Abstract
Background
In clinical reports, the usage of isoflurane and sevoflurane was associated with more surgical field bleeding in endoscopic sinus surgeries as compared to propofol. The activation of platelet receptor αIIbβ3 is a crucial event for platelet aggregation and clot stability. Here we studied the effect of isoflurane, sevoflurane, and propofol on the activation of αIIbβ3.
Methods
The effect of anesthetics on the activation of αIIbβ3 was probed using the activation sensitive antibody PAC-1 in both cell-based (platelets and αIIbβ3 transfectants) and cell-free assays. The binding sites of isoflurane on αIIbβ3 were explored using photoactivatable isoflurane (azi-isoflurane). The functional implication of revealed isoflurane binding sites were studied using alanine-scanning mutagenesis.
Results
Isoflurane and sevoflurane diminished the binding of PAC-1 to wild-type αIIbβ3 transfectants, but not to the high-affinity mutant, β3-N305T. Both anesthetics also impaired PAC-1 binding in a cell-free assay. In contrast, propofol did not affect the activation of αIIbβ3. Residues adducted by azi-isoflurane were near the calcium binding site (an important regulatory site termed SyMBS) just outside of the ligand binding site. The mutagenesis experiments demonstrated that these adducted residues were important in regulating integrin activation.
Conclusions
Isoflurane and sevoflurane, but not propofol, impaired the activation of αIIbβ3. Azi-isoflurane binds to the regulatory site of integrin αIIbβ3, thereby suggesting that isoflurane blocks ligand binding of αIIbβ3 in not a competitive, but an allosteric manner.
Introduction
General anesthesia during surgery is induced and maintained by administration of inhalational (volatile) and/or intravenous anesthetic drugs. While anesthetic drugs primarily act on neuronal cells in the central nervous system [1], thereby inducing general anesthetic states, the report that halothane impairs adenosine diphosphate (ADP)-induced platelet aggregation by Ueda [2] triggered subsequent studies on the effect of hemostasis. Clinical observational investigations into the effects of anesthetics on hemostasis during surgery [3], point to an intriguing trend that intra-operative bleedings were less severe in anesthesia with the intravenous anesthetic propofol than volatile anesthetics isoflurane and sevoflurane [3], [4], [5], [9], [10] ( Table 1 ). However, in vitro mechanistic investigations into the direct effects of propofol [12], [13], isoflurane [10], , and sevoflurane [12], [14] [16] on platelet aggregation, a critical step in hemostasis have shown mixed results thus far.
Table 1. The effect of anesthetics on surgical bleeding.
| Surgical procedure | Anesthetics and number of patients | Study design | Results | Refer-ence |
| Endoscopic sinus surgery | Sevoflurane/remifentanil (n = 20) versus propofol/remifentanil (n = 20) | Prospective, randomized study | Less blood loss and better surgical field in propofol group for patients with extensive chronic sinusitis | [3] |
| Endoscopic sinus surgery | Propofol (n = 30) versus isoflurane (n = 26) | Prospective, randomized study | Better surgical field | [8] |
| Endoscopic sinus surgery | Propofol (n = 12) versus isoflurane (n = 13) | Retrospective review | Decreased blood loss in propofol group | [4] |
| Endoscopic sinus surgery | Propofol/remifentanil (n = 45) versus isoflurane/alfentanil (n = 43) | Prospective, randomized study | Bleeding from surgical field was significantly better in propofol group | [5] |
| Endoscopic sinus-nasal surgery | Propofol/remifentanil (n = 27) versus isoflurane/fentanyl (n = 37) | Prospective, randomized study | Propofol/remifentanil was effective in reducing bleeding | [10] |
| Endoscopic sinus surgery | Sufentanil/Sevoflurane (n = 23) versus remifentanil/propofol (n = 20) versus fentanyl/isoflurane (n = 28) | Retrospective review | Least bleeding in remifentanil/propofol group | [7] |
| Endoscopic sinus surgery | Sevoflurane/fentanyl (n = 28) versus propofol/remifentanil (n = 28) | Prospective, randomized study | Better surgical field in propofol/remifentanil group | [11] |
| Endoscopic sinus surgery | Propofol/fentanyl (n = 16) versus sevoflurane/fentanyl (n = 16) | Prospective, randomized study | Less bleeding in propofol group | [9] |
| Head and neck surgery | Isoflurane (n = 20) versus propofol (n = 18) | Prospective, randomized | Blood loss in isoflurane group tended to be slightly higher. | [6] |
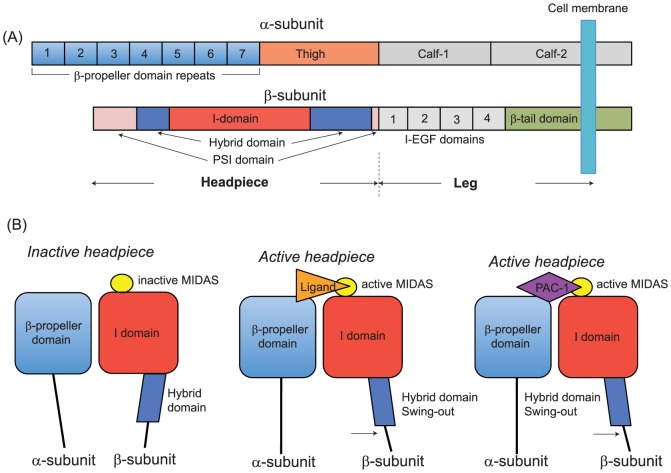
αIIbβ3 is the most abundant receptor in platelets and plays a critical role in platelet aggregation and clot stability through the interaction with its Arg-Gly-Asp (RGD)-motif –containing ligands fibrinogen, von Willebrand factor and fibronectin [17], [18], [19], [20], [21], [22]. αIIbβ3 is a member of the adhesion molecule family integrins, and is composed of non-covalently linked α/β heterodimers, with each subunit consisting of multiple well-characterized domains [23]( Figure 1A ). Only upon activation, αIIbβ3 undergoes the conformational changes referred to as “the hybrid domain swing-out”, which induces the ligand binding site to the high-affinity state [24] ( Figure 1B ). Three metal binding sites (metal-ion dependent adhesion site (MIDAS), SyMBS, and ADMIDAS) located on the top of the β3 I domain differentially regulate the activity of integrin αIIbβ3 during this conformational change. The MIDAS directly binds to the RGD motif of ligands, while SyMBS and ADMIDAS take indirect roles in ligand binding by modulating metal coordinations at the MIDAS [24],[25],[26]. The study by Horn et al. demonstrated that sevoflurane, even at subanesthetic concentrations, significantly abolished the activation of αIIbβ3 in whole blood [27]. Inspired by Horn et al, and building on our previous studies on the effects of volatile anesthetics to leukocyte integrins [28],[29],[30],[31], here we tested the hypothesis that isoflurane and sevoflurane, not propofol directly interacted with platelet integrin αIIbβ3and interfered with its activation.
