Abstract
Objective
The FTO A/T polymorphism (rs9939609) is a strong candidate to influence obesity-related traits. Elite athletes from many different sporting disciplines are characterized by low body fat. Therefore, the aim of this study was to assess whether athletic status is associated with the FTO A/T polymorphism.
Subjects and Methods
A large cohort of European Caucasians from Poland, Russia and Spain were tested to examine the association between FTO A/T polymorphism (rs9939609) and athletic status. A total of 551 athletes were divided by type of sport (endurance athletes, n = 266 vs. sprint/power athletes, n = 285) as well as by level of competition (elite-level vs. national-level). The control group consisted of 1,416 ethnically-matched, non-athletic participants, all Europeans. Multinomial logistic regression analyses were conducted to assess the association between FTO A/T genotypes and athletic status/competition level.
Results
There were no significantly greater/lesser odds of harbouring any type of genotype when comparing across athletic status (endurance athletes, sprint/power athletes or control participants). These effects were observed after controlling for sex and nationality. Furthermore, no significantly greater/lesser odds ratios were observed for any of the genotypes in respect to the level of competition (elite-level vs. national-level).
Conclusion
The FTO A/T polymorphism is not associated with elite athletic status in the largest group of elite athletes studied to date. Large collaborations and data sharing between researchers, as presented here, are strongly recommended to enhance the research in the field of exercise genomics.
Introduction
There is emerging evidence that elite athletes or former elite athletes are predisposed towards longer life expectancy, and lower risk of chronic diseases such as obesity and type 2 diabetes, than matched sedentary controls [1]–[3]. A twenty-year follow-up on former elite athletes revealed that several risk factors (smoking, diabetes, obesity) were never present among the athletes, and the prevalence of other risk factors remained low after twenty years [3].
Genetic factors may contribute to the low predisposition of elite athletes to the aforementioned disease conditions. Specifically, the A/T polymorphism (rs9939609) in the fat mass and obesity associated (FTO) gene (which codes for the protein alpha-ketoglutarate-dependent dioxygenase FTO, also known as fat mass and obesity-associated protein) is a strong candidate to explain how disease modifier polymorphisms may contribute to lower risk for obesity among trained individuals [4]. The FTO A/T polymorphism has initially been identified as a risk factor for obesity by two independent genome-wide association studies (GWAS) [5], [6]. It has been shown that adults who are homozygous for the A-allele weigh on average 1.5 to 3 kg more than those homozygous for the T allele. This finding has now been replicated in multiples obese cohorts [7].
Exercise may attenuate the association between FTO A/T polymorphism and obesity related-traits. The association between FTO A/T polymorphism and body mass index (BMI) is significantly weaker in individuals with higher exercise levels [8]. This phenomenon has been confirmed in Caucasian and African-American cohorts [9], [10]. A recent meta-analysis data, that was calculated from 45 studies of adults (n = 218,166) and 9 studies of children and adolescents (n = 19,268) has shown that the A-allele increased the risk of obesity 30% less in the physically active group than in their inactive peers [4]. Keeping in mind that elite athletes represent the end point of the human physical activity levels, the FTO A/T polymorphism might be a novel target to influence elite athletic status.
Therefore, the aim of the present study was to compare the frequency distribution of the FTO A/T polymorphism (rs9939609) between elite endurance athletes, elite sprint/power athletes, and ethnically-matched, non-athletic control participants in a large group of Europeans (including Spanish, Polish and Russian cohorts). We also examined the association of the FTO A/T polymorphism with respect to the level of achievement of the athletes (‘elite-level’ and ‘national-level’), in both endurance and sprint/power athletes. We hypothesized that the frequency of the A-allele or the AA genotype will be lower among elite athletes compared with control participants.
Materials and Methods
The study was conducted according to the Declaration of Helsinki. Written informed consent was obtained from all participants, and the study was approved by the ethics committees of Universidad Europea de Madrid, Spain, the Pomeranian Medical University, Poland, and the Kazan State Medical University, Russia.
