Abstract
Background
Monoamine releasers such as d-amphetamine that selectively promote release of dopamine/norepinephrine versus serotonin are one class of candidate medications for treating cocaine dependence; however, their clinical utility is limited by undesirable effects such as abuse liability. Clinical utility of these compounds may be increased by development of prodrugs to reduce abuse potential by slowing onset of drug effects. This study examined the behavioral and pharmacokinetic profile of the Schedule III compound phendimetrazine, which may serve as a prodrug for the N-demethylated metabolite and potent dopamine/norepinephrine releaser phenmetrazine.
Methods
Monkeys (n=5) were trained in a two-key food-reinforced discrimination procedure to discriminate cocaine (0.32 mg/kg, IM) from saline, and the potency and time course of cocaine-like discriminative stimulus effects were determined for (+)-phenmetrazine, (−)-phenmetrazine, (+)-phendimetrazine, (−)-phendimetrazine, and (! )-phendimetrazine. Parallel pharmacokinetic studies in the same monkeys examined plasma phenmetrazine and phendimetrazine levels for correlation with cocaine-like discriminative stimulus effects.
Results
Both isomers of phenmetrazine, and the racemate and both isomers of phendimetrazine, produced dose- and time-dependent substitution for the discriminative stimulus effects of cocaine, with greater potency residing in the (+) isomers. In general, plasma phenmetrazine levels increased to similar levels after administration of behaviorally active doses of either phenmetrazine or phendimetrazine.
Conclusions
These results support the hypothesis that phenmetrazine is an active metabolite that contributes to the effects of phendimetrazine. However, behavioral effects of phendimetrazine had a more rapid onset than would have been predicted by phenmetrazine levels alone, suggesting that other mechanisms may also contribute.
Keywords: cocaine, discrimination, rhesus monkey, pharmacokinetic, phenmetrazine, phendimetrazine, addiction
1. INTRODUCTION
Despite decades of research, there is currently no Food and Drug Administration-approved pharmacological treatment strategy for cocaine dependence (Kampman, 2010; Vocci and Ling, 2005). For other drugs of abuse, such as heroin, agonist-substitution therapy has been successful as a maintenance strategy for addiction treatment (Kreek et al., 2002; Nyswander, 1956). The goal of agonist-based pharmacotherapy is to use a medication that has pharmacological effects similar to those of the abused drug, slow onset to reduce abuse liability, and long duration of action to assist in compliance (Grabowski et al., 2004b). Research over the last decade has suggested that this agonist-based strategy may also have utility in treating cocaine dependence (Herin et al., 2010; Rothman et al., 2005).
One example of this medication approach for treatment of cocaine dependence is d-amphetamine, a monoamine releaser that selectively promotes release of dopamine and norepinephrine versus serotonin. Chronic d-amphetamine treatment has been shown to decrease cocaine self-administration by rodents, nonhuman primates and humans (Chiodo et al., 2008; Czoty et al., 2011; Greenwald et al., 2010; Negus, 2003) and to decrease cocaine use in clinical trials (Grabowski et al., 2001, 2004a; Shearer et al., 2003). One manifestation of the shared pharmacological effects of d-amphetamine and cocaine is their similar discriminative stimulus effects in nonhuman primates and other species (Beardsley et al., 2001; de la Garza and Johanson, 1985; Gold and Balster, 1996; Negus et al., 2009; Oliveto et al., 1998). d-Amphetamine also has a long duration of action in nonhuman primates and humans, which is another desirable attribute of an agonist-based pharmacotherapy (Chait et al., 1986; Negus et al., 2009). However, d-amphetamine is a Schedule II psychostimulant with a rapid onset of action that contributes to its high abuse liability, and it also produces undesirable cardiovascular effects that ultimately limit its clinical utility as a pharmacotherapy for cocaine dependence.
One strategy for improving the clinical utility of dopamine/norepinephrine-selective monoamine releasers might be development of a prodrug to slow the rate of onset of drug effects in an attempt to reduce abuse potential (Balster and Schuster, 1973; Huttunen et al., 2011; Schindler et al., 2009). We have previously shown that (+)-phenmetrazine is another dopamine/norepinephrine-selective monoamine releaser that produces cocaine-like discriminative stimulus effects and decreases cocaine self-administration in rhesus monkeys (Banks et al., 2012, 2011; Negus et al., 2009); however, like d-amphetamine, phenmetrazine has a relatively rapid onset and high potential for abuse (Chait et al., 1987; Corwin et al., 1987; de la Garza and Johanson, 1987; Griffiths et al., 1976), and it was withdrawn from the market in the United States. Phendimetrazine in an N-methyl analog of phenmetrazine that itself has low potency to affect monoamine release or uptake, but that may serve as a prodrug for phenmetrazine in vivo (Rothman et al., 2002). Consistent with this characterization, previous studies have demonstrated that phendimetrazine shares d-amphetamine discriminative stimulus effects in monkeys (de la Garza and Johanson, 1987; Evans and Johanson, 1987). More importantly, phendimetrazine appears to have reduced abuse potential compared to phenmetrazine in some drug self-administration assays (Corwin et al., 1987), and phendimetrazine is currently available clinically as a Schedule III agent for treatment of obesity. Taken together, these results support further research of phendimetrazine as a candidate “agonist” pharmacotherapy for treating cocaine dependence (Banks et al., 2011; Negus et al., 2009; Rothman et al., 2002).
