Summary
Background:
Synovial sarcoma (SS) is an uncommon malignant neoplasm arising from primitive pluripotential mesenchyme primarily affecting the soft tissues of the extremities. Rarely other locations are involved, including the mediastinum.
Case Report:
After treatment for mediastinal SS by surgical resection, radiation therapy, and chemotherapy, an 11-year-old boy developed an esophageal stricture and fistula, the latter resulting in a paraesophageal abscess. Management of the esophageal stricture and fistula required a multi-disciplinary approach. We report our experience with the management of this difficult complication, as well as a brief review of the literature on SS.
Conclusions:
Rare conditions, particularly those with unusual complications, present therapeutic challenges requiring a multi-disciplinary team approach. Reporting experiences with difficult cases can benefit providers faced with similar problems in the future.
Keywords: synovial cell sarcoma, paraesophageal abscess, Esophageal Fistula - complications
Background
Synovial sarcoma (SS) is an uncommon malignant neoplasm primarily affecting the soft tissues of the extremities and rarely other locations, including the mediastinum [1–9]. SS is most commonly found in adolescents and young adults [3,10] but is also seen in children [7–9]. Primary SS of the esophagus is exceedingly rare, with only five cases reported in the literature [11–15].
Case Report
An 11-year-old male presented to his primary care provider with complaints of shortness of breath, wheezing, and cough. He was treated empirically for reactive airway disease with no significant improvement. His symptoms recurred intermittently over 2-months with increasing severity requiring hospitalization. There was no history of weight loss, fever, or night sweats. A chest x-ray was obtained that showed a para-tracheal mass (Figure 1A). He was referred to our hospital for further evaluation. Past history was significant for hospitalization for pneumonia at 2 years of age and removal of an enlarged lymph node from his neck at 6 years of age. Chronic medical conditions included asthma, gastroesophageal reflux, and migraine headaches. Family history was non-contributory. Examination showed a thin body habitus. There was no adenopathy. Abdominal examination showed no tenderness, masses, or hepatosplenomegaly. Chest examination revealed good breath sounds without rales, ronchi, or wheezes. Heart examination was normal.
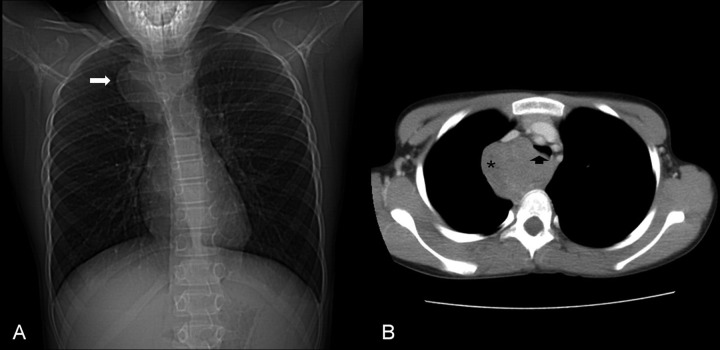
Figure 1.
Initial radiological investigation. Chest x-ray (A) showed a mass in the right upper chest (arrow). Computed tomography scan (B) confirmed the mass (*) compressing the trachea (arrow head).
Computed tomography (CT) scan of the chest showed a large mediastinal mass partially compressing his trachea (Figure 1B). The mass was located just above the aortic arch with loose attachments to the right innominate vein, trachea, and esophagus. A right postero-lateral thoracotomy was performed with excision of this mass. It was found to be smooth, covered by the pleural lining, and rubbery in consistency, suspicious for a ganglioneuroma or neuroblastoma. It was felt that microscopic disease likely remained due to inability to obtain clear margins as the tumor was adherent to the sympathetic chain and esophagus. Histological evaluation was consistent with a synovial cell sarcoma. A metastatic work up including bone scan and CT scans of the head, neck, abdomen, and pelvis were negative.
The patient was enrolled in COG protocol ARST0332 and received ifosfamide (days 1–3 in weeks 1, 4, 7, 10, 13, and 16) and doxorubicin hydrochloride (days 1 and 2 in weeks 1, 4, 13, 16, and 19). Radiotherapy was begun at week 4, the patient received an initial dose of 45 Grays (Gy) at the rate of 180 cGy per fraction using intensity-modulated radiation therapy (IMRT). This was followed by a mediastinal boost of 1,080 cGy. The total dose delivered to the tumor bed was 5,580 cGy, with all treatments given at a rate of 180 cGy per fraction.
