasoning task [WAIS-III Comprehension (Wechsler, 1997)]. Cherner and colleagues (Cherner et al., 2005; Letendre et al., 2005) demonstrated that, after controlling for methamphetamine abuse and HIV status, HCV status predicted impairments in the domain of abstraction/problem-solving [as measured by the WCST, Halstead Category Test, Trail Making Test B, and Stroop Task Interference Ratio (Heaton, Miller, Taylor, & Grant, 2004)]. Although not all investigators have found HCV associated deficits in verbal response inhibition (e.g., Huckans et al., 2009), Martin and colleagues (Martin et al., 2004) found that HCV status was associated with slowed as well as reduced performance on a Stroop task, and Cordoba and colleagues (Cordoba et al., 2003) found that HCV+ adults with decompensated liver cirrhosis evidenced impaired Stroop performance. Likewise, Posada and colleagues (in press) recently reported that HCV+ individuals were nearly three times more likely to self-report clinically elevated behavioral symptoms of disinhibition than seronegative comparison subjects. In short, both higher and lower level executive functions appear to be broadly impacted by HCV.
Although the exact etiology of HCV associated cognitive and executive dysfunction is unknown, multiple theories have been proposed, including virological mechanisms involving neuroimmune activation and cytokine/chemokine mediation (Lee et al., 2004; Meyers, Albitar, & Estey, 2005; Wilson, Finch, & Cohen, 2002), and non-virological mechanisms involving prevalent medical, psychiatric, and substance abuse comorbidities (Golden, O’Dwyer, & Conroy, 2005; Yovtcheva et al., 2001; Loftis & Hauser, 2003). Accumulating evidence supports virological mechanisms and brain involvement in HCV associated cognitive disorder (Forton et al., 2001; McAndrews et al., 2005), while comorbidities such as substance abuse are considered to play a lesser role (Forton, Taylor-Robinson, & Thomas, 2006; Huckans et al., 2009). Based on neuroimaging studies and the prominence of executive dysfunction in HCV+ adults, brain regions thought to be most susceptible to HCV associated injury include the frontal cortex and striatum (Huckans et al., 2009; Perry et al., 2008; Forton et al., 2001; McAndrews et al., 2005; Taylor et al., 2004; Weissenborn et al., 2004).
Impulsivity and Delay Discounting in the Context of Substance Use Disorders and Hepatitis C
HCV is transmitted through blood, and the most common transmission route is injection drug use (IDU) (Seeff & Hoofnagle, 2003). In general, HCV+ adults have high rates of substance use disorders (SUDs) (Thomson & Finch, 2005) and psychiatric comorbidities (Golden, O’Dwyer, & Conroy, 2005). These comorbidities are also common among veterans, and, in our own retrospective database study, 64% of HCV+ veterans using VA facilities in the northwest had a documented history of alcohol or drug use disorder (Huckans et al., 2006).
Impulsive behavior is thought to be central to the development and maintenance of SUD (Petry, 2003; Reynolds, 2006 for a review; Sher et al., 2004). Delay discounting has been described as a behavioral model of impulsivity, in which impulsive individuals are likely to choose smaller, less-valuable immediate rewards over larger, more-valuable delayed rewards (Ainslie, 1975). A variety of delay discounting tasks have been used to operationalize delay discounting (Reynolds, 2006 for a review), and the magnitude of an individual’s tendency towards delay discounting is commonly calculated as a function of the length of various delay periods as well as the value of various immediate versus delayed rewards (Reynolds, 2006). Thus far, research on delay discounting has primarily focused on its relationship to SUDs and suggests that, compared with adults without SUDs, adults with a variety of addictions evidence a significantly greater tendency toward delay discounting. This increased tendency toward delay discounting has been demonstrated with individuals with disordered use of alcohol (e.g., Petry, 2001), nicotine (e.g., Mitchell, 1999), cocaine (e.g., Kirby & Petry, 2004), methamphetamine (e.g., Hoffman et al., 2006), and heroin (e.g., Kirby, Petry, & Bickel, 1999; Madden et al., 1997).
As in SUDs, impulsive behaviors, including high risk drug behaviors such as needle sharing and impulsive decision making, are thought to play an important role in the transmission of infectious diseases (Odum et al., 2000; Seal & Agostinelli, 1994), including HCV (Cohen et al., 2006; Shaptava et al., 2006). However, there is currently no published research on delay discounting in adults with infectious diseases. Furthermore, most research on impulsivity in populations other than those with SUDs has relied on paper-and pencil measures of other aspects of impulsivity (Evenden, 1999 for a review). Studies have shown that delay discounting measures are not highly correlated with paper and pencil measures of general impulsivity (Mitchell, 1999; Reynolds, 2006), suggesting that delay discounting may be a more precise and sensitive measure of one aspect of impulsivity. Therefore, the primary objective of the current study is to examine whether adults with HCV are more likely to discount delayed rewards than adults without HCV. Given the high rates of executive dysfunction among HCV+ adults and the association between high risk behaviors and HCV transmission, our primary hypothesis is that HCV+ adults will be more likely to discount delayed rewards than HCV-Controls.
Impulsivity has been associated with cognitive impairments in the domains of working memory and decision making in healthy volunteers and ecstasy addicted individuals (Cools et al., 2007; Morgan et al., 2006). However, to date, few published studies have specifically examined how delay discounting relates to aspects of neuropsychological functioning. In a previous sample of methamphetamine abusers and non-dependent controls, our group found that increased discounting correlated with worse performance on verbal learning and memory tests (Hoffman et al., 2006). Using experimental models of both working memory load and delay discounting, another study found that individuals are more likely to discount delayed rewards under conditions of increased memory load (Hinson, Jameson, & Whitney, 2003). These same investigators found that delay discounting positively correlated with self-report ratings of aspects of impulsivity and executive dysfunction, suggesting that delay discounting may be associated with cognitive impairments in working memory and perhaps executive functioning. Therefore, a secondary objective of the current study is to better characterize the relationship between delay discounting and neuropsychological functioning, using a comprehensive neuropsychological battery that objectively assesses performance on a full range of cognitive domains. Our secondary hypothesis is that an increased tendency toward delay discounting will be associated with increased cognitive impairment, specifically in the domain of executive functioning.
