Abstract
Background
Antiretroviral treatment (ART) has been introduced in Ethiopia a decade ago and continues to be scaled up. However, there is dearth of literature on the impact of ART on changes in CD4 lymphocyte count and weight among patients on treatment.
Objective
To determine the predictors of change in CD4 lymphocyte count and weight among HIV/AIDS infected patients taking antiretroviral treatment in eastern Ethiopia.
Methods
A retrospective cohort study was conducted among HIV/AIDS patients taking ART from 2005 to 2010. A sample of 1540 HIV infected adult patients who started antiretroviral therapy in hospitals located in eastern Ethiopia were included in the study. The primary outcomes of interest were changes in CD4 count and weight. Descriptive statistics and multivariable regression analyses were performed to examine the outcomes among the cohort.
Results
Both the median CD4 lymphocyte counts and weight showed improvements in the follow up periods. The multivariate analysis shows that the duration of ART was an important predictor of improvements in CD4 lymphocyte count (beta 7.91; 95% CI 7.48–8.34; p 0.000) and weight (beta 0.15; 95% CI 0.13–0.18; p 0.000). Advanced WHO clinical stage, lower baseline CD4 cell count, and baseline hemoglobin levels were factors associated with decline in weight. Actively working patients had higher CD4 lymphocyte count and weight compared to those that were ambulatory (p<0.05).
Conclusion
We detected a substantial increment in weight and CD4 lymphocyte count among the patients who were taking ART in eastern Ethiopia. Patients who are of older age, with low initial CD4 lymphocyte count, late stage of the WHO clinical stages and lower hemoglobin level may need special attention. The reasons for the improved findings on CD4 count and weight throughout the five years of follow up merit further investigation.
Introduction
HIV/AIDS continues to be a global challenge. Sub-Sahara Africa is the most affected part of the world with an estimated 22.9 million people living with HIV and 1.2 million deaths from AIDS among children and adults in 2010 [1]. Immunological suppression and disturbance caused by HIV infection is responsible for the decline in CD4 cell counts and is predictive of both morbidity and mortality from AIDS [2], [3].
The introduction of antiretroviral treatment has altered the course of the HIV/AIDS epidemic [4], [5]. Antiretroviral drugs control the HIV infection by reducing viral replication which in most cases is measured by increases in CD4 lymphocyte count [3]. This has led to drastic reductions in morbidity and mortality from AIDS, and improvement in the quality of life of patients [6], .
Monitoring clinical and diagnostic progression of patients on anti-retroviral treatment (ART) is important to examine responses to the treatment and for clinical decision-making. Patient responses and clinical diagnostic measures to anti-retroviral treatment show differences based on individual and population characteristics, and the type of setting where treatment is delivered [4], [9]. These include drug resistance levels, adherence to treatment, age, source of infection and substance use among others [3], [4], [10], [11], [12]. In Ethiopia, despite an increasing trend of access to ART in the past decade, there is paucity of studies examining the weight and immunological responses of patients in the course of treatment. This study therefore aims to examine predictors of change in CD4 lymphocyte count and weight among HIV infected patients on antiretroviral treatment in country.
Methodology
Ethical Consideration
Ethical clearance was obtained from the Institutional Research Ethics Review Committee (IRERC) of Haramaya University. Further approval was obtained from the hospital management before accessing the patients' medical records. In Ethiopia, patients are not customarily requested for consent before their medical record is to be used for research. This includes at the beginning of the recording process and afterward. Initially we wanted to contact loss to follow up patients through nurses to check if they are alive or dead, however the IREC advised us against this citing interference and emotional burden to patients and their families. As a result, we abandoned this approach and collected only non-identifying data from patients' cards. This was done by anti-retroviral treatment nurses working in the respective hospitals in order to assure confidentiality. The IRERC has approved this procedure.