Three weeks after completion of radiotherapy the patient developed odynophagia and dysphagia limiting his ability to tolerate all oral intake, including liquids. Upper endoscopy was performed revealing esophagitis and a stricture in the upper portion of the esophagus. Dilation was performed with through-the-scope radial balloon dilators under direct visualization, followed by initiation of acid-blockade with an H2-receptor antagonist. Symptoms improved initially, but within one month the patient again complained of odynophagia resulting in decreased oral intake and weight loss. A barium swallow showed narrowing at the previous stricture site but free passage of barium. Acid blockade was changed to a proton-pump inhibitor to provide better acid suppression with minimal improvement in symptoms. Chest CT scan at the completion of chemotherapy showed that the previous soft tissue mass was replaced by a fluid collection with surrounding right upper lobe parenchymal consolidation and atelectasis, as well as pleural thickening. The changes were consistent with postsurgical change and radiation effect, but residual tumor could not be excluded. Also seen was a fistulous tract extending from a thickened esophagus to the fluid collection (Figure 2A, B). An upper gastrointestinal series confirmed the fistula (Figure 3).
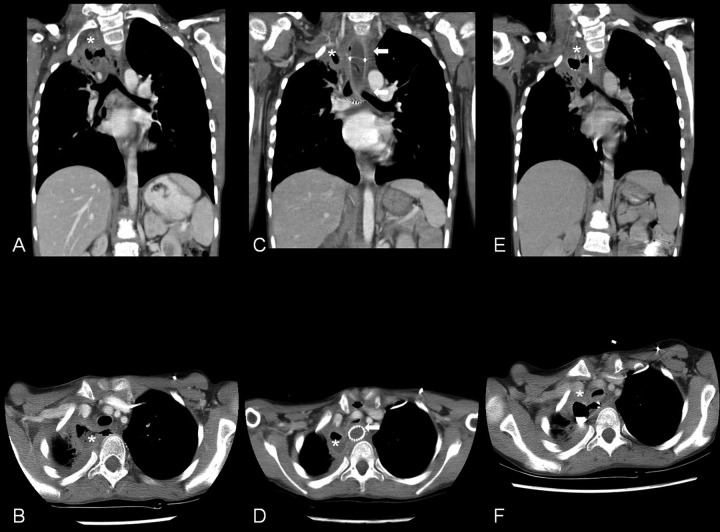
Figure 2.
Sequence of computed tomography (CT) scans following completion of chemo- and radiotherapy. Coronal view (A) of the first CT scan following completion of therapy showed a fluid collection in the right upper chest (*); axial view (B) showed a fistula (arrow head) extending from the esophagus to the fluid collection (*). Coronal view (C) of the CT scan following placement of an esophageal stent (arrow) showed good placement; axial view (D) showed occlusion of the fistula but severe compression of the trachea (arrow head). The fluid filled cavity is seen in both views (*). Coronal view (E) of the CT scan following palliative chemotherapy and nasogastric feeds showed persistence of the fistula and fluid filled cavity (*); axial view (F) showed persistence of the fistula (arrow head) and fluid filled cavity (*).
Figure 3.
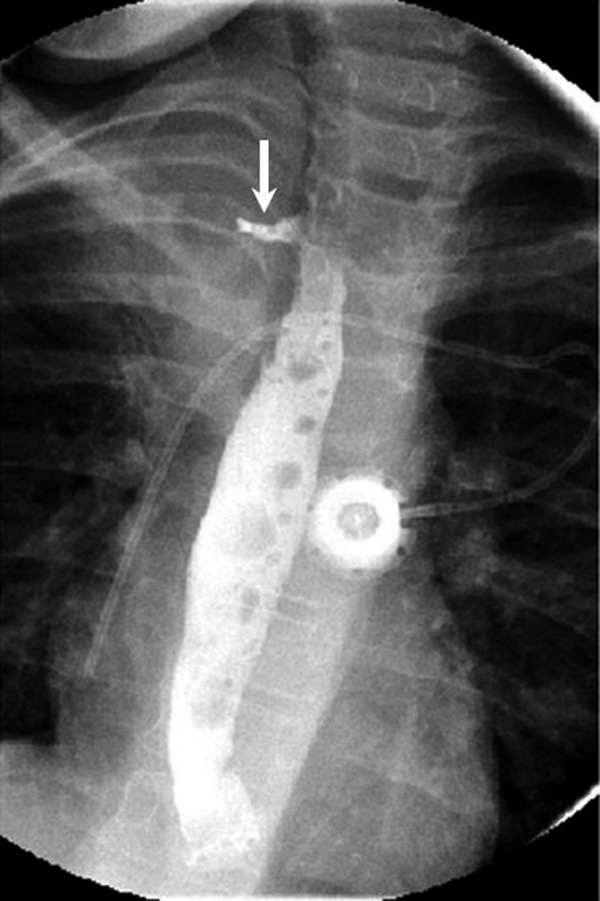
Upper gastrointestinal series. An upper gastrointestinal series confirmed a fistulous tract (arrow) from the esophagus to the fluid filled cavity.