Figure 1. Integrin structure and conformational change.
(A) αIIbβ3 consists of the α subunit (αIIb) and the βsubunit (β3). Domains within the primary structure of α- and β- subunits suggested by X ray crystal structures of αVβ3 and αIIbβ3 [24], [52] are shown. The β-propeller and the thigh domains of the α subunit and the PSI, the hybrid and the I domains of the β subunit constitute the headpiece of αIIbβ3. (B) Schema of conformational change of the headpiece. The metal-ion dependent adhesion site (MIDAS) undergoes conformation change and interacts directly with ligands when it is in an active form. In a conformation where the hybrid domain faces inward toward the α subunit, the MIDAS is inactive. When the hybrid domain swings out, the conformational change of the MIDAS ensues with ligand or PAC-1 binding.
Materials and Methods
Cells
Chinese hamster ovary (CHO) -K1 cells stably transfected with αIIb-wild type (WT)/β3-WT or αIIb-WT/β3-N305T were previously described and kindly given by Dr. Springer [32]. They were cultured in RPMI1640, 10% FBS and geneticin G418 in 5% CO2 at 37°C. 293T cells (ATCC; Manassas, VA, USA) were cultured in DMEM with HEPES modification, 10% FBS at 37°C in 5% CO2.
PAC-1 binding assay using human platelets
The activation of αIIbβ3 was probed using PAC-1, an IgM antibody that binds only to the activated αIIbβ3 [33], [34]. Freshly prepared platelet-rich plasma (PRP) was purchased from Research Blood Components, LLC (Boston, MA, USA). PRP was diluted in Tyrode's buffer (1% bovine serum albumin (BSA), 2 mmol/L MgCl2, 137.5 mmol/L NaCl, 12 mmol/L NaHCO3, 2.6 mmol/L KCl, pH 7.4) as described [35], and stimulated with 20 µM adenosine 5′- diphosphate (ADP) (Sigma; St. Louis, MO, USA) in the presence of PAC-1-FITC (BD Biosciences; San Jose, CA, USA) and anesthetics (isoflurane or propofol) for 30 minutes. Isoflurane was administered to PRP in the closed chamber using a Fluotec vaporizer (Cyprane Ltd., Keighley, UK), and their concentrations were measured using infrared spectroscopy (Datex Instrument Corp., Helsinki, Finland). Following stimulation, samples were fixed with paraformaldehyde (1%) and subject to the flow cytometry anaylsis using a FACScan (BD Biosciences; San Jose, CA, USA). Data were shown as mean fluorescence intensity (MFI).
PAC-1 binding assay using αIIbβ3 transfectants
CHO-K1 cells transfected with αIIbβ3 were detached in HEPES-buffered saline (HBS)/10 mM EDTA and washed three times with HBS. Cells were incubated with 10 µg/ml PAC-1 (BD Biosciences) in HBS containing 1 mM MgCl2/CaCl2 (inactivating condition) or HBS Containing 1 mM MnCl2/0.4 mM CaCl2 (activating condition) in the presence of various concentrations of isoflurane, sevoflurane or propofol. Isoflurane and sevoflurane were administered to cells in the closed chamber using a Fluotec vaporizer, and their concentrations were measured using infrared spectroscopy. Goat anti-mouse IgM-FITC (Santa Cruz biotechnology Inc.; Santa Cruz, CA, USA) was used as a secondary antibody. Cells were analyzed with a FACScan. In addition, the cell surface expression of αIIbβ3 was probed with AP3 antibody (Immune Disease Institute; Boston, MA, USA). PAC-1 binding % was calculated as [(MFI of sample at various concentrations of anesthetics)-(MFI of isotype control sample)]/(MFI of sample without anesthetics)-(MFI of isotype control sample)]×100%.
Protein expression and purification
The purification of full-length ectodomain and headpiece αIIbβ3 was previously described [36], [37]. Integrin αIIbβ3 purified from human platelets was purchased from EMD Millipore (Billerica, MA, USA).
PAC-1 binding to the extracellular portion of αIIbβ3
Capturing antibody AP3 was coated on 96 wells overnight. Wells were blocked with 2% BSA and then incubated with recombinant αIIbβ3 (full length or headpiece). Following washing, wells were incubated with PAC-1 in the presence of various concentrations of isoflurane, sevoflurane or propofol containing 1 mM MgCl2/CaCl2 or 1 mM MnCl2/0.4 mM CaCl2 for 1 hour. Isoflurane and sevoflurane were administered to wells in a closed chamber using a Fluotec vaporizer and their concentrations were measured using infrared spectroscopy. Attached αIIbβ3 was captured with anti-mouse IgM- HRP (Cayman Chemical; Ann Arbor, Michigan, USA). Color was developed with substrate (BD Bioscience; San Jose, CA, USA). Optical density (OD) at 405 nm was read using an ELISA plate reader (Molecular Device; Sunnyvale, CA, USA). PAC-1 binding % was defined as [(OD of sample at various concentrations of anesthetics) – (OD of background)]/[(OD of sample without anesthetics) - (OD of background)]×100%.