Study setting
The study was conducted in Hiwot Fana, Jugal and Dil Chora hospitals located in eastern Ethiopia. According to the 2007 ART guideline of Ethiopia, the eligibility criteria for initiation of ART are dependent on the availability of CD4 count instruments in the treating health facilities. When CD4 count is not available, ART can be initiated to patients in WHO clinical stages III and IV irrespective of total lymphocyte count (TLC), and WHO clinical stage II patients if TLC is less than 1,200/ml. Whereas in the presence of CD4 count measurement instruments in the health facilities, treatment is given to WHO clinical stage IV patients, irrespective of the CD4 count – to WHO clinical stage III, if the CD4 cell count is less than 350/mL; and all WHO clinical stages if the CD4 cell count is below 200/mL [13]. The first line treatment regimens recommended in the national guideline are TDF+FTC+EFV or ZDV+3TC+EFV or ZDV+3TC+NVP. There are also alternative treatment regimens like D4T+3TC+NVP, TDF+3TC+NVP, D4T+3TC+EFV, ABC+3TC+NVP, ABC+3TC+EFV and ABC+3TC+ZDV. Usually, once treatment is recommended for AIDS cases by physicians, nurses trained for 1–3 months provide ART treatment, counseling and follow up. Physicians are available for consultation in case of complications and advanced disease patients that need to be admitted to medical wards.
Study design
A retrospective cohort study was conducted among HIV patients on ART. Data was collected from September to November 2010. A random sample of 1540 patients who started treatment between September 11, 2005 and September 10, 2008 were selected and retrospectively followed until September 10, 2010. The patients' identification numbers were used to extract the necessary data from the database and cards of the HIV patients in the ART clinics.
Socio-demographic characteristics, baseline and follow up clinical and laboratory data, and treatment outcomes were abstracted from patients' cards. The primary outcomes for this study were CD4 lymphocyte count and body weight measurements across time.
Data collection and quality control
A standard questionnaire was used to extract the data from patients' cards. This form is developed using the standardized ART entry and follow up form employed by the ART clinics. Four advanced ART nurses who were trained on comprehensive HIV care and treatment services and were involved in patient follow ups at the time of the study collected the data. Data collection was supervised by the researchers. All completed data collection forms were examined for clarity and consistency. Death was ascertained by reviewing cards and death certificates. Patients who died from unrelated diseases or accidents, as well as those alive at the end of the follow up period were considered censored. CD4 lymphocyte count (mm3) and weight (kg) were measured every six months by the ART nurses, but in some instances such as dysfunction of CD4 counter machines measurements could be extended to longer intervals.
Data analysis
Descriptive statistics such as median, standard deviations (SDs) and tables were used to investigate the characteristics of the cohort. Descriptive statistics were analyzed using SPSS version 16, while regressions were fitted using STATA version 11. Linear regression was used to characterize and screen data for problems of multicollinearity. Mixed models regression was used to examine changes in CD4 cell count and weight after the baseline measurement. Models were fitted to both the raw and transformed data that approximate to a normal curve. Data transformations ranging from inverse to square roots of the CD4 counts and weight were attempted. Both the random intercept and slope-intercept models were examined to see the model fit. To check the reliability of the model based on the 60th month follow up due to changing missing values, outcomes up to the 48th and 54th months were also fitted and compared with. Model fit was examined using the differences in maximum likelihoods of the competing models. The final model was random slopes and intercepts model in which the slopes as well as the coefficients were allowed to vary. The variables were entered in a simultaneous manner. Due to the large number of models that needed to be checked in addition to comparing random intercept or slope models, we did not use step wise models since they increase the risk of a false positive result (type I error) [14]. Regression coefficients of the final model and their 95% confidence intervals were used as measures of association between the predictors and outcome variable. A p-value of less or equal to 0.05 was considered statistically significant.
Results
Characteristics of patients
A total of 1540 patients were included in the study. The median (IQR) age of participants was 32 (28.0–40.0) years and 37.3% (574) were males. Three hundred forty eight (22.7%) and 890 (58.2%) patients were on the WHO clinical stages of II and III at baseline respectively. The median (Inter-quartile range, IQR) of baseline CD4 count was 135 (76.0–198.3) per milliliter (Table 1).