EGD, performed in an attempt to close the fistula, showed narrowing of the esophagus and opening to the fistula to be approximately 3 mm in diameter (Figure 4). Two endoclips were placed across the fistula; however, the fistula could not be adequately closed and the endoclips were removed. Next, a covered stent was placed across the opening to occlude the opening and provide time for the fistula to heal. Immediately upon awakening from the procedure the patient developed chest pain and stridor felt to be due to instrumentation during stent placement. The next morning his stridor worsened and was accompanied by increased work of breathing. Emergent chest CT scan was done revealing the stent to be in good position but causing severe tracheal compression (Figure 2C, D) requiring removal of the stent. The patient was made nil per os (NPO) and a nasogastric tube was placed to provide nutritional support.
Figure 4.
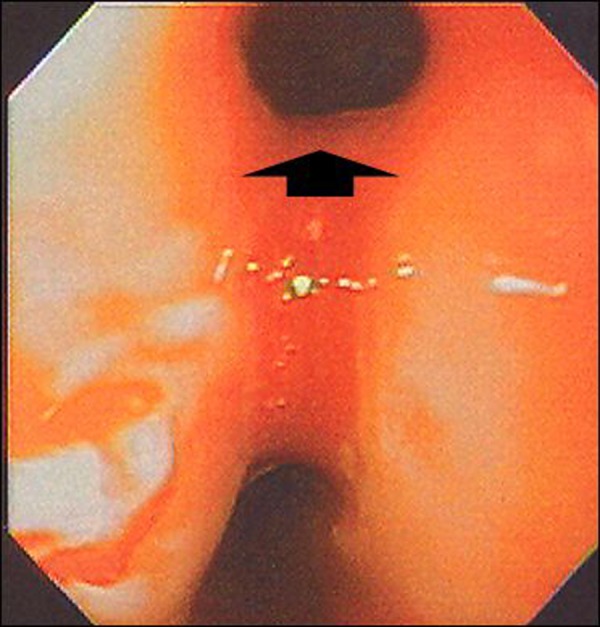
Upper endoscopy findings. At upper endoscopy a large fistulous opening (arrow head) was seen but could not be closed with endoclips.
Chemotherapy was continued with vincristine, dactinomycin, cylophosphamide and Mesna. A chest CT scan (Figure 2E, F) and esophagram performed 6 weeks later showed persistence of the fistulous tract. It was decided to place a gastrostomy tube and keep the patient NPO to avoid anything passing through the esophagus and potentially entering the fistula. Chemotherapy was continued for 4 months after which a repeat chest CT scan showed no interval change in the mass or fistula. Repeat right thoracotomy was performed with multiple biopsies throughout the right chest cavity showed fibrosis with no residual tumor. Despite being kept NPO and receiving only enteral feeds for another 4 months the fistula persisted. An esophagectomy, gastric pull-up with anastomosis to the cervical esophagus, pyloroplasty, and feeding jejunostomy were performed due to persistence of this esophageal fistula. He did well throughout the early postoperative time-period, tolerating a regular diet. Three months postoperatively the patient developed dysphagia to solid foods, and intermittent chest pain with liquids. An esophagram revealed an anastomotic stricture with a small fistula tract leading into the right chest. The patient has had repeated esophageal dilations and minimally invasive attempts at fistula closure including cauterization and injection with hemostatic matrix compounds. These procedures have relieved his dysphagia and chest pain, but a small fistula tract from the cervical anastomosis to the right chest remains 2 years after esophagectomy. He still tolerates a regular diet and supplements his intake with jejunal feeds at night.