Photolabeling experiments
Photolabeling experiments were performed using azi-isoflurane, isoflurane with a diaryzinyl moiety. The details of experiment have been previously described [38], [39]. Briefly, full-length ectodomain αIIbβ3 or αIIbβ3 purified from human platelets was incubated with or without 1 mM azi-isoflurane in quartz cuvettes for 15 minutes, and then exposed to 300 nm UV light for 15 minutes. The protein was separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Bands corresponding to the protein of interest were excised, trypsinized and submitted for nano liquid chromatography (LC)/mass spectrometry (MS) analysis. LC was performed using a 10 cm C18 capillary column at 200 nl/min for 60 minutes with gradient elution. MS-detected peptides were searched for adducts of the appropriate mass (196 Da) and then further fragment patterns (MS/MS) were searched using Sequest software to determine the adduct attachment sites. Mass spectrometry work was performed at the University of Pennsylvania Proteomics Core Facility.
Point mutagenesis and transfection
Alanine scanning mutagenesis was performed using Quikchange XL kit (Stratagene; La Jolla, CA, USA). DNA sequence was confirmed. Transfection was performed using Lipofectamine 2000 (Invitrogen; Carlsbad, CA, USA) per company protocol.
Statistical significance
Data were analyzed using an analysis of variance (ANOVA) with Tukey post hoc pairwise comparisons or student's t-test as indicated in corresponding figure legends. Statistical significance was defined as P<0.05. Statistical analysis was performed using PRISM 5 software (GraphPad Software; La Jolla, CA, USA).
Results
Isoflurane and sevoflurane, but not propofol attenuated PAC-1 epitope exposure in ADP stimulated platelets, but propofol did not
From clinical observational studies on hemostasis as summarized in Table 1 , we hypothesized that isoflurane and sevoflurane would attenuat the activation of integrin αIIbβ3, but propofol would not. In fact, volatile anesthetic sevoflurane attenuated the activation of αIIbβ3 on platelets stimulated by ADP as demonstrated by Horn et al. [27]. We demonstrated that another volatile anesthetic isoflurane at a clinically relevant concentration (2%) attenuated its activation on platelets ( Figure 2 ). The clinical relevant concentration of propofol ranges from 10–50 µM [40], [41], [42]. Propofol (50 µM) did not attenuate the activation of αIIbβ3 on platelets ( Figure 2 ). These results supported our hypothesis. To assess the direct interaction of volatile anesthetics with αIIbβ3, we examined the effect of anesthetics using CHO cells stably transfected with αIIbβ3 and purified proteins in the following sections.
Figure 2. PAC-1 binding assays with anesthetics in platelets.
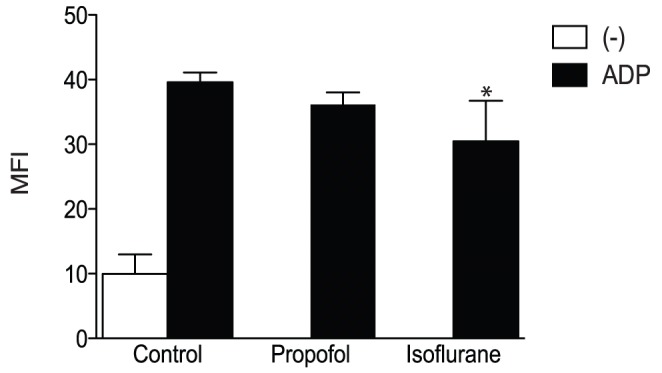
Flow cytometry based PAC-1 binding assays were performed using platelet-rich plasma stimulated with 20 µM adenosine 5′-diphosphate (ADP) in the presence of isoflurane (2%) or propofol (50 µM). Data is shown as mean +/− S.D. of mean fluorescence intensity (MFI) of six independent experiments. Data were analyzed using a one-way analysis of variance with Tukey post hoc pairwise comparisons. * denotes p<0.05 versus ADP-treated control sample.
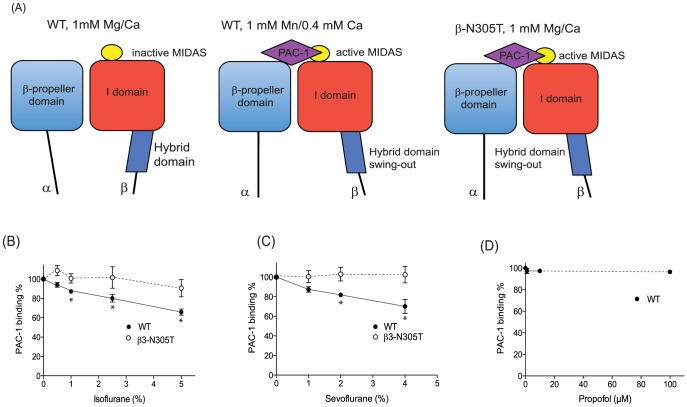
Isoflurane and sevoflurane attenuated the activation of wild type αIIbβ3 on cells
First, we evaluated the effect of anesthetics on αIIbβ3 activation in CHO transfectants. PAC-1 contains the Arg-Tyr-Asp (RYD) sequence that is analogous to the RGD sequence in the complementarity determining region 3 of the heavy chain. This region is speculated to interact with the activated MIDAS [33]. We tested PAC-1 binding either in a resting condition (1 mM Mg2+/Ca2+) or an activating condition (1 mM Mn2+/0.4 mM Ca2+). In 1 mM Mg2+/Ca2+, PAC-1 does not binds to αIIbβ3 WT ( Figure 3A ). On the other hand, PAC-1 binds significantly to αIIbβ3 WT in 1 mM Mn2+/0.4 mM Ca2+ ( Figure 3A ). Isoflurane and sevoflurane diminished PAC-1 binding to WT in 1 mM Mn2+/0.4 mM Ca2+ ( Figure 3B and C ), while they did not alter the expression of αIIbβ3 WT ( Figure 4A ). This suggested that isoflurane and sevoflurane either attenuated the activation of αIIbβ3 WT or directly interacted with PAC-1 binding sites. The β3-N305T mutant was previously designed to introduce N-glycosylation by changing amino acid sequences of the β3 subunit from N303-I304-N305 to N303-I304-T305 [32]. β3-Asn305 is located on the bottom of the I domain, at the interface with the hybrid domain. The introduction of N-glycan at this site opened up the interface between the I domain and the hybrid domain, mimicking the hybrid domain swing-out motion and making this mutant constitutively active [32] ( Figure 3A ). Both isoflurane and sevoflurane failed to modulate PAC-1 binding to β3-N305T mutant ( Figure 3B and C ). Further, exposure to isoflurane and sevoflurane did not alter the expression level of αIIbβ3 on the β3-N305T mutant ( Figure 4B ), suggesting that these volatile anesthetics did not directly interact with PAC-1 binding sites on αIIbβ3. Taking these results together, isoflurane and sevoflurane attenuated the activation of αIIbβ3 WT. In contrast, the intravenous anesthetic propofol failed to modulate PAC-1 binding to αIIbβ3 WT ( Figure 3D ), indicating that propofol did not inhibit the activation of αIIbβ3.
