Abstract
A 59-year-old-male patient with no previous medical history presented with oppressive chest pain; initial electrocardiogram showed ST segment elevation in aVR and V1, with intermittent right bundle branch block. Emergent coronary angiogram showed a proximal sub-occlusive stenosis of the left anterior descending artery, and the patient was hemodynamically unstable during the first 72 hours. Insights into the significance of ST segment elevation in aVR are presented and discussed in light of the current medical data.
Keywords: ST elevation, aVR, V1, acute coronary syndrome
Introduction
Augmented vector right (aVR) lead is commonly “ignored” and designated as the “neglected lead”.1 In the setting of acute coronary syndrome, severe left main coronary artery disease usually presents as widespread ST segment depression, whereas ST segment elevation (STE) in aVR (STE-aVR) is a less recognized finding.2 More importantly, STE-aVR can also manifest in left anterior descending (LAD) artery occlusion/sub-occlusion and it is also an uncommon electrocardiographical sign in this setting.3
Case Presentation
A 59-year-old-male patient with no previous medical history was admitted to the emergency room with oppressive chest pain, present for nearly 7 hours, along with dyspnea and sweating. The patient was a heavy smoker and had no other cardiovascular risk factors. Physical examination at arrival showed severe pallor, a heart rate at 110 bpm, a respiratory rate at 20/min, and a blood pressure of 90/60 mmHg.
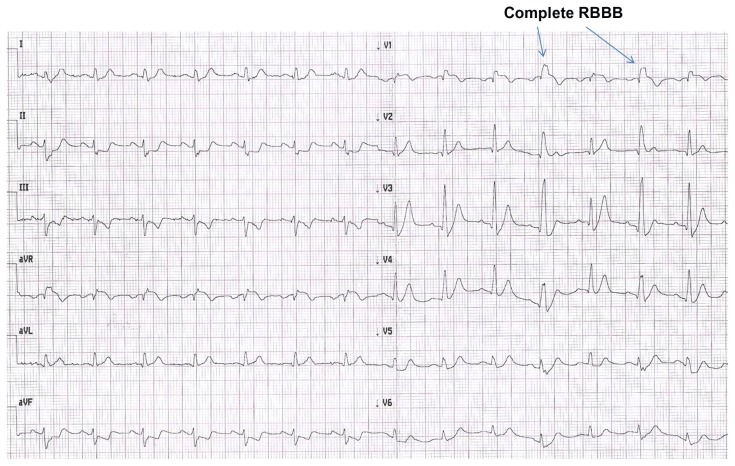
Initial electrocardiogram (Fig. 1) showed intermittent right bundle branch block (RBBB), STE-aVR [J elevation, 2 mm; horizontal STE, 1.5 mm]; in addition, STE was observed in V1 [J elevation, 2 mm; horizontal STE, 1.5 mm when no RBBB was observed] with intermittent incomplete (2nd, 3rd, and 7th complexes) or complete (4th and 6th complexes) RBBB. Along with these changes, there was extensive ST depression (1 to 2 mm) in the inferior and antero-lateral leads, and there was a tall positive symmetrical T wave in anterior leads persisting during the first 6 hours after admission. Moreover, a small Q wave was visualized from V1 until V4, along with absence of Q wave in V5 and V6.
Figure 1.
Electrocardiogram at presentation.
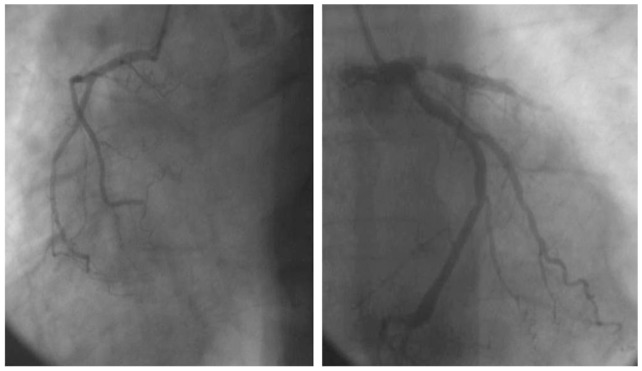
An emergent coronary angiogram (Fig. 2) showed proximal sub-occlusive LAD stenosis (>90%), with poor progression of dye in the mid and distal segments suggesting the presence of multiple luminal clots; additionally, there was a mild diffuse disease in the left circumflex and in the right coronary artery, from which came off a rudimentary conus branch. Emergent percutaneous intervention (door-to-balloon time estimated at 90 minutes) with placement of drug eluting stent in proximal LAD allowed for restoration of flow, with persistence of “slow flow pattern” distally. The patient developed a ventricular fibrillation soon after the percutaneous coronary intervention, and received a 200 J DC shock for defibrillation.
Figure 2.
Coronary angiogram showing sub-occlusive stenosis of the left anterior descending artery along with rudimentary conus branch.