Methods
Participants and Procedures
Three groups of veterans (n = 83) were recruited through the Portland VA Medical Center into three groups: 1) The HCV+/SUD-Group (n = 22) included veterans with chronic HCV and no history of SUD. 2) The HCV+/SUD+ Group (n = 31) included veterans with current HCV and a history of SUD; only adults who had been in full remission for at least 90 days were included in this group. 3) The HCV-/SUD-Group (n = 30) included veterans with no history of HCV or SUD. Lifetime history of SUD was assessed based on DSM-IV-TR (American Psychiatric Association, 2000) criteria for alcohol or drug abuse or dependence on substances other than nicotine or caffeine. Adults were not excluded from the SUD-groups for infrequent or recreational alcohol or drug use, provided they had never met criteria for abuse or dependence. All participants were paid $30 to complete the following study procedures: a) psychiatric clinical interview, b) comprehensive medical record review, c) a comprehensive neuropsychological assessment battery (described below in the section on Measures), and d) a standard version of the Delay Discounting Task (DDT) (Mitchell, 1999) (described below in the section on Measures).
Subjects were excluded from the present study based on the following criteria: 1) History of a major medical condition or currently unstable medical condition that was likely to be associated with current severe neurological or cognitive dysfunction (e.g., stroke, seizures, brain tumors, Parkinson’s disease, neurodegenerative disease, mental retardation, hepatic encephalopathy, HIV). In the interest of generalizability to typical HCV+ populations, adults with well-controlled or stable conditions were included as long as severe cognitive effects were not currently suspected (e.g., well-controlled diabetes, hypertension, or asthma). 2) History of traumatic brain injury with known loss of consciousness ≥ 30 minutes. 3) On the day of testing (< 24 hours), self-reported use of alcohol, illicit substances, medical marijuana, or medications with acute cognitive effects such as sedation or intoxication (e.g., benzodiazepines, opiates, muscle relaxants). 4) Advanced liver disease as indicated by any of the following: a) classified as having stage 4 liver disease or grade 4 inflammation upon biopsy (only a subset of participants underwent biopsies), OR, b) classified by a hepatologist as having probable decompensated cirrhosis based on clinical indicators and standard liver labs, OR, c) aspartate aminotransferase (AST) to platelet ratio index (APRI) ≥ 1.5. The APRI has been previously validated for use in HCV research, and values ≥ 1.5 reliably predict both liver fibrosis and cirrhosis (Lackner et al., 2005; Wai et al., 2003). 5) Current pregnancy. 6) History of schizophrenia or schizoaffective disorder, OR, current psychotic or manic episode. In the interest of generalizability to typical HCV+ populations, adults with other concurrent psychiatric diagnoses were included as long as present symptoms did not preclude valid cognitive testing (e.g., mild depression or anxiety, stable PTSD). 7) History of interferon therapy or chemotherapy for any reason. 8) Low estimated premorbid verbal IQ defined as WRAT3 Reading standard score < 80 [Wide Range Achievement Test, Third Edition, Reading, (Wilkinson, 1993)]. 9) Education > 16 years (to facilitate education matching across groups).
Table 1 summarizes demographic and clinical characteristics for the total sample and each group. There were no significant group differences based on age, race, gender, years of education, or estimated baseline cognitive reserve (operationalized as WRAT3 Reading standard score).
Table 1. Demographic and clinical characteristics by study group.
| Total Sample | HCV−/SUD− | HCV+/SUD− | HCV+/SUD+ | |
|---|---|---|---|---|
| Total N | 83 | 30 | 22 | 31 |
| Demographics | ||||
| Age (mean years ± SD) | 53.8 ± 7.9 | 53.3 ± 11.7 | 53.6 ± 5.4 | 54.4 ± 4.3 |
| Male gender | 77 (92.8%) | 27 (90.0%) | 19 (86.4%) | 31 (100.0%) |
| Caucasian | 75 (90.4%) | 28 (93.3%) | 19 (86.4%) | 28 (90.3%) |
| Years of education (mean ± SD) | 13.8 ± 1.5 | 14.3 ± 1.5 | 14.0 ± 1.7 | 13.4 ± 1.3 |
| Estimated baseline cognitive reserve (WRAT3 Reading mean ± SD) |
101.5 ± 9.7 | 104.7 ± 9.2 | 100.4 ± 10.3 | 99.0 ± 9.0 |
| Psychiatric History | ||||
| Current psychiatric diagnoses | 38 (45.8%) | 12 (40.0%) | 9 (40.9%) | 17 (54.8%) |
| Mood disorders | 31 (37.3% | 11 (36.7%) | 8 (36.4%) | 12 (38.7%) |
| PTSD | 15 (18.1%) | 4 (13.3%) | 5 (22.7%) | 6 (19.4%) |
| Other anxiety disorders | 9 (10.8%) | 2 (6.7%) | 3 (13.6%) | 4 (12.9%) |
| Medical History | ||||
| Current medical diagnoses | 43 (51.8%) | 16 (53.3%) | 13 (59.1%) | 14 (45.2%) |
| Diabetes | 10 (12.0%) | 6 (20.0%) | 1 (4.5%) | 3 (9.7%) |
| Hyperlipidemia | 19 (22.9%) | 10 (33.3%) | 3 (13.6%) | 6 (19.4%) |
| Hypertension | 23 (27.7%) | 8 (26.7%) | 9 (40.9%) | 6 (19.4%) |
| Cardiovascular disease | 9 (10.8%) | 4 (13.3%) | 2 (9.1%) | 3 (9.7%) |
| Asthma/pulmonary | 12 (14.5%) | 3 (10.0%) | 4 (18.2%) | 5 (16.1%) |
Note: Data are expressed as n, with (%) in terms of n over total N, unless otherwise stated. Analyses compared differences between the three study groups. For non-continuous variables, Kruskal Wallis tests were used. For continuous variables, ANOVAs were used. There were no significant (p ≤ 0.050) group differences based on any of the above variables. HCV = hepatitis C. PTSD = post traumatic stress disorder. SD = standard deviation. SUD = history of substance use disorder, currently in remission. WRAT3 = Wide Range Achievement Test, Third Edition.