Participants
A total of 551 athletes (266 endurance athletes and 285 power athletes) and 1416 control participants, from Poland, Russia and Spain, volunteered to participate in this study. All participants were unrelated European men (76%) or women (23%), and all of European descent (as self-reported) for ≥3 generations. The sample included elite athletes (57%) who had competed at an international level (European or World championships, or Olympic Games) and national-level athletes (43%) who participated in their chosen sport at a national level only. The competition level differentiation was made according to the athletes' best individual performances. The athletes were only included if they had never tested positive in anti-doping controls. Control participants were required to be free of any diagnosed cardiorespiratory disease and not participating regularly in any competitive or structured sport or physical activity (i.e. performing less than 3 sessions per week of strenuous exercise such as running, swimming, bicycling or weight lifting).
Spanish cohort
The Spanish cohort (n = 192) were all male and included 81 elite athletes (mean ± SD mass = 62.0±6.3 kg) and 60 control participants (71.9±8.3 kg):
32 elite sprint/power athletes (mean age = 26±3 yr). Thirteen track and field athletes were Olympians during the period 2000–2008.
49 elite endurance athletes (27±4 yr). This sample included 49 elite endurance runners (the top Spanish runners during the 1999–2009 periods, i.e. mainly 5000 m to marathon specialists, virtually all of them Olympians).
60 healthy, non-athletic control participants (20±2 yr). All were undergraduate students from the same university (Universidad Europea de Madrid, Spain).
Polish cohort
The Polish cohort (n = 844), were all male and included 214 athletes (mean ± SD mass = 71.3±6.2 kg) and 630 control participants (79.2±6.1 kg). Of the athletes, 132 were classified as elite and 82 were national-level athletes:
101 power athletes (28±8 yr). This group included weightlifters (n = 42), sprinters (≤400 m, n = 33), and track and field jumpers (n = 26). This group included 63 (62%) elite athletes.
113 endurance athletes (26±6 yr). This group included rowers (n = 53), endurance road cyclists (n = 14), 5,000 m runners (n = 12), marathon runners (n = 12), 800–1,500 m swimmers (n = 11), 15–50 km cross-country skiers (n = 7), and triathletes (n = 4). This group included 69 (61%) elite athletes.
630 healthy, non-athletic control participants (21±2 yr). All the control participants were students of the University of Szczecin.
Russian cohort
The Russian cohort (men and women, n = 982) included 256 athletes (187 men, 69 women; 70.3±16.8 kg) and 726 control participants (328 men and 398 women; 61.2±12.2 kg). Of the athletes 105 were classified as elite and 151 were classified as national-level athletes:
152 power athletes (24±8 yr). This group included: 100–200 m sprinters (n = 18), track and field jumpers (n = 47), short distance speed skaters (500–1000 m; n = 9), 50–100 m swimmers (n = 13), and weight lifters (n = 65). The group included 71 (47%) elite athletes.
104 endurance athletes (20±2 yr). This group included rowers (n = 36), long distance runners (5000 m; n = 27), road cyclists (n = 12), long distance speed skaters (5–10 km; n = 7), skiers (n = 15) and long distance swimmers (800–1500 m; n = 7).The group included 34 (33%) elite athletes.
726 healthy, non-athletic control participants (21±5 yr). All the control participants were citizens of Moscow and Kazan.
Genotyping
We followed recent recommendations for genotype-phenotype association studies provided by Chanock et al. [11], Attia et al. [12] and the latest ‘Strengthening the Reporting of Genetic Association studies’ (STREGA) group report [13].