Figure 3. PAC-1 binding assays using αIIbβ3 transfectants in the presence of anesthetics.
(A) Scheme of PAC-1 interaction with αIIbβ3 WT and β3-N305T mutant. While αIIbβ3 wild-type (WT) binds to PAC-1 only in an activating condition (1 mM Mn2+/0.4 mM Ca2+), activating β3-N305T mutant can bind to PAC-1 in a resting condition (1 mM Mg2+/Ca2+) due to its constitutive swing-out of the hybrid domain. (B–D) Flow cytometry based PAC-1 binding assays were performed using CHO cells stably transfected with wild type αIIbβ3 or N305T mutant in the presence of isoflurane (B) or sevoflurane (C) at various concentrations. For propofol, only wild type αIIbβ3 was tested (D). PAC-1 binding % was calculated as [(mean fluorescence intensity (MFI) at various concentrations of anesthetics) – (MFI of isotyoe control)]/[(MFI without anesthetics) – (MFI of isotype control)]×100 (%). Data is shown as mean +/− S.D. of three independent experiments. Binding experiment was done at 1 mM Mn2+/0.4 mM Ca2+. One-way analysis of variance with Tukey post hoc pairwise comparisons was used to compare the data at different anesthetic concentrations within wild-type or mutant transfectants. * denotes p<0.05 versus mock-treated sample (no anesthetic).
Figure 4. The effect of anesthetics on αIIbβ3 cell surface expression.
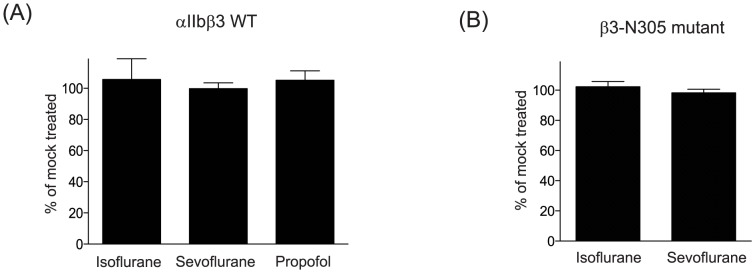
Surface expression of αIIbβ3 WT (A) or β3-N305T (B) was probed by AP3 antibody and expressed using mean fluorescence intensity (MFI). Data was shown as [(MFI of αIIbβ3 exposed to anesthetic)/(MFI of αIIbβ3 of sample not exposed to anesthetic)]×100%, and expressed as mean +/− S.D. of three independent experiments. Isoflurane, sevoflurane, and propofol used were 5%, 4%, and 100 µM, respectively.
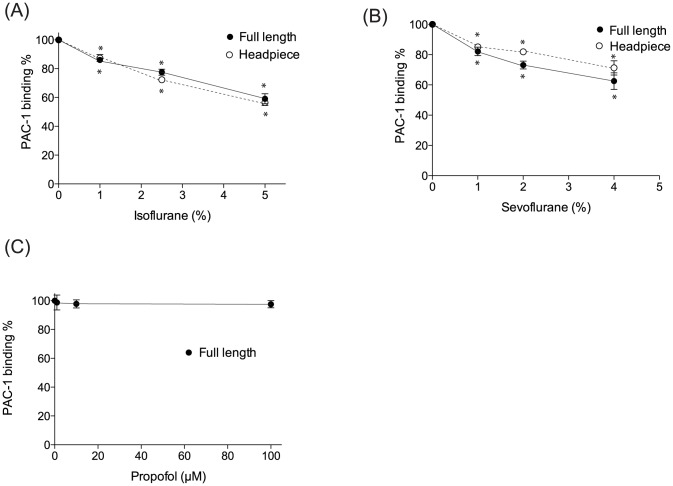
Isoflurane and sevoflurane attenuated the activation of αIIbβ3 protein
We demonstrated that isoflurane and sevoflurane attenuated the activation of αIIbβ3 in cell-based assays using CHO transfectants. Anesthetics are well appreciated as promiscuous molecules [43], and thus we cannot conclude if the results reflected the direct effect of the anesthetics on αIIbβ3, or the indirect effect (for example, the effect on the plasma membrane or intracellular proteins). We examined the effect of anesthetics on the activation of purified αIIbβ3 protein in cell-free ELISA type assay, which excluded the components of the plasma membrane and intracellular proteins. Both isoflurane and sevoflurane impaired the activation of αIIbβ3 ( Figure 5A and B ). Interestingly, there was no difference in the degree of inhibition between headpiece and full-length αIIbβ3, suggesting that isoflurane and sevoflurane interacted with the headpiece portion ofαIIbβ3. Propofol did not affect the activation of αIIbβ3 in this cell-free assay ( Figure 5C ), as predicted by the previous result ( Figure 3D ).
Figure 5. Cell-free PAC-1 binding assays with anesthetics.