While all adults were stable enough to meet inclusion criteria, the total sample included adults with high rates of current psychiatric diagnoses (45.8%) and medical conditions (51.8%). However, groups did not significantly differ in terms of rates of these conditions. The HCV+/SUD+ group (n = 31) included adults with at least ninety days of remission. However, most adults reported several years of remission (average years since remission = 9.1 ± 7.7). Prior to remission, most participants reported many years of abuse [average years of abuse = 19.1 ± 12.4], at an average level of abuse that could be categorized as moderate based on a brief and valid measure of substance dependence severity [Severity of Dependence Scale (SDS) (Gossop et al., 2002; Gossop et al., 1995), average SDS score = 7/25 ± 3.5]. Participants met DSM-IV-TR (American Psychiatric Association, 2000) criteria for previous abuse of or dependence on the following substances: alcohol (83.9%), stimulants (83.9%), marijuana (54.8%), opiates (51.6%), hallucinogens (6.5%), and other drugs of abuse (9.7%). Most participants reported polysubstance abuse (90.3%), so these categories are not mutually exclusive.
Within the HCV+/SUD-group (n = 22), the following risk factors were reported as the most likely HCV transmission route: blood transfusion (22.7%, n = 5), accidental exposure at work (22.7%, n = 5), blood exposure during combat (9.1%, n = 2), and other or unknown risk factors (36.4%, 2 plasma or blood donations, 1 military immunization, 1 tattoo, 4 unknown). Although two (9.1%) remaining individuals in the HCV+/SUD-group reported contracting HCV via remote IDU, their reported use was described as infrequent and experimental only, and neither of these individuals ever met criteria for SUD of any type. Within the HCV+/SUD+ group (n = 31), the following risk factors were reported as the most likely transmission route: IDU (61.3%, n = 19), accidental exposure at work (6.5%, n = 2), blood transfusion (9.7%, n = 3), blood exposure during combat (9.7%, n = 3), and other or unknown risk factors (12.9%, 1 military immunization, 3 unknown).
Measures
Delay Discounting Task (DDT) (Mitchell, 1999)
For each item on the DDT, a subject is asked which of two monetary rewards they would prefer: $100 following a delay, or another amount, of equivalent or lesser value, available now (See Figure 1). The immediate (“now”) reward varies across items and ranges from $1 to $99. The delayed reward is always fixed at $100, but the delay period varies across items, including one of six options (now, 7 days, 30 days, 90 days, 180 days, 365 days). For each delay period, we calculated a subject’s indifference point, which is the point (or immediate reward value) at which the subject switched their preference to the smaller immediate reward instead of the larger delayed reward ($100). A non-linear regression was then used to solve for the function that best fit these indifference points. This function is termed the indifference curve and is represented as where I represents the value of the immediate reward, t represents the delay time and K is a constant that characterizes the degree of discounting (Green, et al., 1997; Johnson & Bickel, 2003; Baker et al., 2003). Higher values of K represent a greater tendency to discount delayed rewards. Because K is not normally distributed, the natural log of K [ln(K)] was used in statistical analyses. Less negative values of ln(K) indicate greater delay discounting.
Figure 1. Delay Discounting Task – Example Item.
Subjects are asked: “At this moment, which would you prefer?”
Neuropsychological Battery
The neuropsychological battery included a depression inventory [Beck Depression Inventory, Second Edition (BDI-II); Beck, Steer, & Brown, 1996] as well as well-validated and widely available cognitive measures testing a full range of cognitive domains. Appendix 1 lists the cognitive subtests by cognitive domain along with the source of their respective normative samples. Raw scores were converted to standard scores based on these norms which corrected for age, gender, race, and years of education, as available by subtest. Average z scores were additionally calculated for each cognitive domain [i.e., sum of all subtest scores (z scores) in a cognitive domain, divided by total number of subtests within that domain].
Data Analysis
All analyses were conducted with SPSS (version 15). Unless otherwise specified, p values ≤ 0.05 were considered significant. Between group analyses of demographic variables and cognitive domain scores (average z scores) were conducted using analysis of variance (ANOVA) and post hoc Scheffe tests for continuous variables; for binary responses, we used three-sample Kruskal-Wallis tests because multiple cells contained low frequencies (<5). For between group comparisons in terms of DDT performance [ln(K)], we conducted ANOVA and post hoc Scheffe tests; η2 was used as a measure of effect size. Pearson R correlations were used to explore the relationship between DDT performance [n(K)] and neuropsychological performance by cognitive domain (average z scores) and by individual subtest score (standardized score), first within the total sample, then within the HCV+ groups combined, and finally within the HCV-group.
Results
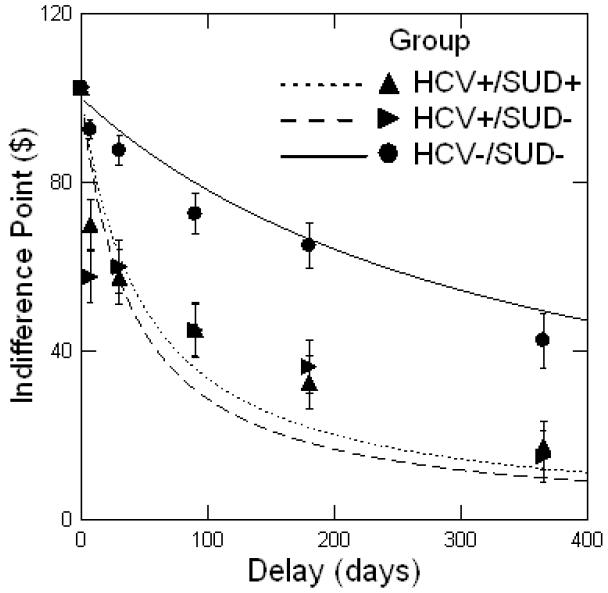
As summarized in Table 2 and Figure 2, ANOVA revealed significant group differences in delay discounting. Specifically, post hoc comparisons (Scheffe) indicated that adults with HCV (HCV+ groups) were significantly more likely to choose smaller immediate rewards over larger delayed rewards compared with adults without HCV (HCV-/SUD-group). This was true even for participants with HCV who had no history of SUD (HCV+/SUD-group). There were no differences in delay discounting across the two HCV+ groups.