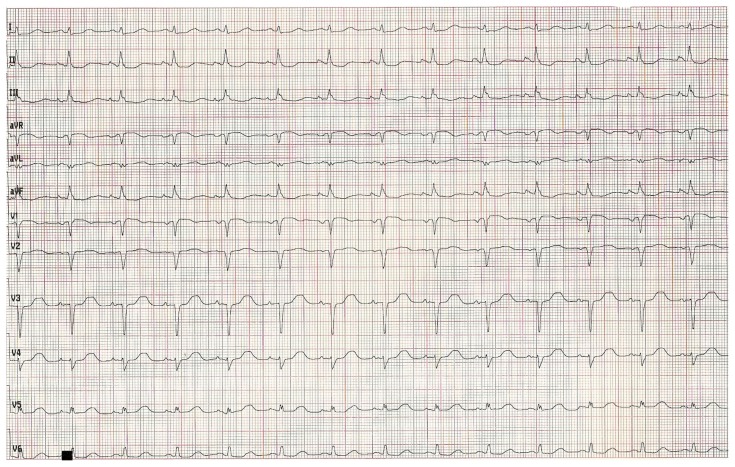
Relevant laboratory findings at 24 hours showed serum creatinine at 1.25 mg/dL, potassium at 4.1 mmol/L, troponin at 11.3 nanog/mL, CPK at 17693 u/L, and CPK-MB at 1462 u/L. Initial evolution was marked by an initial hemodynamic compromise; cardiac echogram at 24 hours showed a severe systolic dysfunction (ejection fraction at 37%) with basal, mid-septal and septo-apical akinesis. The patient clinical condition improved and stabilized progressively and he was discharged on day 14; pre-discharge EKG (Fig. 3) showed a pathological Q wave from V1 to V3, along with poor progression of R wave in V4–V6, absent Q wave in V5–V6. Pre-discharge echogram showed persistent antero-septal and apical akinesis with persistent severe systolic dysfunction.
Figure 3.
Electrocardiogram at discharge.
Discussion
Total left main thrombosis usually leads to sudden cardiac death and affected patients generally die before arriving at hospital. Conversely, patients with subtotal left main occlusion may present with acute coronary syndrome and with electrocardiographic pattern of widespread ST depression in the anterior and inferior leads.4 In the setting of acute coronary syndromes, STE-aVR (≥1 mm) with diffuse ST depression in other leads is usually a sign of severe left main disease; this is often associated with a poor outcome.2,4 Ionescu et al5 reported that electrocardiographic manifestations of left main coronary artery thrombosis may be nonspecific. However, STE-aVR should raise suspicions of severe left main disease. In addition, Taglieri et al6 found that STE-aVR, and reciprocal ST depression in other leads in the setting of non STE acute coronary syndrome is highly predictive of severe left main disease.
Kühl et al7 showed that STE-aVR may represent a proximal LAD artery lesion and that this electrocardiographical sign is useful to differentiate proximal from more distal lesion in the setting of acute coronary syndrome. Along with STE-aVR, STE in V1 may be observed in anterior myocardial infarction and this finding depends on the coronary anatomy.8 Lead V1 reflects the right basal septal area, which is supplied by septal branches from the LAD artery alone or together with the conal branch of the right coronary artery (dual circulation). Accordingly, STE-aVR and STE in V1 predict LAD lesion proximal to the first septal branch, along with insufficient or absent flow from the conus branch.9,10
The mechanism of STE-aVR is not fully understood.3 Lead aVR is electrically opposite to leads D1, D2, aVL, and V3–V6, and therefore an ST depression in these leads produces reciprocal STE-aVR; 11 in addition, lead aVR directly reflects the electrical activity of the right upper portion of the heart, including the basal portion of the interventricular septum and consequently, a transmural infarction in this area theoretically produces STE-aVR. Accordingly, STE-aVR is thought to result from either one of the two following mechanisms: diffuse antero-lateral subendocardial ischemia with reciprocal change in aVR or transmural infarction of the basal portion of the heart.11 In addition, an anatomical variant of the Purkinje fibers has been evoked3 to explain the absence of STE in anterior leads in some patients, despite transmural anterior infarction.
In this patient, we estimate that STE-aVR and V1 reflects basal antero-septal infarction (transmural) related to LAD artery sub-occlusion proximal to the first septal, along with rudimentary conus branch. Similarly, we hypothesize that ST depression in anterior and inferior leads reflects either concomitant subendocardial ischemia, and/or reciprocal changes to STE in aVR. RBBB in this setting is probably related to a transient ischemia of the right bundle branch in the right basal septal area;12 it was intermittent and disappeared completely within 24 hours. Kleemann12 found that RBBB was encountered on admission in 7.1% of non STE myocardial infarction patients and in 4.4% of STE myocardial infarction patients; Kleeman also found that RBBB in STE myocardial infarction was associated with increased in-hospital and long-term mortality.