Table 1. Baseline characteristics of HIV infected patients initiating antiretroviral therapy.* .
| All patients, N¥ (%) | Patients with CD4 count ≤200 (n = 1149) | Patients with CD4 count >200 (n = 361) | |
| Sex | |||
| Male | 574 (37.3) | 431 (37.6) | 131 (36.3) |
| Female | 963 (62.5) | 715 (62.4) | 230 (63.7) |
| Age (Median, IQR) | 32 (28–40) | 32 (28.0–40.0) | 35 (28.0–40.0) |
| Religion | |||
| Muslim | 305 (19.8) | 220 (19.1) | 76 (21.1) |
| Orthodox | 1074 (69.9) | 812 (70.7) | 241 (66.8) |
| Protestant | 144 (9.4) | 103 (9.0) | 41 (11.4) |
| Others | 14 (0.9) | 14 (1.2) | 3 (0.8) |
| Education | |||
| No education | 261 (17.0) | 185 (16.1) | 67 (18.6) |
| Primary | 681 (44.3) | 490 (42.7) | 178 (49.3) |
| Secondary | 500 (32.5) | 391 (34.1) | 102 (28.3) |
| Tertiary | 96 (6.2) | 81 (7.1) | 14 (3.9) |
| Marital status | |||
| Never Married | 287 (18.6) | 217 (18.9) | 65 (18.0) |
| Married | 606 (39.4) | 475 (41.4) | 124 (34.3) |
| Separated | 288 (18.7) | 206 (17.9) | 74 (20.5) |
| Divorced | 116 (7.5) | 89 (7.8) | 22 (6.1) |
| Widowed | 242 (15.7) | 161 (14.0) | 76 (21.1) |
| Occupation | |||
| Merchant | 75 (7.4) | 60 (7.7) | 15 (6.7) |
| Gov. Employee | 157 (15.5) | 122 (15.6) | 33 (14.8) |
| Non-Gov. Employee | 41 (4.1) | 34 (4.4) | 7 (3.1) |
| Day Laborer | 174 (17.2) | 130 (16.7) | 44 (19.7) |
| Job-less | 402 (39.7) | 303 (38.8) | 94 (42.2) |
| Other | 163 (16.2) | 131 (16.8) | 30 (13.5) |
| Past co-trimoxazole treatment | |||
| Yes | 758 (49.2) | 576 (50.1) | 171 (47.4) |
| No | 782 (50.8) | 573 (49.9) | 190 (52.6) |
| WHO stage at baseline | |||
| Stage I | 92 (6.0) | 76 (6.7) | 15 (4.2) |
| Stage II | 348 (22.7) | 282 (24.7) | 63 (17.5) |
| Stage III | 890 (58.2) | 633 (55.5) | 239 (66.6) |
| Stage IV | 200 (13.1) | 150 (13.1) | 42 (11.7) |
| Weight at baseline in Kgs, median (IQR) | 50.0 (44.0–56.0) | 50 (44.0–56.0) | 50 (45.0–57.0) |
Number and percentages unless indicated otherwise. IQR, inter-quartile rage.
The frequency in cells may be lesser than the cohort size (n = 1540) due to missing values on each of the variables reported.
Follow up outcomes of the cohort
From the registered patients in this cohort analysis, the outcome of patients as active, deceased, lost to follow up and transferred out were 1005 (67.2%), 86 (5.9%), 210 (14.0%) and 192 (12.8%) respectively.