Spanish cohort
Genomic DNA was isolated from buccal epithelium or peripheral blood during the years 2004–2008 and genotyping was performed during 2012 in the Genetics Laboratory of Ariel University Centre, Israel. Polymerase chain reaction (PCR) was performed in order to amplify the sequence containing the mutation. A fragment of 105 bp was amplified with the following primers: FTO- F 5′- GGT TCC TTGCGA CTG CTG TGA AAT T '3 and FTO-R 5' GCT TTT ATGCTC TCC CAC TC '3. The PCR conditions were as follows: initial denaturing at 95°C 5 min; 35 cycles at 95°C 30 s, 60°C 30 s, 72°C 30 s and a final extension at 72°C 10 min. FTO genotypes were established by enzymatic digestion of amplicons with ApoI and by allelic discrimination assay on a Real-Time Polymerase Chain Reaction (PCR) instrument (Stratagene Mx3000D) with Taqman® probes (Genotyping ToughMix®). Following recent recommendations [11], we replicated the genotype results of the Spanish cohort (in 40% of samples) using a different method, i.e. direct sequencing. The results from the two different methods were in 100% agreement.
Polish cohort
Genomic DNA was isolated from buccal epithelium using GenElute Mammalian Genomic DNA Miniprep Kit (Sigma, Germany) during the years 2008–2010, according to the producer protocol. All samples were genotyped during 2012, in the Pomeranian Medical University using an allelic discrimination assay on a Rotor-Gene Real-Time Polymerase Chain Reaction (PCR) instrument (Corbett, Australia) with Taqman® probes. For the discrimination of FTO A and T alleles (rs9939609), a TaqMan® Pre-Designed SNP Genotyping Assay was used (Applied Biosystems, USA) (assay ID: C__30090620_10), including primers and fluorescently labelled (FAM and VIC) MGB™ probes for the detection of both alleles.
Russian cohort
Genomic DNA was isolated from epithelial mouth cells using a DNK-sorb-A sorbent kit according to the manufacturer's instruction (Central Research Institute of Epidemiology, Moscow, Russia). Genotyping for the FTO gene polymorphism was performed during 2012, at the Laboratory of Molecular Genetics, Kazan State Medical University by PCR on a multicanal amplificator Tercyk (DNA Technology, Moscow, Russia) and restriction enzyme digestion [14].
Following recent recommendations [11], we replicated the genotype results of a subset of samples (i.e. 40% of samples of the Russian cohort) using a different method, i.e. by MALDI-TOF mass spectrometry [15].
Statistical analysis
Chi squared tests were used to test for the presence of Hardy-Weinberg equilibrium (HWE). Genotype and allele frequencies were compared according to athletic status (i.e. control participants, endurance, or sprint/power athlete) using Fisher's exact test. Multinomial logistic regression analyses were conducted to assess the association between genotype and athletic status/competition level. In each case, gender and nationality were controlled for; and analyses were made comparing AA (reference group) vs. AT; AA vs. TT (co-dominant effect); AA vs. TT and TA combined (dominant effect); AA and TA combined (reference group) vs. TT (recessive effect). Significance was accepted when p≤0.05.
Results
Replication analysis with a different genotyping method yielded 100% agreement. There were no significant differences in age between cohorts. This added to the homogeneity of the study population and allowed us to pool the three cohorts to examine the association between physical performance level and FTO A/T polymorphism.
Table 1 shows the genotype and allele frequency distributions amongst all participants according to their nationality. Genotype distributions of all control and athletic groups met HWE (all p>0.1). In the Russian sample, no differences were observed in the proportion of men and women participating in endurance and power sports (p>0.05) and genotype and allele frequencies were similar according to gender (p>0.05; data not shown). For these reasons, gender was considered as a covariate only in further analyses (see below).