The clinical implication is that STE in aVR (+/−V1) coupled with ST depression in other leads suggests severe left main coronary artery or proximal LAD disease. It represents a critical clinical condition, particularly when the patient presents with hemodynamic compromise, and accordingly the condition requires prompt management. Nevertheless the prognostic value of STE-aVR in acute coronary syndrome is still debated. Though it was found to be a strong and independent predictor of 30-day mortality in non STE myocardial infarction by Szymański et al,13 it was not considered an independent predictor of in-hospital or 6-month mortality by GRACE investigators.14
Conclusion
STE-aVR in the setting of acute coronary syndrome is often related to severe disease of the left main coronary or proximal LAD artery and clinicians must be forewarned of this critical condition. Along with STE-aVR, STE in V1 with RBBB represents an ischemic lesion in the basal septal area, often coupled with deficient backup circulation to the basal right septum from the conus branch.
Acknowledgments
We would like to thank Mr. Schwenn Gull for study editorial assistance.
Footnotes
Author Contributions
Conceived and designed the experiments: AK. Analyzed the data: AK. Wrote the first draft of the manuscript: AK. Contributed to the writing of the manuscript: AK. Agree with manuscript results and conclusions: AK. Jointly developed the structure and arguments for the paper: AK. Made critical revisions and approved final version: AK. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the author has provided signed confirmation of compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Kireyev D, Arkhipov MV, Zador ST, Paris JA, Boden WE. Clinical utility of aVR-The neglected electrocardiographic lead. Ann Noninvasive Electrocardiol. 2010;15:175–80. doi: 10.1111/j.1542-474X.2010.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang M, Kelly DJ, Devlin G. Left main stem stenosis in the unstable patient—forewarned is forearmed. N Z Med J. 2011;124:111–3. [PubMed] [Google Scholar]
- 3.de Winter RJ, Verouden NJ, Wellens HJ, Wilde AA. A New ECG Sign of Proximal LAD Occlusion. N Engl J Med. 2008;359:2071–3. doi: 10.1056/NEJMc0804737. [DOI] [PubMed] [Google Scholar]
- 4.Fiol M, Carrillo A, Rodríguez A, Pascual M, Bethencourt A, Bayés de Luna A. Electrocardiographic changes of ST-elevation myocardial infarction in patients with complete occlusion of the left main trunk without collateral circulation: Differential diagnosis and clinical considerations. J Electrocardiol. 2012;45:487–90. doi: 10.1016/j.jelectrocard.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Ionescu CN, Donohue TJ. ECG findings in acute left main coronary artery thrombosis: a case report and review of the literature. Conn Med. 2009;73:333–5. [PubMed] [Google Scholar]
- 6.Taglieri N, Marzocchi A, Saia F, et al. Short- and long-term prognostic significance of ST-segment elevation in lead aVR in patients with non-ST-segment elevation acute coronary syndrome. Am J Cardiol. 2011;108:21–8. doi: 10.1016/j.amjcard.2011.02.341. [DOI] [PubMed] [Google Scholar]
- 7.Kühl JT, Berg RM. Utility of lead aVR for identifying the culprit lesion in acute myocardial infarction. Ann Noninvasive Electrocardiol. 2009;14:219–25. doi: 10.1111/j.1542-474X.2009.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Gal T, Sclarovsky S, Herz I, et al. Importance of the conal branch of the right coronary artery in patients with acute anterior wall myocardial infarction: electrocardiographic and angiographic correlation. J Am Coll Cardiol. 1997;29:506–11. doi: 10.1016/s0735-1097(96)00536-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhong-qun Z, Wei W, Chong-quan W, Shu-yi D, Chao-rong H, Jun-feng W. Acute anterior wall myocardial infarction entailing ST-segment elevation in lead V3R, V1 or aVR: electrocardiographic and angiographic correlations. J Electrocardiol. 2008;41:329–34. doi: 10.1016/j.jelectrocard.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Vasudevan K, Manjunath CN, Srinivas KH, et al. Electrocardiographic localization of the occlusion site in left anterior descending coronary artery in acute anterior myocardial infarction. Indian Heart J. 2004;56:315–9. [PubMed] [Google Scholar]
- 11.Jong GP, Ma T, Chou P, Shyu MY, Tseng WK, Chang TC. Reciprocalchanges in 12-lead electrocardiography can predict left main coronary artery lesion in patients with acutemyocardialinfarction. Int Heart J. 2006;47:13–20. doi: 10.1536/ihj.47.13. [DOI] [PubMed] [Google Scholar]
- 12.Kleemann T, Juenger C, Gitt AK, et al. MITRA PLUS Study Group. Incidence and clinical impact of right bundle branch block in patients with acute myocardial infarction: ST elevation myocardial infarction versus non-ST elevation myocardial infarction. Am Heart J. 2008;156:256–61. doi: 10.1016/j.ahj.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Szymański FM, Grabowski M, Filipiak KJ, Karpiński G, Opolski G. Admission ST-segment elevation in lead aVR as the factor improving complex risk stratification in acute coronary syndromes. Am J Emerg Med. 2008;26:408–12. doi: 10.1016/j.ajem.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Yan AT, Yan RT, Kennelly BM, et al. GRACE Investigators. Relationship of ST elevation in lead aVR with angiographic findings and outcome in non-ST elevation acute coronary syndromes. Am Heart J. 2007;154:71–8. doi: 10.1016/j.ahj.2007.03.037. [DOI] [PubMed] [Google Scholar]