Table 2. Adults with hepatitis C (HCV) are more likely to discount delayed rewards on the Delay Discounting Task, even without a history of substance use disorder (SUD).1.
| HCV−/SUD− (n=30) |
HCV+/SUD− (n=22) |
HCV+/SUD+ (n=31) |
p-value | Effect Size (η2) |
|
|---|---|---|---|---|---|
|
ln(K)2 (least square mean) |
−5.88a b | −3.92a | −3.69b | 0.001 | 0.162 |
| Standard Deviation | 1.66 | 2.23 | 2.90 | -- | -- |
ANOVA was used to compare differences between the three study groups. Variables with the same superscript were significantly different (p ≤ 0.050) using post hoc Scheffe tests. The magnitude of lnK significantly differed between the HCV+ and HCV− groups, but not between the two HCV+ groups.
ln(K) (natural logarithm of K) is reported because K is not normally distributed. Higher (i.e., less negative) values of ln(K) represent a stronger tendency to discount larger delayed rewards in favor of smaller immediate rewards. HCV = hepatitis C. SUD = history of substance use disorder, currently in remission.
Figure 2. Average discounting function by study group.
Note: Performance on the Delay Discounting Task (DDT) is summarized as the function that best fits the mean ln(K) (natural logarithm of K) values by group. This function is termed the indifference curve and is represented as where I represents the value of the immediate reward, t represents the delay time, and K is a constant that characterizes the degree of discounting. Points depicted above are the median indifference points (the immediate monetary value at which a participant switches preferences from the larger delayed reward to the smaller immediate reward) for a given delay period, and the bars represent the standard error. HCV = hepatitis C. SUD = history of substance use disorder, currently in remission.
As summarized in Table 3, both HCV+ groups performed significantly worse than the HCV-group on tests within the Verbal Memory and Executive Functioning domains (average z scores); the two HCV+ groups did not significantly differ in terms of performance within these domains. The HCV+/SUD+ group (but not the HCV+/SUD-group) also performed significantly worse than the HCV-group on tests within the Speeded Visual Information Processing/Attention domain.
Table 3. Between group comparisons of neuropsychological performance by cognitive domain (average z score).
| HCV−/SUD− (n=30) |
HCV+/SUD− (n=22) |
HCV+/SUD+ (n=31) |
p-value | |
|---|---|---|---|---|
| Language Fluency | 0.066 ± 0.84 | 0.147 ± 0.71 | −0.102 ± 0.81 | .508 |
| Verbal Memory | 0.179 ± 0.67a b | −0.577 ± 0.82a | −0.558 ± 1.01b | .001*** |
| Visuospatial Memory | −0.222 ± 0.76 | −0.691 ± 0.86 | −0.592 ± 0.83 | .110 |
|
Auditory Attention/Working Memory |
0.233 ± 0.92 | −0.174 ± 0.60 | 0.022 ± 0.55 | .131 |
| Speeded Visual Information | 0.095 ± 0.70a | −0.258 ± 0.59 | −0.318 ± 0.61a | .036* |
| Processing/Attention | ||||
| Motor Speed | −0.688 ± 0.56 | −0.653 ± 0.69 | −0.751 ± 0.66 | .859 |
| Visuomotor Construction | −0.443 ± 0.97 | −0.691 ± 1.01 | −0.383 ± 0.95 | .505 |
| Executive Functioning | 0.363 ± 0.69a b | −0.157 ± 0.78a | −0.269 ± 0.61b | .001*** |
Note: Data expressed as the mean ± standard deviation. p values reflect between group comparisons (ANOVA) across the three study groups. Variables with the same superscript were significantly (p ≤ 0.05) different using post hoc Scheffe tests. HCV = hepatitis C. SUD = history of substance use disorder, currently in remission.
p ≤0.050.
p ≤0.001.
As summarized in Table 4, within the total sample and within the HCV+ groups combined, increased delay discounting was significantly correlated with worse Executive Functioning domain scores (average z scores) and with worse individual subtest scores within this domain; these correlations were not significant, however, within the HCV-group. There were also significant correlations between increased delay discounting and worse Auditory Attention/Working Memory domain scores within the total sample, and between increased delay discounting and worse performance on one subtest (WAIS-III Digit Span) in this domain within both the total sample and the HCV+ groups; again, these correlations were not statistically significant within the HCV-group.
Table 4. Bivariate correlations between delay discounting (1nK) and neuropsychological functioning by cognitive domain (average z score) and individual subtest score (standardized score).