Table 1. FTO A/T polymorphism genotype and allele frequencies amongst all participants according to their nationality.
| Spanish cohort | Polish cohort | Russian cohort | |||||||
| Control | Endurance | Power | Control | Endurance | Power | Control | Endurance | Power | |
| All | 60 | 49 | 32 | 630 | 113 | 101 | 726 | 104 | 152 |
| AA | 5 (8.3) | 5 (10.2) | 4 (12.5) | 119 (18.9) | 13 (11.5) | 19 (18.8) | 111 (15.3) | 17 (16.3) | 27 (17.8) |
| AT | 7 (11.7) | 14 (28.6) | 7 (21.8) | 318 (50.5) | 65 (57.5) | 52 (51.5) | 324 (44.6) | 54 (51.9) | 68 (44.7) |
| TT | 48 (80) | 30 (61.2) | 21 (65.6) | 193 (30.6) | 35 (31.0) | 30 (29.7) | 291 (40.1) | 33 (31.7) | 57 (37.5) |
| MAF | 0.141 | 0.245 | 0.234 | 0.441 | 0.403 | 0.446 | 0.376 | 0.423 | 0.401 |
| HWE-P value | .001 | 0.282 | 0.086 | 0.838 | 0.115 | 0.914 | .001 | 0.810 | 0.696 |
Abbreviations: HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency.
Table 2 shows the association between genotype and athletic status for all participants. There were no significantly greater/lesser odds of harbouring any type of genotype when comparing the power, endurance and control groups. Likewise, no differences were observed when comparing all athletes to the control group. These effects were observed after controlling for the effects of gender and nationality.
Table 2. The association between FTO A/T genotypes and athletic status in three cohorts of European participants.
| Power vs. Control | Endurance vs. Power | Endurance vs Control | All athletes vs Control | |||||||||
| OR | CI | p | OR | CI | p | OR | CI | p | OR | CI | p | |
| AA (ref) | 1 | 1 | 1 | 1 | ||||||||
| AT | 0.95 | (0.66–1.36) | 0.782 | 1.48 | (0.89–2.45) | 0.129 | 1.46 | (0.97–2.18) | 0.070 | 1.15 | (0.86–1.54) | 0.349 |
| TT | 1.01 | (0.70–1.46) | 0.968 | 1.16 | (0.69–1.96) | 0.569 | 1.30 | (0.85–1.98) | 0.229 | 1.11 | (0.82–1.50) | 0.484 |
| AT-TT (AA ref) | 0.98 | (0.70–1.37) | 0.892 | 1.33 | (0.82–2.14) | 0.238 | 1.39 | (0.94–2.05) | 0.097 | 1.14 | (0.86–1.49) | 0.363 |
| TT (AA-AT ref) | 1.05 | (0.80–1.36) | 0.742 | 0.87 | (0.61–1.24) | 0.426 | 0.98 | (0.74–1.29) | 0.858 | 1.00 | (0.82–1.24) | 0.969 |
Note. OR: Odds ratio; CI: Confidence intervals; p: 2-tailed p value with significance assumed at p<0.05.
Table 3 shows the association between genotype and competition level (elite vs. national level) for the endurance and power athletes from all countries. No significantly greater/lesser odds ratios were observed for any of the genotypes in either competition level. As above, gender and nationality were controlled for in the regression analyses.
Table 3. The association between FTO A/T genotypes and athletic status according to level of competition (elite compared to national level), in three cohorts of European participants.
| Endurance | Power | |||||
| OR | CI | p | OR | CI | p | |
| AA (ref) | 1 | 1 | ||||
| AT | 0.79 | (0.22–2.87) | 0.719 | 1.61 | (0.79–3.27) | 0.187 |
| TT | 2.08 | (0.83–5.23) | 0.116 | 1.93 | (0.93–4.03) | 0.079 |
| AT-TT (AA ref) | 2.14 | (0.93–4.96) | 0.076 | 1.75 | (0.90–3.39) | 0.099 |
| TT (AA-AT ref) | 1.13 | (0.63–2.05) | 0.682 | 1.37 | (0.81–2.33) | 0.241 |
Note. OR: Odds ratio; CI: Confidence intervals; p: 2-tailed p value with significance assumed at p<0.05.