ELISA type PAC-1 binding assays were performed using full-length ectodomain or headpiece αIIbβ3 in the presence of isoflurane (A) or sevoflurane (B) at various concentrations. For propofol, experiments were performed using full-length αIIbβ3 (C). PAC-1 binding % was calculated as [(OD at various concentrations of anesthetics)- (OD of background)]/[(OD of mock treated sample)-(OD of background)]×100 (%). Data is shown as mean +/− S.D. of three independent experiments. Binding experiment was done at 1 mM Mn2+/0.4 mM Ca2+. One-way analysis of variance with Tukey post hoc pairwise comparisons was used to compare the data at different anesthetic concentrations within full-length or headpiece protein. * denotes p<0.05 versus mock-treated sample (no anesthetic).
Azi-isoflurane bound to the βI domain
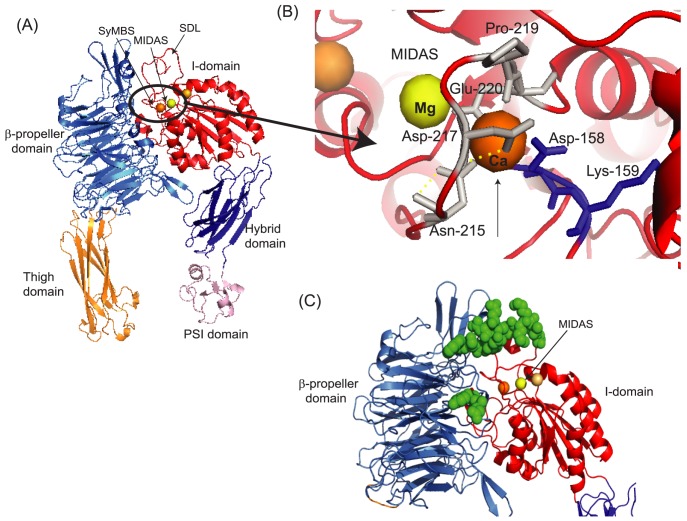
Our cell-free and cell-based assays strongly suggested that isoflurane and sevoflurane directly interacted with αIIbβ3 and attenuated its activation. Previously we reported that a novel photoactivatable compound, azi-isoflurane [38], reliably probed isoflurane binding sites on apoferritin, integrin αL I domain [39], and LFA-1 [31]. Thus, we used azi-isoflurane to reveal isoflurane binding sites on both full-length recombinant αIIbβ3 and purified αIIbβ3 from platelets. Azi-isoflurane bound to the I domain at Asp-158 and/or Lys-159 ( Table 2 , Figure 6 , 7A and 7B ) in both samples. Asp-158 and Lys-159 are close to the calcium binding site as shown in Figure 7B . This calcium binding site is called the synergistic metal binding site (SyMBS) or the ligand associated metal binding site (LIMBS) [26]. The adducted residues were in the headpiece region of αIIbβ3, which was in line with our result of cell-free assays. The epitope mapping of PAC-1 by Puzon-McLaughlin et al. showed that they were within residues 156–162 and 229–230 of the αIIb subunit and residues 179–183 of the β3 subunit ( Figure 7C ) [44]. Our adducted residues did not belong to these residues, which suggested that volatile anesthetics did not compete with PAC-1 directly as we indicated based on the results of cell-based assays. Unfortunately, a photoactive version of sevoflurane is not currently available, and we were not able to explore the binding site of sevoflurane using this approach. However, sevoflurane and isoflurane have similar physicochemistry, and we strongly suspect that sevoflurane interacts with the same site.
Table 2. The photolabeled residues of integrin αIIbβ3 by azi-isoflurane.
| Purified αIIbβ3 from platelets. | ||
| Sequence coverage | Photolabeled residues | |
| α subunit | 29.58% | N/A |
| β subunit | 33.22% | K159 |
Figure 6. Amino acid residues of the β I domain covered by mass spectrometry.
The amino acid residues of the β I domain are shown. Covered residues by mass spectrometry are shown in red. Adducted residues are shown in asterisk.
Figure 7. αIIbβ3 headpiece structure and adducted residues.
(A) The X ray crystal structure of αIIbβ3 headpiece was obtained from protein data bank (PDB; 3FCS). There are three metal binding sites in the I domain of the β subunit. Mg2+ in the MIDAS (this site is directly involved in ligands binding) is shown in yellow sphere, while Ca2+ in the SyMBS and ADMIDAS are shown in orange and light orange spheres, respectively. (B) The blowout of residues around metal binding sites from Figure 7 (A) is shown. The adducted residues of photolabeling experiments are shown in blue. Again, Mg2+ in the MIDAS is shown in yellow sphere, while Ca2+ in the SyMBS is shown in orange sphere. Both figures were created using PYMOL. (C) The structure of αIIbβ3 in the open conformation was obtained from Protein data bank (http://www.rcsb.org/pdb/home/home.do; PDB 3FCU). Residues shown as green spheres on αIIbβ3 are suggested PAC-1 binding sites by Puzon-McLaughlin et al. [44]. This figure was created using PYMOL.
D158A mutant reduced the activation of αIIbβ3
To confirm the functional role of the azi-isoflurane adducted residues (Asp-158 and Lys-159), we made β3-D158A and –K159A mutants to alter the chemical texture of this site. As shown in Figure 8A , β3-D158A mutant completely abolished the activation of αIIbβ3 integrin in activating conditions (1 mM Mn2+/0.4 mM Ca2+), indicating the importance of this residue. This was consistent with the previously reported results of the mutants of the other SyMBS forming residues [26]. The β3-K159A mutant significantly diminished the cell surface expression of αIIbβ3 ( Figure 8B ), suggesting that Lys-159 was a critical residue for expression rather than activation.
Figure 8. β3 mutants of adducted residues.