|
Correlation
Total Sample1 |
p-value |
Correlation
HCV+ Groups2 |
p-value |
Correlation
HCV− Group3 |
p-value | |
|---|---|---|---|---|---|---|
| Language Fluency | .060 | .589 | .078 | .578 | .104 | .584 |
| Letter Fluency | .010 | .927 | −.012 | .931 | .134 | .481 |
| Category Fluency | .093 | .403 | .136 | .330 | .009 | .963 |
| Verbal Memory | −.185 | .094 | −.050 | .721 | .039 | .837 |
| CVLT-II Total Immediate | −.197 | .074 | −.124 | .377 | .067 | .726 |
| CVLT-II Long Delay Free Recall | −.161 | .146 | −.088 | .532 | .048 | .800 |
| CVLT-II Recognition Correct Hits | −.117 | .294 | .048 | .735 | −.049 | .797 |
| Visuospatial Memory | −.110 | .356 | .030 | .848 | −.106 | .586 |
| BVMT-R Total Immediate Recall | −.057 | .626 | .040 | .790 | .132 | .487 |
| BVMT-R Delayed Recall | −.106 | .364 | −.011 | .940 | .023 | .907 |
| RCF Immediate Recall | −.157 | .176 | −.045 | .769 | −.355 | .054 |
| RCF Delayed Recall | −.074 | .505 | .033 | .814 | −.222 | .238 |
|
Auditory Attention/Working Memory |
−.279 | .011** | −.211 | .129 | −.307 | .099 |
| WAIS-III Digit Span | −.333 | .002** | −.297 | .031* | −.267 | .153 |
| WAIS-III Letter Number Sequencing | −.133 | .232 | −.025 | .856 | −.263 | .160 |
|
Speeded Visual Information Processing/Attention |
−.026 | .822 | .131 | .364 | .058 | .767 |
| Trails A | .042 | .705 | .015 | .914 | .214 | .256 |
| Trails B | −.009 | .936 | .029 | .838 | .242 | .198 |
| WAIS-III Digit Symbol | −.125 | .268 | .016 | .912 | .125 | .509 |
| D-KEFS CWIT Inhibition | .033 | .773 | .197 | .165 | −.077 | .692 |
| D-KEFS CWIT Switching | −.058 | .611 | .098 | .492 | −.325 | .085 |
| Motor Speed | −.049 | .677 | −.067 | .653 | .026 | .897 |
| Finger Tapping Dominant | .017 | .880 | −.019 | .897 | .378 | .073 |
| Finger Tapping NonDominant | .009 | .941 | −.128 | .380 | .301 | .106 |
| Pegboard Dominant | −.103 | .365 | −.029 | .841 | −.326 | .084 |
| Pegboard NonDominant | −.003 | .980 | .041 | .782 | −.134 | .488 |
| Visuomotor Construction | .016 | .882 | .054 | .702 | −.024 | .900 |
| RCF Copy | .016 | .882 | .054 | .702 | −.024 | .900 |
| Executive Functioning | −.391 | .001*** | −.321 | .019** | −.193 | .308 |
| D-KEFS Sorting Correct Sorts | −.320 | .003** | −.290 | .035* | −.099 | .601 |
| D-KEFS Proverbs Free Inquiry | −.341 | .002** | −.286 | .038* | −.247 | .188 |
| WAIS-III Matrix Reasoning | −.262 | .017* | −.156 | .263 | −.116 | .540 |
Note: Pearson product moment correlations were conducted first within the total sample , then within the combined HCV+ groups2, and finally within the HCV− control group3. Cognitive domain scores (average z score across all subtests in a domain) and individual subtest scores (standardized scores) were used for analyses. BVMT-R = Brief Visuospatial Memory Test, Revised. CVLT = California Verbal Learning Test. CWIT= Color Word Interference Test. D-KEFS = Delis Kaplan Executive Functioning Scale. RCF = Rey Complex Figure. WAIS = Wechsler Adult Intelligence Scale.
p ≤0.050.
p ≤0.010.
p ≤0.001.
Post hoc analyses were conducted to explore the relationship between depression (BDI-II scores) and cognitive dysfunction (average z scores) and delay discounting (lnK). ANOVA [F (2, 80) = 3.545, p = 0.033] followed by post hoc Scheffe tests revealed that the HCV+/SUD+ group reported significantly higher levels of depression than the HCV-control group (HCV-/SUD-= 6.8±7.4; HCV+/SUD-= 11.2±12.8; HCV+/SUD+ = 14.1± 11.9; p = 0.035) but that no other between group differences reached statistical significance. Within the HCV+ groups, depression significantly correlated with Motor Speed (r = −.394, p = .006) but not with other cognitive domains and so did not appear to account for between group differences in cognitive performance (other non-significant correlations ranged from −.228 to .023, and the non-significant correlation between Executive Function and depression was −.141). Correlations between delay discounting and depression did not reach statistical significance (p > 0.050) within the total sample (r = 0.106), within the HCV+ groups (r = 0.047), nor within the HCV-control group (r = − 0.225).
Discussion
The primary objectives of the present study were to determine whether HCV+ adults have an increased tendency to discount delayed rewards compared with HCV-adults, and to better characterize the relationship between neuropsychological functioning and delay discounting. To our knowledge, our study is the first to examine delay discounting in adults with infectious diseases, and specifically in HCV+ adults with and without a history of substance abuse.
As hypothesized, we found that, compared with adults without HCV, adults with HCV were significantly more likely to choose smaller immediate rewards over larger delayed rewards, even when they had no history of SUD. Because a primary aim of the study was to rule out SUD as a confounding factor to HCV effects, it is not surprising that we found no differences between our HCV+/SUD- and HCV+/SUD+ groups in terms of delay discounting or neuropsychological performance. Indeed within the HCV+/SUD+ group, SUD history was rather remote, substances of abuse were mixed, and abuse and dependence were combined. These characteristics enabled us to more clearly demonstrate HCV associated effects in the absence of a SUD. However, these same characteristics did not allow us to adequately test for possible additive effects of substance use history and therefore represent a limitation to our study.
In short, our results clearly indicate that, in our sample, increased discounting was attributable to HCV status rather than common comorbidities. Although our study design does not allow us to identify the direction of causality nor the neurobiological mechanisms underlying our findings, we have several hypotheses worthy of further investigation: 1) Because impulsive behavior is thought to play an important role in the transmission of infectious diseases including HCV (Cohen et al., 2006; Seal & Agostinelli, 1994; Shaptava et al., 2006), one possibility is that impulsive individuals are more likely to contract HCV. However, the majority (20/22; 91.9%) of our HCV+/SUD-adults contracted HCV through accidental exposure (e.g., transfusions, work exposure) rather than IDU or high risk behaviors, and, while the majority (19/31; 61.3%) of our HCV+/SUD+ adults contracted HCV through IDU, a substantial minority (12/31; 38.7%) contracted HCV through other causes. Contraction data and substance use history were based on medical records and self-report, so we cannot definitively rule out the possibility that the HCV+/SUD- and HCV-/SUD-groups under-reported high risk health behaviors. Nevertheless, it appears more plausible that, in our sample, increased discounting arose following HCV infection. 2) For example, adults with HCV, and perhaps other chronic diseases or disorders, may be more likely to view their futures as tenuous or to believe that they will soon become seriously ill or die, which could lead them to devalue delayed rewards. In support of this theory, Petry et al. (1998) found that heroin addicts who discounted delayed rewards had significantly shortened time horizons as measured by the Stanford Time Perception Inventory (STPI) (Zimbardo, 1999) and the Future Time Perspective (FTP) measure (Wallace, 1956). Heroin addicts also had decreased sensitivity to the delayed consequences of their behaviors, perhaps because their shortened perception of the future did not involve these consequences. Although a tendency toward shortened time horizons might be an attractive and perhaps parsimonious explanation for our sample’s increased tendency toward delay discounting, it would not explain why delay discounting was correlated with specific cognitive impairments within our total sample and within our HCV+ groups, but not within our HCV-control group. 3) Therefore, a third hypothesis is that chronic HCV infection causes cognitive dysfunction which then alters an individual’s decision making style, capacity to evaluate outcomes, and/or ability to overcome the negative salience of delayed rewards. In line with this theory, we found that compared with HCV-controls, HCV+ adults performed significantly worse on several cognitive domains (Executive Function, Verbal Memory, and Speeded Visual Information Processing/Attention) and that HCV associated Executive Function and Verbal Memory deficits existed even in the absence of a history of substance use disorder. Moreover, within the total sample and within the HCV+ groups, but not within the HCV-group, increased delay discounting significantly correlated with worse performance on several tests of Attention/Working Memory and Executive Function: D-KEFS Sorting, D-KEFS Proverbs, WAIS-III Matrix Reasoning (total sample only), and WAIS-III Digit Span. D-KEFS Sorting is a task that measures problem-solving, concept formation, and mental flexibility, and D-KEFS Proverbs and WAIS-III Matrix Reasoning measure verbal and nonverbal abstraction and reasoning, all of which are considered aspects of the larger construct of executive functioning. WAIS-III Digit Span is considered an attention/working memory task, but it can also be described as an executive functioning task because it requires an individual to manipulate information and strategize. In short, it makes sense that, in our sample, delay discounting was related to impairments on these tests of attention/working memory and reasoning/mental flexibility because these tests each rely on executive functions.