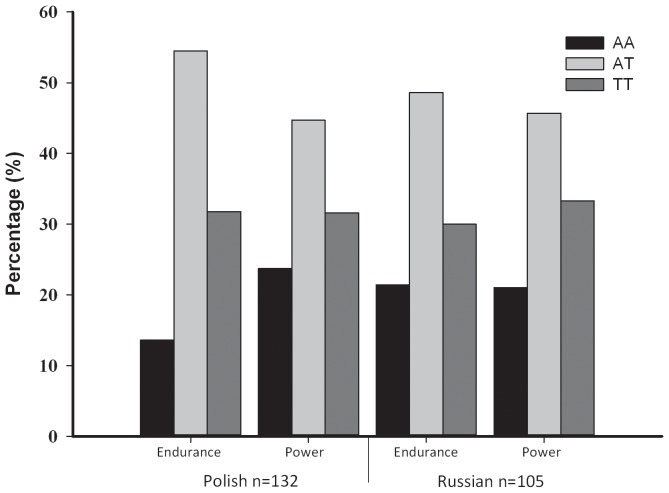
Figure 1 shows the percentage of genotypes present in elite-level athletes according to nationality and athletic status. No significant genotype differences were observed between elite endurance athletes and elite power athletes across nationalities.
Figure 1. Genotype distributions in elite-level athletes according to nationality and athletic status.
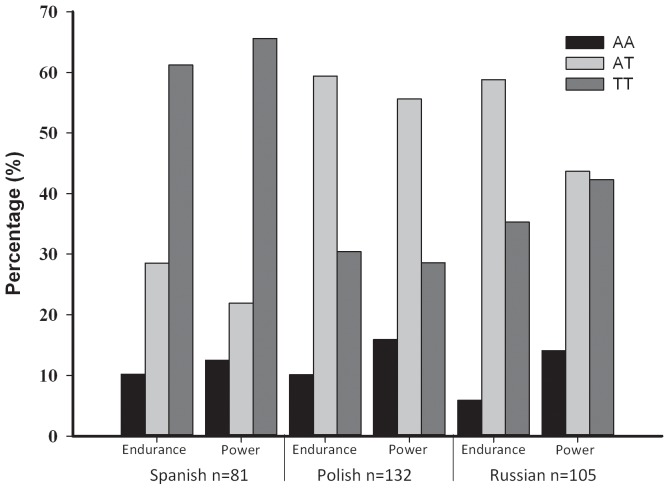
Figure 2 shows the percentage of genotypes present in national-level athletes according to nationality and athletic status. No significant genotype differences were observed between national-level endurance athletes and national-level power athletes across nationalities.
Figure 2. Genotype distributions in national-level athletes according to nationality and athletic status.
Discussion
We have analysed, for the first time, the association between FTO A/T polymorphism (rs9939609) and elite athletic status in a large cohort of European athletes. No significant differences were observed in genotype/allele frequencies when comparing between the three groups (endurance athletes, sprint/power athletes, and non-athletic controls) or when comparing between the groups with respect to the level of performance (elite vs. national-level), suggesting that this polymorphic marker is not related to elite athletic performance.
In the present study we explored the association between the FTO A/T polymorphism and athletic performance in elite athletes from several cohorts. Taken individually, the results from our three cohorts are probably limited by relatively small sample size of the individual cohorts, and low statistical power. In an attempt to overcome the barrier of sample size, and the difficulty of gathering a homogenous cohort of elite athletes in each sporting discipline, we have recruited a total of 551 athletes (266 endurance athletes and 285 power/sprint athletes), all of European descent for ≥3 generations. Indeed, it has been estimated that testing a single variant using a case (athletes):control (non-athletes) design would require ∼250 cases to obtain a statistical power of 80% [16]. A sufficient sample size of elite athletes, together with following recent recommendations for association studies, are probably necessary to reach solid conclusions in the field of genes and elite performance [17].