Discussion
Synovial sarcoma (SS) is an uncommon malignant neoplasm primarily affecting the soft tissues of the extremities and rarely other locations, including the mediastinum [1–9]. Evidence suggests that SS arises from primitive pluripotential mesenchyme capable of synovial differentiation explaining non-extremity involvement [2,4]. SS is most commonly found in adolescents and young adults [3,10] but is also seen in children [7–9]. In several large studies, children accounted for 10% to 17% of patients with SS [7–9], with an age-adjusted incidence rate of 0.81 per million children [7]. Primary intrathoracic tumors are rare accounting for 1.5% to 4.1% of all SS [7,8], being more common in adults (4.7%) than in children (0.9%) [7]. Presenting symptoms include cough, dyspnea, chest pain, back pain, dysphagia, hoarseness, hemoptysis, and as an incidental finding [1–6]. Due to the paucity of mediastinal SS no standard treatment protocols have been developed. Therapy typically consists of wide surgical excision followed by adjuvant radiotherapy, chemotherapy, or both [16]. Good prognostic factors for survival of a primary SS include tumor < 5 cm, clear margin at resection, low mitotic activity, tumor in extremities, lack of tumor necrosis, and young patient age [7,17,18]. Increasing age has been identified as an adverse prognostic factor, with a cancer-specific mortality in adults (34%) twice that of children (16%) [7]. A trend is seen within the pediatric age group as well, with children <10 years old having better outcomes than adolescents [7].
Primary SS of the esophagus is exceedingly rare, with only five cases reported in the literature [11–15]. These lesions have all been described as intraluminal polypoid lesions causing varying degrees of esophageal obstruction [11–15]. Esophageal invasion by SS has also been reported in conjunction with mediastinal tumors, again resulting in large intraluminal masses [1]. In the patient described herein, there was no evidence of tumor within the esophageal lumen, rather a fistulous tract formed between the esophagus and the tumor. It can not be ascertained whether the fistula arose from tumor invasion, due to mucosal injury following radiotherapy, or secondary to esophageal dilation. An upper gastrointestinal series three weeks after dilation did not reveal a fistula, suggesting that dilation was not causative. Previous studies have shown that esophageal dilation can be safely performed after radiotherapy [19,20], without increased risk of perforation [19].
The development of an esophageal fistula presented a difficult challenge in this patient, both diagnostically and therapeutically. Inability to ascertain if the fistula was due to tumor invasion resulted in continued chemotherapy. Subsequent repeat biopsy showed that the residual mass was scar tissue allowing discontinuation of chemotherapy. Attempt at endoscopic closure of the fistula with endoclips and a stent were unsuccessful. Historically, esophageal stents have been used primarily for palliation in esophageal cancer as they could not be removed once placed. However, development of covered, removable stents has expanded the uses for stents including benign esophageal strictures in children [21]. Attempts at by-passing the fistula with a nasogastric tube to allow closure was also unsuccessful necessitating esophageal resection and gastric pull-up. Post-surgery, a new stricture and fistula developed, which have been treated with scheduled dilations.
Conclusions
In conclusion, we report a child with a rare presentation of an uncommon childhood malignancy. Short term follow-up revealed no recurrence of tumor; however, the course was complicated by development of an esophageal fistula and resulting para-esophageal abscess formation. We report our experience to shed light on the difficulties encountered in managing an esophageal fistula following chemo- and radiotherapy in the hope that it will be instructive to other practitioners.