Although a thorough review of the anatomical correlates of executive functioning and delay discounting are far beyond the scope of the present paper, numerous imaging studies have demonstrated that executive function processes are largely controlled by frontal and frontal-striatal circuits (Aron & Paulus, 2007; Collette et al., 2006 for a review) related to the “cognitive” (i.e., dorsal anterior cingulate cortex, dorsal lateral prefrontal cortex, posterior parietal cortex, superior temporal gyrus) and “affective” (i.e., amygdala, ventral striatum, ventral lateral prefrontal cortex, ventral anterior cingulate cortex, anterior insula) circuits that have been consistently activated during the DDT in previous imaging studies (Ernst & Paulus, 2005; Hoffman et al., 2008; McClure et al., 2004; Monterosso et al., 2006; Wittman et al., 2007; Boettiger et al., 2007; Monterosso et al., 2007). While our study design does not allow us to make causal or neuroanatomical inferences, our study does provide preliminary evidence that the ability to choose larger delayed rewards over smaller immediate rewards (i.e., to delay gratification) is in part dependent on a range of executive functions. Moreover, our results suggest that HCV associated impairments in executive functioning may lead to alterations in delay discounting, and perhaps in decision-making and impulsivity more generally. Lastly, our results indicate that future studies utilizing functional neuroimaging to better delineate the effects of HCV on cerebral function and a broader range of impulsive behaviors and dysexecutive problems is warranted.
Several additional study limitations should be considered. First, the sample consisted of primarily Caucasian male veterans with some college education. Therefore, findings may not be generalizable to wider HCV+ populations. Moreover, although our groups were similar in terms of many important characteristics (e.g., age, education, estimated pre-morbid intellectual ability, rates of psychiatric and medical comorbidities), it is unclear to what extent group differences on unknown factors (e.g., motivation, values, pre-morbid characteristics) may have contributed to our results. Another limitation is that our study design did not allow us to more carefully examine the clinical and functional significance of HCV associated alterations in delay discounting. Non-health related risk behaviors were not queried, and high risk health behaviors related to HCV transmission may have been under-reported. Future studies should, therefore, explore the extent to which a tendency to discount delayed rewards predicts, for example, completion of ADLs, treatment compliance, a broad range of risk behaviors (e.g., needle sharing, high-risk sexual behaviors, gambling, illegal activities, high-risk sports), violence or hostility, behavioral regulation (e.g., disinhibition) and metacognition (e.g., poor planning) in the daily lives of adults with HCV. Lastly, it should be noted that the significant correlations between delay discounting and executive function within the total sample and the HCV+ groups are small to moderate and explain only a small percentage of the variance in delay discounting; thus, future studies should examine what factors besides cognitive abilities contribute to delay discounting and other impulsive behaviors.
In summary, our results indicate that HCV+ adults are more likely to exhibit executive dysfunction and to discount delayed rewards than adults without HCV, even in the absence of a history of substance abuse or dependence. Additionally, executive dysfunction is significantly correlated with, and may therefore contribute toward, an increased tendency to discount larger delayed rewards in favor of smaller immediate rewards in HCV+ and other populations.
ACKNOWLEDGEMENTS
The first author would like to thank Diane Howieson for her initial input into the analysis plan and conceptualization of this study; Daniel Kriz, Hannah Luber, Renee Anderson, and Michael Kolessar for their many contributions as current research assistants within Dr. Huckans’ research program; Emily Kizer, Danell Bjornson, Laura Parisi, and Samantha Ruimy for administrative and recruitment support over the years; Daniel Storzbach and Arthur Vandenbark for their continued mentorship and consultation; Betsy Zucker, Anna Sasaki, Michael Chang, and the other providers of the PVAMC Liver Clinic for their on-going collaborative support of Dr. Huckans’ research.
This material is based upon work in part supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development, as well as by the Oregon Health and Science University. This study was supported in part by a Northwest Health Foundation Grant and a VA Career Development Award to Dr. Huckans.