Changes in CD4 lymphocyte count and weight
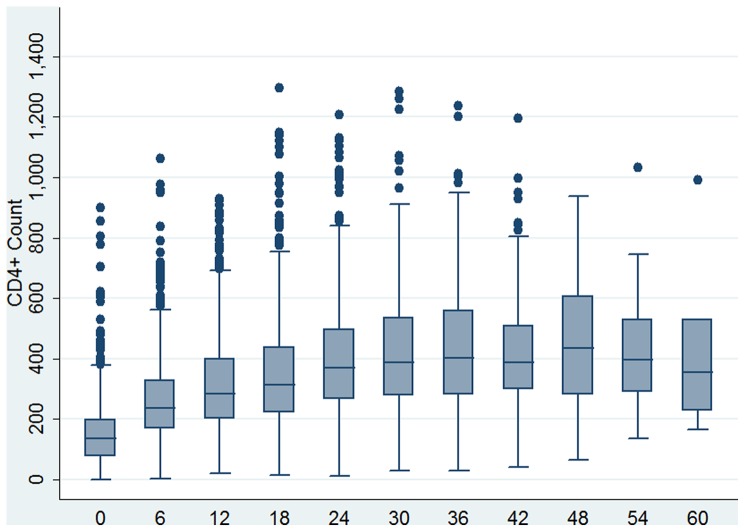
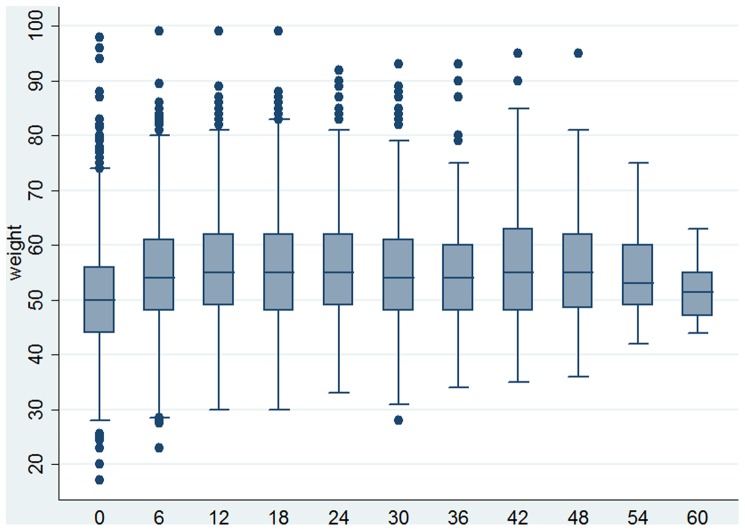
As shown in Figure 1, the median CD4 lymphocyte count had improved over the five year period except at the 54th and 60th months where the median CD4 cell count showed a slight decline (Figure 1 and Table 2). In figure 2 we show the changes in body weight over time. The median weight improved after the 6th month follow up period and declined in the 54th and 60th months but they were still higher than that of the baseline values (Figure 1 and Table 2). The random effects models regression was fitted to both the raw and the square root of CD4 lymphocyte count and patients body weight. The length of time in ART (Beta = 7.91; 95% CI 7.48–8.34; p value 0.000) rather than other variables predicted improvement in CD4 counts. However, actively working patients had better CD4 lymphocyte count than ambulatory patients (P-value 0.038). The model from the square root transformed data was used for interpretation since functional status was significant; the model from the raw data is shown additionally in Table 3 to facilitate comparison. To examine sensitivity of the model to missing values we refitted it using observations up to the 48th and 54th months. However, we did not find differences with the model based on 60th month follow up (Table 3).
Figure 1. Box plot of changes in CD4+ count among the cohort of patients on anti-retroviral treatment across months of retrospective follow up over five years.
Table 2. Baseline and follow up values of weight and CD4 count.* .
| Follow up period | Weight (in kilo grams) | CD4 count | ||
| N | median (IQR) | N | median (IQR) | |
| Baseline | 1489 | 50.0 (44.0–56.0) | 1510 | 135.0(76.0–198.3) |
| 6th month | 1181 | 54.0 (48.0–61.0) | 1098 | 237.0 (169.0–328.0) |
| 12th month | 1088 | 55.0 (49.0–62.0) | 954 | 285.0 (200.0–398.0) |
| 18th month | 971 | 55.0 (48.0–62.00) | 875 | 315.0 (221.0–480.0) |
| 24th month | 784 | 55.0 (49.0–62.0) | 698 | 370.0 (266.8–498.3) |
| 30th month | 562 | 54.0 (48.0–61.0) | 509 | 387.0 (278.0–536.5) |
| 36th month | 351 | 53.0 (48.0–60.0) | 305 | 403.0 (279.5–559.5) |
| 42nd month | 197 | 55.0 (48.0–63.0) | 180 | 387.5 (298.8–508.5) |
| 48th month | 80 | 55.0 (48.3–62.0) | 74 | 435.0 (279.3–606.0) |
| 54th month | 19 | 53.0 (49.0–60.0) | 17 | 396.0 (287.5–577.5) |
| 60th month | 6 | 51.5 (46.3–57.0) | 6 | 355.0 (213.0–644.8) |
The frequency in cells may be lesser than the cohort size (n = 1540) due to missing values on each of the variables reported.