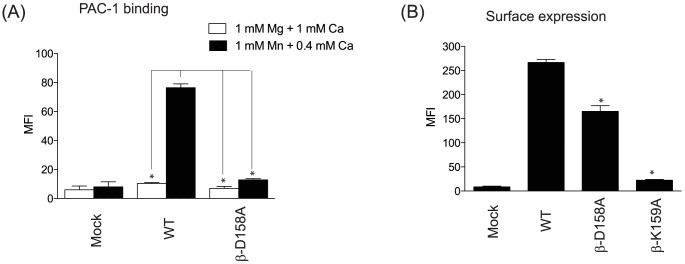
(A) PAC-1 binding to mock, αIIbβ3 wild type or mutant in 1 mM Mg2+/Ca2+ or 1 mM Mn2+/0.4 mM Ca2+. MFI; mean fluorescence intensity. One-way analysis of variance with Tukey post hoc analysis was performed to compare different groups (excluding mock group). * denotes p<0.05 versus wild type, 1 mM Mn2+/0.4 mM Ca2+ group. (B) Surface expression of mock, αIIbβ3 wild-type or mutants probed by AP3 is shown. Data is shown as mean +/− S.D. of three independent experiments. One-way analysis of variance with Tukey post hoc analysis was performed (excluding mock group). * denotes p<0.05 versus wild type.
Discussion
In this study, we demonstrated that isoflurane and sevoflurane, but not propofol, attenuated the activation of integrin αIIbβ3. Furthermore, the photolabeling experiment using azi-isoflurane suggested that isoflurane bound to the residues around the SyMBS of the I domain of the β subunit. That these two findings were linked was suggested by the mutagenesis experiments, which indicated the importance of this site for expression and activation.
With the appreciation of its profound effect on platelet aggregation, αIIbβ3 has been an attractive therapeutic target to prevent platelet aggregation in specific disease states. For example, peptides containing the RGD sequence competitively prevent αIIbβ3 from binding to its ligands [22], and have thus served as a basis for antagonist design [17]. Currently, abciximab (Reo-Pro, Eli Lilly, Indianapolis, IN), eptifibatide (Integrelin, Cor therapeutics, Cambridge, MA) and tirofiban (Aggrastat, Merck, Whitehouse Station, NJ) are approved for clinical usage to reduce ischemic events in patients with acute coronary syndrome undergoing percutaneous coronary intervention [45], [46]. When these drugs were developed, there was no structural information how these compounds interacted with αIIbβ3. Now we know that the majority of αIIbβ3 small molecule antagonists including eptifibatide and tirofiban bind to a small pocket on the top of the αIIbβ3 head formed by loops from the αIIb β-propeller and the βI domain [24]. These compounds interact with the MIDAS Mg2+ ion of the I domain via one of the oxygen atoms in the compound's Asp carboxyl or an equivalent carboxyl [24], [25]. The exception is abciximab, the β3 specific-7E3 Fab, which blocks ligand binding by binding to residues in the specificity determining loop (SDL) [47] ( Figure 7A ). Macromolecules such as fibrinogen recognize a rather larger area at the interface between the β-propeller domain of the αIIb subunit and the I domain of the β subunit, and interact with the β3 SDL and αIIb β-propeller loops. Therefore, blocking SDL wth abciximab prevents fibrinogen binding [24].
Surprisingly, the adducted residues of photolabeling experiments were located around the SyMBS, which was not at the binding pocket of the aforementioned αIIbβ3 small molecule antagonists and Fab. The SyMBS coordinates Ca2+ and allosterically activates integrins for ligand binding by stabilizing the MIDAS site [36]. The side chain carboxyl of β3-Glu-220 coordinates the SyMBS Ca2+ and MIDAS Mg2+ at the same time [36] ( Figure 7B ). Therefore, any alteration of residues surrounding the SyMBS could influence the orientation of MIDAS, and therefore αIIbβ3 activation. The result of β3-D158A mutant supported this idea. Also, the SyMBS coordinates with the SDL and disruption of this interaction resulted in impaired activation, as shown by blockade of ligand binding by abciximab [48]. Thus, allosteric inhibition of activation via binding to SyMBS is feasible. However, azi-isoflurane is structurally altered from isoflurane, and it is possible that the sites reported could be different from isoflurane binding site(s). However, the crystallographically proven identity of azi-isoflurane and isoflurane protein binding sites in our previous reports argues against this possibility. Co-crystallization of isoflurane with αIIbβ3 may answer this question in the future. Additionally, we cannot exclude the existence of other binding site(s) on regions of the protein that we were not able to detect using mass spectrometry.
Interestingly, we found the adducted residues only on the I domain of the β3 subunit in αIIbβ3 with two different preparations. In addition to the αIIb subunit, the β3 subunit couples with the αV subunit to form integrin αVβ3. The number of αVβ3 copies on platelets is small compared with that of αIIbβ3 [20], but αVβ3is highly expressed on endothelial cells. Both αIIbβ3 and αVβ3integrins bind to fibrinogen, but at different sites, forming a cooperative interaction between αIIbβ3 and αVβ3 that allows the platelet thrombus to be anchored on the endothelium through αVβ3 [49], [50]. It is possible that sevoflurane and isoflurane also impair the activation of αVβ3 as well to diminish the anchoring of platelets on the endothelium, which will be an additional effect to impair hemostasis by volatile anesthetics.
Clinical significance of functional alternation in αIIbβ3 is apparent from a familial bleeding disease, Glanzmann thrombasthenia. Bleeding in this disorder derives from the failure of platelet aggregation due to reduced or absent αIIbβ3 [51]. Therefore, the fact that sevoflurane and isoflurane directly modulate the activation of αIIbβ3 can be clinically significant. Our results are entirely consistent with this and with the previous clinical reports of endoscopic sinus surgeries. Although many studies have been performed in this surgical population, the numbers of patients enrolled in each study are not large ( Table 1 ). Future clinical investigation will be extremely important, particularly on cases at high risk of bleeding such as scoliosis and vascular surgeries. Since blood products are not unlimited resources and not entirely risk-free, this is an important health care consideration. The choice of anesthetic drugs may need to be considered from hemostasis standpoint.