Genetic variants such as the FTO A/T polymorphism studied here are associated with increased BMI and energy intake [9], [18], and are thus candidates to influence obesity and other disease-related phenotypes. Conversely, such variants may also influence elite athletic performance because body composition and BMI are well-characterised phenotypes in athletic populations that, to some extent at least, differentiate between athletes of different achievement levels, and between athletes and non-athletes. However, there are some complex interrelationships between increased/decreased BMI and both physical activity levels (i.e., energy expenditure) and energy intake, affected by interconnected metabolic processes [18], [19]. Indeed, physical activity was recently shown to attenuate the influence of FTO variants on obesity risk [4].
The duality of specific polymorphisms associated with both obesity and athletic performance has been well demonstrated. A good example is the peroxisome proliferator-activated receptor gamma coactivator1α (PPARGC1A) Gly482Ser polymorphism, in which the 482Ser allele is associated with increased risk of obesity and type 2 diabetes [20], whereas the ‘favourable’ Gly482 allele is associated with elite athletic performance [21]–[24]. Interestingly, the minor Ser482 allele is associated with risk of obesity in inactive individuals [20], [25] supporting the notion that genetic susceptibility to obesity is enhanced by physical inactivity. The link between PPARGC1A gene and fat oxidative metabolism suggest that this gene may influence athletic performance on one hand, and prevention of obesity on the other hand. Additional examples of polymorphisms that were found to be associated with both obesity and elite athletic performance are the ADRB2 Arg16Gly (rs1042713) [26], [27], and the ADRB3 Trp64Arg (rs4994) [28], [29].
The FTO A/T polymorphism (rs9939609) is located in the first intron of the FTO gene, which is expressed mainly in the brain, skeletal muscles and adipose tissue [30]. The mechanism by which it influences adiposity and attenuates physical activity is mostly unknown and probably multifaceted. Mechanistic research involving mice models demonstrated alternation in food intake in mice expressing several copies of the FTO gene, and significant reduction in adipose tissue and lean body mass [31]. A knockout mice model revealed that the FTO gene is functionally involved in energy homeostasis, mitochondrial coupling and substrate cycling by controlling energy expenditure [32]. The FTO A/T polymorphism was shown to affect energy efficiency potentially by influencing mitochondrial coupling in human type I (oxidative) muscle fibres [33], and FTO mRNA expression in human skeletal muscle correlates with whole-body substrate oxidation rates [34]. Thus, it could have been hypothesized that elite endurance performance in particular, which traditionally requires a high proportion of type I skeletal muscle fibres in the locomotory muscles and high mitochondrial coupling, would be influenced by the FTO A/T polymorphism.
To summarize, (i) the potential involvement of the FTO gene in energy metabolism and muscle function, (ii) the fact that other gene polymorphisms have been shown to be associated with both obesity and athletic performance (e.g. PPARGC1A Gly482Ser, ADRB3 Trp64Arg, and the ADRB2 Arg16Gly), (iii) the interaction between the FTO A/T polymorphism and physical activity levels, (iv) the low percentage of body fat characteristic of elite athletes excelling in both endurance and many sprint/power events (v) the lower risk of obesity in former elite athletes, and (vi) the suggestive role of the FTO gene in muscle performance, encouraged us to hypothesize that the FTO A/T polymorphism was associated with elite athletic status. However, no association was found between the FTO A/T polymorphism and athletic status in the largest group of elite athletes studied to date. Elite athletic status is a polygenic trait with several candidate gene variants (most of which likely remain unidentified) playing a certain role, either alone (which does not seem to be the case with the FTO A/T variation), or through complex, gene-gene and gene-environment interactions [35], [36]. Large collaborations and data sharing between researchers, as presented here, are strongly recommended to enhance the research in the field of exercise genomics.
Acknowledgments
The Authors declare no acknowledgments for this work.
Funding Statement
The authors have no support or funding to report.