Figure 2. Box plot of changes in weight among the cohort of patients on anti-retroviral treatment in months of retrospective follow up over five years.
Table 3. Baseline variables associated with changes in CD4 count among the cohort.* .
| Model 1 | CD4 count (non-transformed) | |||
| Predictor variables | Coefficient | SE | 95% CI | P-value |
| Age | −0.97 | 0.59 | −2.13, 0.18 | 0.099 |
| Hgb level | 0.003 | 0.66 | −1.28, 1.29 | 0.994 |
| WHO stage | ||||
| Stage I | ref | ref | Ref | Ref |
| Stage II | 9.28 | 23.31 | −36.40, 54.97 | 0.690 |
| Stage III | 25.63 | 22.73 | −18.91, 70.18 | 0.259 |
| Stage IV | 15.69 | 28.55 | −40.26, 71.64 | 0.583 |
| Weight | 0.12 | 0.51 | −0.88, 1.12 | 0.818 |
| Functional status | ||||
| Working | ref | ref | Ref | Ref |
| Ambulatory | −20.70 | 12.98 | −46.15, 4.75 | 0.111 |
| Bedridden | −43.28 | 29.73 | −101.56, 14.99 | 0.146 |
| Time of visit in months | 7.91 | 0.22 | 7.48, 8.34 | 0.000 |
| Model 2 | Based on square root transformation of CD4 count measurements | |||
| Age | −0.018 | 0.02 | −0.05, 0.02 | 0.295 |
| Hgb level | 0.001 | 0.02 | −0.04, 0.04 | 0.975 |
| WHO stage | ||||
| Stage I | ref | ref | Ref | Ref |
| Stage II | 0.18 | 0.68 | −1.15, 1.51 | 0.789 |
| Stage III | 0.65 | 0.66 | −0.64, 1.95 | 0.323 |
| Stage IV | 0.006 | 0.83 | −1.62, 1.63 | 0.994 |
| Weight | −0.002 | 0.01 | −0.03, 0.03 | 0.886 |
| Functional status | ||||
| Working | ref | ref | Ref | Ref |
| Ambulatory | −0.78 | 0.38 | −1.52, −0.04 | 0.038 |
| Bedridden | −1.62 | 0.86 | −3.31, 0.06 | 0.058 |
| Time of visit in months | 0.24 | 0.01 | 0.23, 0.25 | 0.000 |
Model 2 was used for interpreting the results; the findings are based on all observations of CD4 counts up to the 60th month.
The length of stay on ART was an important predictor of improvement in weight (Beta 0.15; 95% CI 0.13–0.18; p-value 0.000). Advanced WHO clinical stages, lower baseline CD4 lymphocyte count, and baseline hemoglobin levels were associated with lower body weight. Higher age predicted improvement in weight (Beta 0.15; 95% CI 0.07–0.23; p-value 0.000). The model from the square root transformed data revealed similar findings with the one based on the raw data. We show both models in the tables. To examine sensitivity of the model to missing values we refitted it using values of observations up to the 48th and 54th. Since these models were not different from the model based on the 60th month observations, the final model included all observations up to the 60th month of follow up (Table 4).
Table 4. Baseline variables associated with changes in weight (in kilograms) among the cohort.* .