Appendix 1. Subtests by cognitive domain and the source of their respective normative samples
| Norms | |
|---|---|
| Language Fluency | |
| Letter Fluency-Controlled Oral Word Association (COWA) (Benton & Hamsher, 1989) |
Delis Kaplan Executive Function System (D-KEFS) Manual (Delis, Kaplan & Kramer, 2001) |
| Category Fluency-Animals (Rosen, 1980) |
(Lezak, Howieson, & Loring, 2005) |
| Verbal Memory | |
| California Verbal Learning Test Second Edition (CVLT-II) |
CVLT-II Manual/Computer Printout (Delis et al., 1987) |
| Visuospatial Memory | |
| Benton Visual Memory Test Revised (BVMT-R) | BVMT-R Manual (Benedict, 1997) |
| Rey-Osterrieth Complex Figure (RCF) Immediate and Delayed Recall (Osterrieth, 1944) |
(Mitsrushina et al., 2005) |
| Auditory Attention | |
| Wechsler Adult Intelligence Scale Third Edition (WAIS-III) Digit Span |
WAIS-III Manual (Wechsler, 1997) |
| WAIS-III Letter Number Sequencing | WAIS-III Manual (Wechsler, 1997) |
| Speeded Visual Information Processing/Attention | |
| Trails A and B (Reitan & Wolfson, 1985) |
Revised Heaton Norms (Heaton et al., 2004) |
| WAIS-III Digit Symbol | WAIS-III Manual (Wechsler, 1997) |
| D-KEFS Color-Word Interference Test (CWIT) | D-KEFS Manual (Delis, Kaplan & Kramer, 2001) |
| Motor Speed | |
| Finger Tapping (Halstead, 1947; Reitan, 1955) |
Revised Heaton Norms (Heaton et al., 2004) |
| Grooved Pegboard (Klove, 1963) |
Revised Heaton Norms (Heaton et al., 2004) |
| Visuomotor Construction | |
| RCF Copy (Osterrieth, 1944) |
(Mitsrushina et al., 2005) |
| Executive Functioning | |
| D-KEFS Sorting | D-KEFS Manual (Delis, Kaplan & Kramer, 2001) |
| D-KEFS Proverbs | D-KEFS Manual (Delis, Kaplan & Kramer, 2001) |
| WAIS-III Matrix Reasoning | WAIS-III Manual (Wechsler, 1997) |
References
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual for mental disorders. American Psychiatric Association; Washington, DC: 2000. Fourth Edition, Text Revision ed. [Google Scholar]
- Aron JL, Paulus MP. Location, location: Using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102:33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discouting in current and never-before cigarette smokers: similarities and differences among commodity, sign, and magnitude. Journal of Abnormal Psychology. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Beck A.t., Steer RA, Brown GK. Beck Depression Inventory Manual, Second Edition. Psychological Corporation; New York: 1996. [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test - Revised. Psychological Assessment Resources, Inc.; Lutz, FL: 1997. [Google Scholar]
- Benton AL, Hamsher K. deS. Multilingual Aphasia Examination. AJA Associates; Iowa City, Iowa: 1989. [Google Scholar]
- Bieliauskas LA, Back-Madruga C, Linday KL, Snow KK, Kronfol Z, Lok AS, Padmanabhan L, Fontana RJ. Clinical relevance of cognitive scores in hepatitis C patients with advanced fibrosis. Journal of Clinical and Experimental Neuropsychology. 2006;28:1346–1361. doi: 10.1080/13803390500473720. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-o-methyltransferase 158 genotype. The Journal of Neuroscience. 2007;27(52):14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, et al. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64(8):1343–1347. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- Cohen DE, Russel CJ, Golub SA, Mayer KH. Prevalence of hepatitis C virus infection among men who have sex with men at a Boston community health center and its association with markers of high-risk behavior. AIDS Patient Care STDS. 2006;20:557–64. doi: 10.1089/apc.2006.20.557. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. The Journal of Neuroscience. 2007;27(20):5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba J, Flavia M, Jacas C, Sauleda S, Esteban JI, Vargas V, Esteban R, Guardia J. Quality of life and cognitive function in hepatitis C at different stages of liver disease. Journal of Hepatology. 2003;39:231–238. doi: 10.1016/s0168-8278(03)00189-2. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Functioning System. Harcourt Assessment Company; New York: 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan e., Ober BA. California Verbal learning Test: Adult Version Manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Dominitz JA, Boyko EJ, Koepsell TD, Heagert PJ, Maynard C, Sporleder JL. Prevalence of Hepatitis C Infection in Veterans: VA Cooperative Study #488. VA Health Services Research & Development Meeting; Washington, D.C.: Mar 4, 2004. 2004. Paper presented at the. [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58(8):597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Forton DM, Allsop JM, Main J, Foster GR, Thomas HC, Taylor-Robinson SD. Evidence for a cerebral effect of the hepatitis C virus. Lancet. 2001;358(9275):38–39. doi: 10.1016/S0140-6736(00)05270-3. [DOI] [PubMed] [Google Scholar]
- Forton DM, Taylor-Robinson SD, Thomas HC. Central nervous system changes in hepatitis C virus infection. European Journal of Gastroenterological Hepatology. 2006;18:333–338. doi: 10.1097/00042737-200604000-00005. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, McFadden E. Rate of temporal discounting decreases with amount of reward. Memory & Cognition. 1997;25:715–723. doi: 10.3758/bf03211314. [DOI] [PubMed] [Google Scholar]
- Global burden of disease (GBD) for hepatitis C Journal of Clinical Pharmacology. 2004;44(1):20–29. doi: 10.1177/0091270003258669. [DOI] [PubMed] [Google Scholar]
- Golden J, O’Dwyer AM, Conroy RM. Depression and anxiety in patients with Hepatitis C: Prevalence, detection rates and risk factors. General Hospital Psychiatry. 2005;27:431–438. doi: 10.1016/j.genhosppsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Gossop M, Marsden J, Stewart D. Dual dependence: assessment of dependence upon alcohol and illicit drugs, and the relationship of alcohol dependence among drug misusers to patterns of drinking, illicit drug use and health problems. Addiction. 2002;97(2):169–178. doi: 10.1046/j.1360-0443.2002.00028.x. [DOI] [PubMed] [Google Scholar]
- Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, Strang J. The Severity of Dependence Scale (SDS): Psychometric properties in English and Australian samples of heroin, cocaine, and amphetamine users. Addiction. 1995;90:607–614. doi: 10.1046/j.1360-0443.1995.9056072.x. [DOI] [PubMed] [Google Scholar]
- Halstead WC. Brain and Intelligence. University of Chicago Press; Chicago, IL: 1947. [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test. Psychological Assessment Resources; Odessa, FL: 1981. [Google Scholar]
- Heaton R, Miller W, Taylor M, Grant I. Revised Comprehensive Norms for an Expanded Halsted-Reitan Battery. Psychological Assessment Resources, Inc.; Lutz, FL: 2004. [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. Journal of Experimental Psychology. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological functions and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188:162–70. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans M, McFarland BH, Meiri G, Stevens AA, Mitchell S. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckans MS, Blackwell AD, Harms TA, Indest DW, Hauser P. Integrated hepatitis C virus treatment: addressing comorbid substance use disorders and HIV. AIDS. 2006;19(suppl 3):S106–S115. doi: 10.1097/01.aids.0000192078.49185.b0. [DOI] [PubMed] [Google Scholar]