References:
- 1.Kaira K, Ishizuka T, Sunaga N, et al. Primary mediastinal synovial sarcoma: a report of 2 cases. J Comput Assist Tomogr. 2008;32:238–41. doi: 10.1097/RCT.0b013e31806ad1f8. [DOI] [PubMed] [Google Scholar]
- 2.Witkin GB, Miettinen M, Rosai J. A biphasic tumor of the mediastinum with features of synovial sarcoma. A report of four cases. Am J Surg Pathol. 1989;13:490–99. doi: 10.1097/00000478-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Trupiano JK, Rice TW, Herzog K, et al. Mediastinal synovial sarcoma: report of two cases with molecular genetic analysis. Ann Thorac Surg. 2002;73:628–30. doi: 10.1016/s0003-4975(01)03110-1. [DOI] [PubMed] [Google Scholar]
- 4.Cheng LS, Tse GM, Li WW, et al. Mediastinal synovial sarcoma: a case report and literature review. Can Respir J. 2003;10:393–95. doi: 10.1155/2003/681919. [DOI] [PubMed] [Google Scholar]
- 5.Katakura H, Fukuse T, Shiraishi I, et al. Mediastinal synovial sarcoma. Thorac Cardiovasc Surg. 2009;57:183–85. doi: 10.1055/s-2006-955886. [DOI] [PubMed] [Google Scholar]
- 6.Jeganathan R, Davis R, Wilson L, et al. Primary mediastinal synovial sarcoma. Ulster Med J. 2007;76:109–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Sultan I, Rodriguez-Galindo C, Saab R, et al. Comparing children and adults with synovial sarcoma in the surveillance, epidemiology, and end results program, 1983 to 2005: an analysis of 1268 patients. Cancer. 2009;115:3537–47. doi: 10.1002/cncr.24424. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101:627–34. doi: 10.1002/cncr.20386. [DOI] [PubMed] [Google Scholar]
- 9.Palmerini E, Staals EL, Alberghini M, et al. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer. 2009;115:2988–98. doi: 10.1002/cncr.24370. [DOI] [PubMed] [Google Scholar]
- 10.Eilber FC, Dry SM. Diagnosis and management of synovial sarcoma. J Surg Oncol. 2008;97:314–20. doi: 10.1002/jso.20974. [DOI] [PubMed] [Google Scholar]
- 11.Palmer BV, Levene A, Shaw HJ. Synovial sarcoma of the pharynx and oesophagus. J Laryngol Otol. 1983;97:1173–76. doi: 10.1017/s0022215100096171. [DOI] [PubMed] [Google Scholar]
- 12.Amr SS, Shihabi NK, Al Hajj H. Synovial sarcoma of the esophagus. Am J Otolaryngol. 1984;5:266–69. doi: 10.1016/s0196-0709(84)80037-x. [DOI] [PubMed] [Google Scholar]
- 13.Bloch MJ, Iozzo RV, Edmunds LH, Jr, Brooks JJ. Polypoid synovial sarcoma of the esophagus. Gastroenterology. 1987;92:229–33. doi: 10.1016/0016-5085(87)90865-1. [DOI] [PubMed] [Google Scholar]
- 14.Antón-Pacheco J, Cano I, Cuadros J, et al. Synovial sarcoma of the esophagus. J Pediatr Surg. 1996;31:1703–5. doi: 10.1016/s0022-3468(96)90057-3. [DOI] [PubMed] [Google Scholar]
- 15.Habu S, Okamoto E, Toyosaka A, et al. Synovial sarcoma of the esophagus: report of a case. Surg Today. 1998;28:401–4. doi: 10.1007/s005950050149. [DOI] [PubMed] [Google Scholar]
- 16.Abe K, Maebayashi T, Shizukuishi T, et al. Radiological assessment following thermoradiation therapy for primary pleural synovial sarcoma: case report. Med Oncol. 2010;27:1027–30. doi: 10.1007/s12032-009-9328-3. [DOI] [PubMed] [Google Scholar]
- 17.Kawai A, Woodruff J, Healey JH, et al. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998;338:153–60. doi: 10.1056/NEJM199801153380303. [DOI] [PubMed] [Google Scholar]
- 18.Bergh P, Meis-Kindblom JM, Gherlinzoni F, et al. Synovial sarcoma: identification of low and high risk groups. Cancer. 1999;85:2596–607. doi: 10.1002/(sici)1097-0142(19990615)85:12<2596::aid-cncr16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Ng TM, Spencer GM, Sargeant IR, et al. Management of strictures after radiotherapy for esophageal cancer. Gastrointest Endosc. 1996;43:584–90. doi: 10.1016/s0016-5107(96)70196-7. [DOI] [PubMed] [Google Scholar]
- 20.Wax MK, Amirali A, Ulewicz DE, Lough R. Safety of esophagoscopy in the irradiated esophagus. Ann Otol Rhinol Laryngol. 1997;106:297–300. doi: 10.1177/000348949710600406. [DOI] [PubMed] [Google Scholar]
- 21.Kramer RE, Quiros JA. Esophageal stents for severe strictures in young children: experience, benefits, and risk. Curr Gastroenterol Rep. 2010;12:203–10. doi: 10.1007/s11894-010-0105-4. [DOI] [PubMed] [Google Scholar]