References
- 1. Kujala UM, Tikkanen HO, Sarna S, Pukkala E, Kaprio J, et al. (2001) Disease-specific mortality among elite athletes. JAMA 285: 44–45. [DOI] [PubMed] [Google Scholar]
- 2. Sarna S, Sahi T, Koskenvuo M, Kaprio J (1993) Increased life expectancy of world class male athletes. Med Sci Sports Exerc 25: 237–244. [PubMed] [Google Scholar]
- 3. Mengelkoch LJ, Pollock ML, Limacher MC, Graves JE, Shireman RB, et al. (1997) Effects of age, physical training, and physical fitness on coronary heart disease risk factors in older track athletes at twenty-year follow-up. J Am Geriatr Soc 45: 1446–1453. [DOI] [PubMed] [Google Scholar]
- 4. Kilpelainen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, et al. (2011) Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med 8: e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, et al. (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scuteri A, Sanna S, Chen WM, Uda M, Albai G, et al. (2007) Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 3: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fawcett KA, Barroso I (2010) The genetics of obesity: FTO leads the way. Trends Genet 26: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roth SM, Rankinen T, Hagberg JM, Loos RJ, Perusse L, et al. (2012) Advances in exercise, fitness, and performance genomics in 2011. Med Sci Sports Exerc 44: 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demerath EW, Lutsey PL, Monda KL, Linda Kao WH, Bressler J, et al. (2011) Interaction of FTO and physical activity level on adiposity in African-American and European-American adults: the ARIC study. Obesity (Silver Spring) 19: 1866–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andreasen CH, Stender-Petersen KL, Mogensen MS, Torekov SS, Wegner L, et al. (2008) Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes 57: 95–101. [DOI] [PubMed] [Google Scholar]
- 11. Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, et al. (2007) Replicating genotype-phenotype associations. Nature 447: 655–660. [DOI] [PubMed] [Google Scholar]
- 12. Attia J, Ioannidis JP, Thakkinstian A, McEvoy M, Scott RJ, et al. (2009) How to use an article about genetic association: B: Are the results of the study valid? JAMA 301: 191–197. [DOI] [PubMed] [Google Scholar]
- 13. Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, et al. (2009) Strengthening the reporting of genetic association studies (STREGA): an extension of the STROBE Statement. Hum Genet 125: 131–151. [DOI] [PubMed] [Google Scholar]
- 14. Lopez-Bermejo A, Petry CJ, Diaz M, Sebastiani G, de Zegher F, et al. (2008) The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J Clin Endocrinol Metab 93: 1501–1505. [DOI] [PubMed] [Google Scholar]
- 15. Ross P, Hall L, Smirnov I, Haff L (1998) High level multiplex genotyping by MALDI-TOF mass spectrometry. Nat Biotechnol 16: 1347–1351. [DOI] [PubMed] [Google Scholar]
- 16. Hong E, Park J (2012) Sample size and statistical power calculations. 10: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eynon N, Ruiz JR, Oliveira J, Duarte JA, Birk R, et al. (2011) Genes and elite athletes: a roadmap for future research. J Physiol 589: 3063–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eynon N, Meckel Y, Alves AJ, Yamin C, Sagiv M, et al. (2009) Is there an interaction between PPARD T294C and PPARGC1A Gly482Ser polymorphisms and human endurance performance? Exp Physiol 94: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 19. Cook CM, Schoeller DA (2011) Physical activity and weight control: conflicting findings. Curr Opin Clin Nutr Metab Care 14: 419–424. [DOI] [PubMed] [Google Scholar]
- 20. Ridderstrale M, Johansson LE, Rastam L, Lindblad U (2006) Increased risk of obesity associated with the variant allele of the PPARGC1A Gly482Ser polymorphism in physically inactive elderly men. Diabetologia 49: 496–500. [DOI] [PubMed] [Google Scholar]