| Model 1 | Based on non-transformed weight measurements | |||
| Predictor variables | Coefficient | SE | 95% CI | P-value |
| Age | 0.15 | 0.04 | 0.07, 0.23 | 0.000 |
| Hgb level | 0.11 | 0.04 | 0.02, 0.19 | 0.013 |
| WHO stage | ||||
| Stage I | ref | ref | Ref | ref |
| Stage II | −4.69 | 1.59 | −7.82, −1.57 | 0.003 |
| Stage III | −3.80 | 1.56 | −6.85, −0.74 | 0.015 |
| Stage IV | −3.94 | 1.93 | −7.72, 0.15 | 0.042 |
| CD4 count | 0.01 | 0.00 | 0.01, −0.00 | 0.021 |
| Functional status | ||||
| Working | ref | ref | Ref | ref |
| Ambulatory | −4.50 | 0.88 | −6.23, −2.77 | 0.000 |
| Bedridden | −7.66 | 1.83 | −11.25, −4.06 | 0.000 |
| Time of visit in months | 0.15 | 0.01 | 0.13, 0.18 | 0.000 |
| Model 2 | Based on square root transformation of weight measurements | |||
| Age | 0.01 | 0.00 | 0.00, 0.01 | 0.000 |
| Hgb level | 0.01 | 0.00 | 0.01, 0.01 | 0.016 |
| WHO stage | ||||
| Stage I | ref | ref | Ref | ref |
| Stage II | −0.29 | 0.11 | −0.51, −0.08 | 0.006 |
| Stage III | −0.24 | 0.11 | −0.45, −0.03 | 0.006 |
| Stage IV | −0.27 | 0.13 | −0.53, −0.01 | 0.040 |
| CD4 count | 0.0004 | 0.00 | 0.00, 0.00 | 0.012 |
| Functional status | ||||
| Working | ref | ref | Ref | ref |
| Ambulatory | −0.31 | 0.06 | −0.42, 0.19 | 0.000 |
| Bedridden | −0.53 | 0.13 | −0.78, −0.28 | 0.000 |
| Time of visit in months | 0.01 | 0.00 | 0.01, 0.01 | 0.000 |
Model 1 was used for interpretation; the findings are based on all observations of CD4 counts up to the 60th month.
Discussion
This longitudinal study aimed to examine the progress of CD4 lymphocyte count and weight, and their predictors among a cohort of patients taking antiretroviral treatment in eastern Ethiopia. Our findings indicate that the duration of ART was an important predictor of improvements in CD4 lymphocyte count (beta 7.91; 95% CI 7.48–8.34; p 0.000) and weight (beta 0.15; 95% CI 0.13–0.18; p 0.000). Patient's functional status at the beginning of treatment also predicted improvements in both body weight and CD4 lymphocyte count. Furthermore, higher age, earlier WHO clinical stage, higher CD4 count and high hemoglobin levels were associated with improvements in weight.
The research literature indicates that declines in CD4 lymphocyte count and weight loss among patients on anti-retroviral treatment are associated with increased mortality and morbidity manifesting in the form of deterioration of clinical conditions and decreased functional status in both adults [15] and children [16]. Similarly, in a previous study by our research group, the increment of CD4 count and weight gain were found to be important factors predicting patient survival [17]. Silva and colleagues reported that protease-inhibitor-containing antiretroviral therapy is associated with a weight gain and improvements in body mass index (BMI) [18]. In a cohort study, Mangili and colleagues showed that lower CD4 lymphocyte cell counts were associated with lower weight. When Mangili et al. classified patients being provided nutritional supplementation into CD4+ cell count strata, each 100-cell/mm3 decrease in CD4+ cell count was associated with an average of 1.9 kg reduction in weight [19].
Studies have shown that longer duration on ART leads to an improvement of CD4 lymphocyte counts. Similarly, in this study, longer duration on treatment was significantly associated with improvements in CD4 count and weight. A study among 358 patients on ART from southern Ethiopia has documented similar findings [20]. In a multi-country study among sub-Saharan African patients, median CD4 cell counts increased from a baseline of 97 cells/μl to 261 cells/μl at 48 weeks; whereas the proportion of patients with a CD4 cell count <100 cells/μl was reduced from 50% at baseline to 4% at 48 weeks [21]. The main reason for clinical improvement associated with longer duration on therapy is thought to be due to the reduction in viremia [22]. Patients that are able to stay longer on treatment are also more likely to have other parallel healthy behaviors like adherence to treatment as well as social support that improve their clinical outcomes.
In this study we found that older age is associated with improvement in weight. A similar finding has been reported by Mariz and colleagues where older age among patients on ART was associated not only with increased weight gain but also with becoming overweight among patients in Brazil [23]. They reported that patients more than 40 years of age were at risk of overweight or even obesity. Among patients on ART in Tanzania, Li and colleagues found a slightly higher odds of weight loss among patients between the ages of 15 and 29, and patients above the age of 50 three months after initiation of treatment [24]. However, it is difficult to interpret the latter study since its report is based on short term measurements (3 months), while in this study we reported outcomes that are based on follow up of patients for up to 5 years. This highlights a need for future research to examine the relationship between age and weight among patients on ART.