In conclusion, we have demonstrated that the inhalational anesthetics isoflurane and sevoflurane, not but the intravenous anesthetic propofol, impairs the activation of integrin αIIbβ3 via a direct novel allosteric mechanism.
Funding Statement
This work is in part supported by the National Institute of Health, P01GM55876 and K08GM101345. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1. Franks NP (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9: 370–386. [DOI] [PubMed] [Google Scholar]
- 2. Ueda I (1971) The effects of volatile general anesthetics on adenosine diphosphate-induced platelet aggregation. Anesthesiology 34: 405–408. [DOI] [PubMed] [Google Scholar]
- 3. Ahn HJ, Chung SK, Dhong HJ, Kim HY, Ahn JH, et al. (2008) Comparison of surgical conditions during propofol or sevoflurane anaesthesia for endoscopic sinus surgery. Br J Anaesth 100: 50–54. [DOI] [PubMed] [Google Scholar]
- 4. Blackwell KE, Ross DA, Kapur P, Calcaterra TC (1993) Propofol for maintenance of general anesthesia: a technique to limit blood loss during endoscopic sinus surgery. Am J Otolaryngol 14: 262–266. [DOI] [PubMed] [Google Scholar]
- 5. Eberhart LH, Folz BJ, Wulf H, Geldner G (2003) Intravenous anesthesia provides optimal surgical conditions during microscopic and endoscopic sinus surgery. Laryngoscope 113: 1369–1373. [DOI] [PubMed] [Google Scholar]
- 6. Law NL, Ng KF, Irwin MG, Man JS (2001) Comparison of coagulation and blood loss during anaesthesia with inhaled isoflurane or intravenous propofol. Br J Anaesth 86: 94–98. [DOI] [PubMed] [Google Scholar]
- 7. Manola M, De Luca E, Moscillo L, Mastella A (2005) Using remifentanil and sufentanil in functional endoscopic sinus surgery to improve surgical conditions. ORL J Otorhinolaryngol Relat Spec 67: 83–86. [DOI] [PubMed] [Google Scholar]
- 8. Pavlin JD, Colley PS, Weymuller EA Jr, Van Norman G, Gunn HC, et al. (1999) Propofol versus isoflurane for endoscopic sinus surgery. Am J Otolaryngol 20: 96–101. [DOI] [PubMed] [Google Scholar]
- 9. Sivaci R, Yilmaz MD, Balci C, Erincler T, Unlu H (2004) Comparison of propofol and sevoflurane anesthesia by means of blood loss during endoscopic sinus surgery. Saudi Med J 25: 1995–1998. [PubMed] [Google Scholar]
- 10. Tirelli G, Bigarini S, Russolo M, Lucangelo U, Gullo A (2004) Total intravenous anaesthesia in endoscopic sinus-nasal surgery. Acta Otorhinolaryngol Ital 24: 137–144. [PubMed] [Google Scholar]
- 11. Wormald PJ, van Renen G, Perks J, Jones JA, Langton-Hewer CD (2005) The effect of the total intravenous anesthesia compared with inhalational anesthesia on the surgical field during endoscopic sinus surgery. Am J Rhinol 19: 514–520. [PubMed] [Google Scholar]
- 12. Dogan IV, Ovali E, Eti Z, Yayci A, Gogus FY (1999) The in vitro effects of isoflurane, sevoflurane, and propofol on platelet aggregation. Anesth Analg 88: 432–436. [DOI] [PubMed] [Google Scholar]
- 13. Tuerkan HSA, Beyan C, Guzeldemir ME, Yalcin A (1995) Effect of propofol on platelet aggregation. Br J Anaesth 74: S81. [Google Scholar]
- 14. Hirakata H, Ushikubi F, Toda H, Nakamura K, Sai S, et al. (1996) Sevoflurane inhibits human platelet aggregation and thromboxane A2 formation, possibly by suppression of cyclooxygenase activity. Anesthesiology 85: 1447–1453. [DOI] [PubMed] [Google Scholar]
- 15. Hirakata H, Ushikubi F, Narumiya S, Hatano Y, Nakamura K, et al. (1995) The effect of inhaled anesthetics on the platelet aggregation and the ligand-binding affinity of the platelet thromboxane A2 receptor. Anesth Analg 81: 114–118. [DOI] [PubMed] [Google Scholar]
- 16. Nozuchi S, Mizobe T, Aoki H, Hiramatsu N, Kageyama K, et al. (2000) Sevoflurane does not inhibit human platelet aggregation induced by thrombin. Anesthesiology 92: 164–170. [DOI] [PubMed] [Google Scholar]
- 17. Scarborough RM, Gretler DD (2000) Platelet glycoprotein IIb-IIIa antagonists as prototypical integrin blockers: novel parenteral and potential oral antithrombotic agents. J Med Chem 43: 3453–3473. [DOI] [PubMed] [Google Scholar]
- 18. Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, et al. (1996) Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood 88: 907–914. [PubMed] [Google Scholar]
- 19. Topol EJ, Byzova TV, Plow EF (1999) Platelet GPIIb-IIIa blockers. Lancet 353: 227–231. [DOI] [PubMed] [Google Scholar]
- 20. Leclerc JR (2002) Platelet glycoprotein IIb/IIIa antagonists: lessons learned from clinical trials and future directions. Crit Care Med 30: S332–340. [DOI] [PubMed] [Google Scholar]
- 21. Jackson SP (2007) The growing complexity of platelet aggregation. Blood 109: 5087–5095. [DOI] [PubMed] [Google Scholar]
- 22. Pytela R, Pierschbacher MD, Ginsberg MH, Plow EF, Ruoslahti E (1986) Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp–specific adhesion receptors. Science 231: 1559–1562. [DOI] [PubMed] [Google Scholar]
- 23. Shimaoka M, Springer TA (2003) Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov 2: 703–716. [DOI] [PubMed] [Google Scholar]
- 24. Xiao T, Takagi J, Coller BS, Wang JH, Springer TA (2004) Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 432: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Springer TA, Zhu J, Xiao T (2008) Structural basis for distinctive recognition of fibrinogen gammaC peptide by the platelet integrin alphaIIbbeta3. J Cell Biol 182: 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raborn J, Wang W, Luo BH (2011) Regulation of integrin alphaIIbbeta3 ligand binding and signaling by the metal ion binding sites in the beta I domain. Biochemistry 50: 2084–2091. [DOI] [PubMed] [Google Scholar]