- Huckans M, Seelye A, Parcel T, Mull L, Woodhouse J, Bjornson D, Fuller B, Loftis JM, Morasco B, Sasaki AW, Storzbach D, Hauser P. The cognitive effects of hepatitis C in the presence and absence of a history of substance use disorder. Journal of the International Neuropsychological Society. 2009;15:69–82. doi: 10.1017/S1355617708090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H. Clinical Neuropsychology. Medical Clinics of North America. 1963;47:1647–1658. [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41(6):1376–1382. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, Myers CA, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, et al. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. Aids. 2005;19(Suppl 3):S72–78. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Loring D. Neuropsychological Assessment. Oxford University Press; New York: 2005. [Google Scholar]
- Loftis JM, Hauser P. Hepatitis C in patients with psychiatric disease and substance abuse: Screening strategies and comanagement models of care. Current Hepatitis Report. 2003;2:93–100. [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Martin EM, Novak RM, Fendrich M, Vassileva J, Gonzalez R, Grbesic RM, Nunnally G, Sworowski L. Stroop performance in drug users classificed by HIV and hepatitis C serostatus. Journal of the International Neuropsychological Society. 2004;10:298–300. doi: 10.1017/S135561770410218X. [DOI] [PubMed] [Google Scholar]
- McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S, Heathcote EJ. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41:801–808. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DL, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetaminedependent and comparison subjects performing a delay discounting task. Human Brain Mapping. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Impallomeni LC, Pirona A, Rogers RD. Elevated impulsivity and impaired decision-making in abstinent ecstasy (MDMA) users compared to polydrug and drug-naïve controls. Neuropsychopharmacology. 2006;31:1562–1573. doi: 10.1038/sj.npp.1300953. [DOI] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Badger GJ, Bickel WK. Needle sharing in opioid-dependent outpatients: psychological processes underlying risk. Drug and Alcohol Dependence. 2000;60:259–66. doi: 10.1016/s0376-8716(00)00111-3. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complexe. Archives de Psychologie. 1944;30:206–356. translated by Corwin J, Bylsma FW. The Clinical Neuropsychologist. 1993;7:9–15.
- Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic Hepatitis C: A review. Digestive Disease Sciences. 2008;53:307–321. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]; Seal DW, Agostinelli G. Individual differences with high-risk sexual behaviour: implications for intervention programmes. AIDS Care. 1994;6:393–7. doi: 10.1080/09540129408258653. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of money, health, and freedom in substance abusers and controls. Drug and Alcohol Dependence. 2003;71:133–141. doi: 10.1016/s0376-8716(03)00090-5. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93(5):729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Posada C, Moore DJ, Woods SP, Vigil O, Perry W, Hassanein T, Letendre SL, Grant I, HNRC Group Hepatitis C virus is associated with elevated behavioral symptoms of frontal systems dysfunction. Journal of Clinical and Experimental Neuropsychology. in press. [Google Scholar]
- Reitan RB. Investigation of the validity of Halstead’s measures of biological intelligence. Archives of Neurology and Psychiatry. 1955;73:28–35. doi: 10.1001/archneurpsyc.1955.02330070030005. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Neuropsychological Press; Tuscson, AZ: 1985. [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behavioral Pharmacology. 2006;17(8):651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Rosen WG. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980;2:135–146. [Google Scholar]
- Seal DW, Agostinelli G. Individual differences associated with high-risk sexual behaviour: implications for intervention programmes. AIDS Care. 1994;6(4):393–397. doi: 10.1080/09540129408258653. [DOI] [PubMed] [Google Scholar]
- Seeff LB, Hoofnagle JH. Appendix: The National Institutes of Health Consensus Development Conference Management of Hepatitis C 2002. Clin Liver Dis. 2003;7(1):261–287. doi: 10.1016/s1089-3261(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Shapatava E, Nelson KE, Tsertsvadze T, del Rio C. Risk behaviors and HIV, hepatitis B, and hepatitis C seroprevalence among injection drug users in Georgia. Drug and Alcohol Dependence. 2006;82:35–8. doi: 10.1016/s0376-8716(06)80006-2. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. 2004 doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Letendre SL, Schweinsburg BC, Alhassoon OM, Brown GG, Gongvatana A, Grant I, HNRC Hepatitis C virus infection is associated with reduced white matter N-acetylaspartate in abstinent methamphetamine users. Journal of the International Neuropsychological Society. 2004;10:110–113. doi: 10.1017/S1355617704101161. [DOI] [PubMed] [Google Scholar]
- Vigil O, Posada C, Woods SP, Atkinson JH, Heaton RK, Perry W, Hassanein TI, Grant I, Letendre SL, HNRC group Impairments in fine-motor coordination and speed of information processing predict declines in everyday functioning in hepatitis C infection. Journal of Clinical and Experimental Neuropsychology. 2008;4:1–11. doi: 10.1080/13803390701802354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- Wallace M. Future time perspective in schizophrenia. Journal of Abnormal Psychology. 1956;52(2):240–245. doi: 10.1037/h0039899. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, Administration and Scoring Manual. Third Edition Psychological Corporation; New York: 1997. [Google Scholar]
- Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schuler A, Ennen JC, Ahl B, Manns MP, Boker KW. Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. Journal of Hepatology. 2004;41:845–851. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. WRAT3 Administration Manual. Wide Range; Delaware: 1993. [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition-the case for a head-to-toe inflammatory paradigm. Journal of the American Geriatrics Society. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Experimental Brain Research. 2007;179:643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Yovtcheva SP, Aly Rifai M, Moles JK, Van Der Linden BJ. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001;42:411–415. doi: 10.1176/appi.psy.42.5.411. [DOI] [PubMed] [Google Scholar]
- Zimbardo PG, Boyd JN. Putting time in perspective: A valid, reliable individual-difference metric. Journal of Personality and Social Psychology. 1999;77:1271–1288. [Google Scholar]