We found that high baseline hemoglobin level predicts improvement in weight gain. Our finding is similar to previous literature in that BMI levels are predicted by not only CD4 counts but also by anemia and hemoglobin levels [23]. For instance, a study carried out among Rwandan women shows risk factors for anemia to be associated with lower body mass index (OR 3.4; 95% CI 2.4–4.1), and CD4 lymphocyte counts below 200 cells/μL (OR 2.41; 95% CI 2.01–3.07). While patients were on treatment, the mean hemoglobin level of 10.9 at ART initiation significantly increased to 12.3 in eight months' time (P<0.001) [25]. However, reverse causation was also documented in another study; lower hemoglobin level at baseline was associated with the risk of significant weight loss at 3 months after initiation among patients on ART in Tanzania [26]. However, it seems that, without regard to the causal relationship between weight gain and anemia, a targeted nutritional supplementation and counseling could help in both weight gain and reduction of anemia among patients.
This study has some limitations. CD4 lymphocyte counts were not available for all patients due to lack of resources at the hospitals where the study was conducted. On top of this HIV RNA measurements were not collected since they are not part of the clinical monitoring of patients in Ethiopia because of their very prohibitive cost. Similarly, diagnostic tests that would confirm the presence of certain opportunistic infections were also limited. Due to this we were not able to include them in our analysis to examine and account for their potential contribution to CD4 count and weight changes. The retrospective study design limited our ability to gather data about factors that may influence morbidity, CD4 count and weight levels, such as food insecurity, social support and depression. However, our study has strengths in that we used a relatively large sample size, multiple referral hospitals, a robust analysis and a longer follow up period. It also has strengths in that it provides evidence about the implementation of ART and its impacts on clinical indicators in the context of treatment provided by lower level health workers in a resource poor country.
We detected a substantial increment in weight and CD4 lymphocyte count among the patients who were taking ART in eastern Ethiopia. The findings indicate that the length of time patients stay on ART and patients' functional status improves both CD4 count and weight gain. Higher age groups, earlier WHO stage, higher CD4 count and high hemoglobin levels were associated with improvements in weight. Ambulatory patients during the initiation of ART should be monitored carefully for immunological response. Patients who are in old age, with low CD4 lymphocyte count, late stage of WHO and lower hemoglobin level may need special diet considerations such as food by prescription programs to help them gain weight over time.
Acknowledgments
We thank all the health facilities, and the staff for their cooperation. We appreciate the anonymous patients whose medical records forms the base of this study. We thank ART nurses and other paid individuals who provided valuable support during the data collection and entry process.
Funding Statement
Haramaya University funded this study (www.haramaya.edu.et). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS (2010) Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS). [Google Scholar]
- 2. Levy JA (1993) HIV pathogenesis and long-term survival. AIDS 7: 1401–1410. [DOI] [PubMed] [Google Scholar]
- 3. Jacobson LP, Phair JP, Yamashita TE (2004) Virologic and immunologic response to highly active antiretroviral therapy. Curr HIV/AIDS Rep 1: 74–81. [DOI] [PubMed] [Google Scholar]
- 4. Yamashita TE, Phair JP, Muñoz A, Margolick JB, Detels R, et al. (2001) Immunologic and virologic response to highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. AIDS 15: 735–746. [DOI] [PubMed] [Google Scholar]
- 5. Resino S, Bellón JM, Sánchez-Ramón S, Gurbindo D, Ruiz-Contreras J, et al. (2002) Impact of antiretroviral protocols on dynamics of AIDS progression markers. Arch Dis Child 86: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siegel K, Lekas H-M (2002) AIDS as a chronic illness: Psychosocial implications. AIDS 16: S69–S76. [DOI] [PubMed] [Google Scholar]