- 27. Horn NA, de Rossi L, Robitzsch T, Hecker KE, Hutschenreuter G, et al. (2001) Sevoflurane inhibits unstimulated and agonist-induced platelet antigen expression and platelet function in whole blood in vitro. Anesthesiology 95: 1220–1225. [DOI] [PubMed] [Google Scholar]
- 28. Zhang H, Astrof NS, Liu JH, Wang JH, Shimaoka M (2009) Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems. FASEB J 23: 2735–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuki K, Astrof NS, Bracken C, Soriano SG, Shimaoka M (2010) Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology 113: 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuki K, Astrof NS, Bracken C, Yoo R, Silkworth W, et al. (2008) The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J 22: 4109–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yuki K, Bu W, Xi J, Sen M, Shimaoka M, et al. (2012) Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo BH, Springer TA, Takagi J (2003) Stabilizing the open conformation of the integrin headpiece with a glycan wedge increases affinity for ligand. Proc Natl Acad Sci U S A 100: 2403–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shattil SJ, Hoxie JA, Cunningham M, Brass LF (1985) Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem 260: 11107–11114. [PubMed] [Google Scholar]
- 34. Michelson AD (1996) Flow cytometry: a clinical test of platelet function. Blood 87: 4925–4936. [PubMed] [Google Scholar]
- 35. Ginsberg MH, Frelinger AL, Lam SC, Forsyth J, McMillan R, et al. (1990) Analysis of platelet aggregation disorders based on flow cytometric analysis of membrane glycoprotein IIb-IIIa with conformation-specific monoclonal antibodies. Blood 76: 2017–2023. [PubMed] [Google Scholar]
- 36. Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, et al. (2008) Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell 32: 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu J, Negri A, Provasi D, Filizola M, Coller BS, et al. Closed headpiece of integrin alphaIIbbeta3 and its complex with an alphaIIbbeta3-specific antagonist that does not induce opening. Blood 116: 5050–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eckenhoff RG, Xi J, Dailey WP Inhalational anesthetic photolabeling. Methods Mol Biol 617: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eckenhoff RG, Xi J, Shimaoka M, Bhattacharji A, Covarrubias M, et al. Azi-isoflurane, a Photolabel Analog of the Commonly Used Inhaled General Anesthetic Isoflurane. ACS Chem Neurosci 1: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Short TG, Aun CS, Tan P, Wong J, Tam YH, et al. (1994) A prospective evaluation of pharmacokinetic model controlled infusion of propofol in paediatric patients. Br J Anaesth 72: 302–306. [DOI] [PubMed] [Google Scholar]
- 41. Gepts E, Sonck WA, Camu F, Vercruysse A (1987) Pharmacokinetics of intravenously administered meptazinol during general anaesthesia in man. Eur J Anaesthesiol 4: 35–43. [PubMed] [Google Scholar]
- 42. Albanese J, Martin C, Lacarelle B, Saux P, Durand A, et al. (1990) Pharmacokinetics of long-term propofol infusion used for sedation in ICU patients. Anesthesiology 73: 214–217. [DOI] [PubMed] [Google Scholar]
- 43. Eckenhoff RG (2001) Promiscuous ligands and attractive cavities: how do the inhaled anesthetics work? Mol Interv 1: 258–268. [PubMed] [Google Scholar]
- 44. Puzon-McLaughlin W, Kamata T, Takada Y (2000) Multiple discontinuous ligand-mimetic antibody binding sites define a ligand binding pocket in integrin alpha(IIb)beta(3). J Biol Chem 275: 7795–7802. [DOI] [PubMed] [Google Scholar]
- 45. Boersma E, Akkerhuis KM, Theroux P, Califf RM, Topol EJ, et al. (1999) Platelet glycoprotein IIb/IIIa receptor inhibition in non-ST-elevation acute coronary syndromes: early benefit during medical treatment only, with additional protection during percutaneous coronary intervention. Circulation 100: 2045–2048. [DOI] [PubMed] [Google Scholar]
- 46. Kleiman NS, Lincoff AM, Flaker GC, Pieper KS, Wilcox RG, et al. (2000) Early percutaneous coronary intervention, platelet inhibition with eptifibatide, and clinical outcomes in patients with acute coronary syndromes. PURSUIT Investigators. Circulation 101: 751–757. [DOI] [PubMed] [Google Scholar]
- 47. Artoni A, Li J, Mitchell B, Ruan J, Takagi J, et al. (2004) Integrin beta3 regions controlling binding of murine mAb 7E3: implications for the mechanism of integrin alphaIIbbeta3 activation. Proc Natl Acad Sci U S A 101: 13114–13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pan Y, Zhang K, Qi J, Yue J, Springer TA, et al. Cation-pi interaction regulates ligand-binding affinity and signaling of integrin alpha4beta7. Proc Natl Acad Sci U S A 107: 21388–21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cheresh DA, Berliner SA, Vicente V, Ruggeri ZM (1989) Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell 58: 945–953. [DOI] [PubMed] [Google Scholar]
- 50. Smith JW, Ruggeri ZM, Kunicki TJ, Cheresh DA (1990) Interaction of integrins alpha v beta 3 and glycoprotein IIb-IIIa with fibrinogen. Differential peptide recognition accounts for distinct binding sites. J Biol Chem 265: 12267–12271. [PubMed] [Google Scholar]
- 51. Baker EK, Tozer EC, Pfaff M, Shattil SJ, Loftus JC, et al. (1997) A genetic analysis of integrin function: Glanzmann thrombasthenia in vitro. Proc Natl Acad Sci U S A 94: 1973–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, et al. (2001) Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science 294: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]