- 21. Eynon N, Meckel Y, Sagiv M, Yamin C, Amir R, et al. (2010) Do PPARGC1A and PPARalpha polymorphisms influence sprint or endurance phenotypes? Scand J Med Sci Sports 20: e145–150. [DOI] [PubMed] [Google Scholar]
- 22. Lucia A, Gomez-Gallego F, Barroso I, Rabadan M, Bandres F, et al. (2005) PPARGC1A genotype (Gly482Ser) predicts exceptional endurance capacity in European men. J Appl Physiol 99: 344–348. [DOI] [PubMed] [Google Scholar]
- 23. Ahmetov, II, Williams AG, Popov DV, Lyubaeva EV, Hakimullina AM, et al. (2009) The combined impact of metabolic gene polymorphisms on elite endurance athlete status and related phenotypes. Hum Genet 126: 751–761. [DOI] [PubMed] [Google Scholar]
- 24. Maciejewska A, Sawczuk M, Cieszczyk P, Mozhayskaya IA, Ahmetov II (2012) The PPARGC1A gene Gly482Ser in Polish and Russian athletes. J Sports Sci 30: 101–113. [DOI] [PubMed] [Google Scholar]
- 25. Esterbauer H, Oberkofler H, Linnemayr V, Iglseder B, Hedegger M, et al. (2002) Peroxisome proliferator-activated receptor-gamma coactivator-1 gene locus: associations with obesity indices in middle-aged women. Diabetes 51: 1281–1286. [DOI] [PubMed] [Google Scholar]
- 26. Tsunekawa K, Yanagawa Y, Aoki T, Morimura T, Araki O, et al. (2011) Association between accumulation of visceral fat and the combination of beta3 adrenergic receptor Trp64Arg, beta2 adrenergic receptor Arg16Gly and uncoupling protein 1 -3826A>G polymorphisms detected by Smart Amplification Process 2. Endocr J 58: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 27. Wolfarth B, Rankinen T, Muhlbauer S, Scherr J, Boulay MR, et al. (2007) Association between a beta2-adrenergic receptor polymorphism and elite endurance performance. Metabolism 56: 1649–1651. [DOI] [PubMed] [Google Scholar]
- 28. Baturin AK, Pogozheva AV, Sorokina E, Makurina ON, Tutel'ian VA (2012) [The Trp64Arg polymorphism of beta3-adrenoreceptor gene study in persons with overweight and obesity]. Vopr Pitan 81: 23–27. [PubMed] [Google Scholar]
- 29. Santiago C, Ruiz JR, Buxens A, Artieda M, Arteta D, et al. (2011) Trp64Arg polymorphism in ADRB3 gene is associated with elite endurance performance. Br J Sports Med 45: 147–149. [DOI] [PubMed] [Google Scholar]
- 30. Fredriksson R, Hagglund M, Olszewski PK, Stephansson O, Jacobsson JA, et al. (2008) The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 149: 2062–2071. [DOI] [PubMed] [Google Scholar]
- 31. Church C, Lee S, Bagg EA, McTaggart JS, Deacon R, et al. (2009) A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet 5: e1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, et al. (2009) Inactivation of the Fto gene protects from obesity. Nature 458: 894–898. [DOI] [PubMed] [Google Scholar]
- 33. Grunnet LG, Brons C, Jacobsen S, Nilsson E, Astrup A, et al. (2009) Increased recovery rates of phosphocreatine and inorganic phosphate after isometric contraction in oxidative muscle fibers and elevated hepatic insulin resistance in homozygous carriers of the A-allele of FTO rs9939609. J Clin Endocrinol Metab 94: 596–602. [DOI] [PubMed] [Google Scholar]
- 34. Grunnet LG, Nilsson E, Ling C, Hansen T, Pedersen O, et al. (2009) Regulation and function of FTO mRNA expression in human skeletal muscle and subcutaneous adipose tissue. Diabetes 58: 2402–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruiz JR, Gomez-Gallego F, Santiago C, Gonzalez-Freire M, Verde Z, et al. (2009) Is there an optimum endurance polygenic profile? J Physiol 587: 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams AG, Folland JP (2008) Similarity of polygenic profiles limits the potential for elite human physical performance. J Physiol 586: 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]