- 7. Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, et al. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338: 853–860. [DOI] [PubMed] [Google Scholar]
- 8. Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, et al. (2001) Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Annals of Internal Medicine 135: 7–26. [DOI] [PubMed] [Google Scholar]
- 9. Oyomopito R, Lee MP, Phanuphak P, Lim PL, Ditangco R, et al. (2010) Measures of site resourcing predict virologic suppression, immunologic response and HIV disease progression following highly active antiretroviral therapy (HAART) in the TREAT Asia HIV Observational Database (TAHOD). HIV Medicine 11: 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirschel B, Opravil M (1999) The year in review: antiretroviral treatment. AIDS 13: S177–S187. [PubMed] [Google Scholar]
- 11.Huruy K, Kassu A, Mulu A, Wondie Y (2020) Immune restoration disease and changes in CD4+ T-cell count in HIV-infected patients during highly active antiretroviral therapy at Zewditu memorial hospital, Addis Ababa, Ethiopia. AIDS Research and Therapy 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Modjarrad K, Vermund SH (2010) Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infectious Diseases 10: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HAPCO MOH (2007) Guideline for the management of opportunistic infections and anti-retroviral treatment in adolescents and adults in Ethiopia. In: Ethiopian HIV/AIDS Prevention and Control Office, editor. Addis Ababa: Ethiopia Ministry of Health. [Google Scholar]
- 14.Rabe-Hasketh S, Everitt BS (2007) Multiple regression. A handbook of statistical analyses using Stata. Florida: Chapman & Hall/CRC. [Google Scholar]
- 15. Grinspoon S, Mulligan K (2003) Weight loss and wasting in patients infected with human immunodeficiency virus. Clin Infect Dis 36: S69–78. [DOI] [PubMed] [Google Scholar]
- 16. Verweel G, van Rossum AMC, Hartwig NG, Wolfs TFW, Scherpbier HJ, et al. (2002) Treatment with highly active antiretroviral therapy in Human Immunodeficiency Virus Type 1-infected children is associated with a sustained effect on growth. Pediatrics 109: e25. [DOI] [PubMed] [Google Scholar]
- 17. Biadgilign S, Reda AA, Digafe T (2012) Predictors of mortality among HIV infected patients taking antiretroviral treatment in Ethiopia: a retrospective cohort study. AIDS Res Ther 9: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva M, Skolnik PR, Gorbach SL, Spiegelman D, Wilson IB, et al.. (1998) The effect of protease inhibitors on weight and body composition in HIV- infected patients. AIDS 12: 1645– 1651. [DOI] [PubMed] [Google Scholar]
- 19. Mangili A, Murman DH, Zampini AM, Wanke CA (2006) Nutrition and HIV Infection: Review of Weight Loss and Wasting in the Era of Highly Active Antiretroviral Therapy from the Nutrition for Healthy Living Cohort. Clinical Infectious Diseases 42: 836–842. [DOI] [PubMed] [Google Scholar]
- 20. Tafese Z, Berhan Y, Abebe H (2012) Changes in nutritional, functional and immunological status of HIV-infected adults with antiretroviral therapy. Ethiop Med J 50: 75–87. [PubMed] [Google Scholar]
- 21.Lawn SD, Myer L, Bekker L-G, Wood R (2006) CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al-Harthi L, Voris J, Patterson BK, Becker S, Eron J, et al. (2004) Evaluation of the impact of highly active antiretroviral therapy on immune recovery in antiretroviral naive patients. HIV Medicine 5: 55–65. [DOI] [PubMed] [Google Scholar]
- 23. Mariz Cde A, Albuquerque Mde F, Ximenes RA, Melo HR, Bandeira F, et al. (2011) Body mass index in individuals with HIV infection and factors associated with thinness and overweight/obesity. Cad Saude Publica 27: 1997–2008. [DOI] [PubMed] [Google Scholar]
- 24. Li N, Spiegelman D, Drain P, Mwiru RS, Mugusi F, et al. (2012) Predictors of weight loss after HAART initiation among HIV-infected adults in Tanzania. AIDS 26: 577–585. [DOI] [PubMed] [Google Scholar]
- 25. Masaisa F, Gahutu JB, Mukiibi J, Delanghe J, Philippé J (2011) Anemia in Human Immunodeficiency Virus–Infected and Uninfected Women in Rwanda. Am J Trop Med Hyg 84: 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li N, Spiegelman D, Drain P, Mwiru RS, Mugusi F, et al. (2012) Predictors of Weight Loss after Highly-Active Antiretroviral Therapy (HAART) Initiation among HIV-Infected Adults in Tanzania. AIDS 26: 577–585. [DOI] [PubMed] [Google Scholar]