Although existing data suggest that phendimetrazine may be an inactive parent drug that serves as a prodrug for the active metabolite phenmetrazine, this issue has not been directly addressed in pharmacokinetic studies. Accordingly, the aim of the present study was to correlate cocaine-like discriminative stimulus effects of phenmetrazine and phendimetrazine with plasma phenmetrazine levels in rhesus monkeys. Phendimetrazine is clinically available as a racemate of the more potent (+) and less potent (−) isomers, and this study assessed effects of both isomers of phenmetrazine and phendimetrazine as well as of racemic phendimetrazine. We hypothesized that, in accordance with the relative potencies of phenmetrazine isomers to promote dopamine release (Rothman et al., 2002), the (+)-isomers of phenmetrazine and phendimetrazine would be more potent than the (−) isomers in producing cocaine-like discriminative stimulus effects. Furthermore, we hypothesized that phendimetrazine would yield behaviorally active levels of phenmetrazine as an active metabolite, and that the potency and time course of cocaine-like discriminative stimulus effects of phendimetrazine would correlate with generation of the phenmetrazine metabolite. Overall, these expected results would provide a basis for further studies with phendimetrazine as a prodrug for phenmetrazine and a candidate “agonist” pharmacotherapy for cocaine dependence.
2. METHODS
2.1 Animals
Studies were conducted in 5 adult male rhesus monkeys (Macaca mulatta). Monkeys were maintained on a diet of fresh fruit and food biscuits (Lab Diet High Protein Monkey Biscuits, PMI Feeds, Inc., St. Louis, MO) provided in the afternoon after the operant session. In addition, monkeys could earn up to 70 1-gm banana-flavored pellets (Grain-based Precision Primate Pellets, Test Diets, Richmond, IL) during daily experimental sessions (see below). Water was continuously available. A 12 hr light-dark cycle was in effect (lights on from 7AM to 7PM). Environmental enrichment consisting of foraging boards, novel treats, videos or radio were also provided in the afternoons after behavioral sessions. All monkeys had prior exposure to monoaminergic and opioidergic compounds (unpublished results) before training in the two-key food-reinforced cocaine vs. saline discrimination procedure. The facility was licensed by both the United States Department of Agriculture and the Association for Assessment and Accreditation of Laboratory Animal Care, and protocols were approved by the Institutional Animal Care and Use Committee.
2.2 Drug Discrimination Procedures
2.2.1 Apparatus
Experimental sessions were conducted in each monkey’s home cage. The front wall was equipped with an operant response panel that included three square response keys arranged horizontally. Each housing chamber was also equipped with a pellet dispenser (Med Associates, ENV-203-1000, St. Albans, VT). Operation of the operant panels and data collection were accomplished with computers and software purchased from Med Associates.
2.2.2 Discrimination Training
Monkeys were trained to discriminate 0.32 mg/kg cocaine intramuscularly (IM) from saline in a two-key, food-reinforced drug discrimination procedure. Discrimination training was conducted 5 days/week during daily sessions composed of multiple components. Each component consisted of a 5-min response period, during which the right and left response keys were transilluminated red and green, respectively, and monkeys could earn up to 10 food pellets by responding under a fixed-ratio (FR) 30 schedule of food presentation. Training sessions were composed of three components presented at 2-hr intervals, and either saline or 0.32 mg/kg cocaine was administered IM 15 min prior to the start of each component. Thus, on training days, monkeys would receive a sequence of saline (S) and cocaine (C) injections in the order SSS, SSC, SCS, CSS, SCC, CSC, CCS, or CCC, and training sequences were randomly presented. The goal of this training regimen was to engender daily experience with randomized sequences of saline- and cocaine-appropriate components. The 2hr duration of inter-component intervals was selected to exceed the time course of discriminative stimulus effects produced by the training dose of cocaine in rhesus monkeys (Lamas et al., 1995) and to thereby minimize effects of cocaine administered in earlier trials on performance during later trials on the same day. Following administration of saline, only responding on the green key (the saline-appropriate key) produced food, whereas following administration of 0.32 mg/kg cocaine, only responding on the red key (the cocaine-appropriate key) produced food. Responses on the inappropriate key reset the FR requirement on the appropriate key. The criterion for accurate discrimination was >80% injection-appropriate responding before delivery of the first reinforcer, >90% injection-appropriate responding for the entire component, and response rates >0.1 responses/sec (sufficient to earn at least one pellet) for all components during 7 of 8 consecutive sessions.
2.2.3 Discrimination Testing
Test sessions were identical to training sessions except that (a) responding on either key produced food, (b) monkeys received only one injection of vehicle or a dose of the test drug at the start of the session, and (c) 5-min response components began 10, 30, 56, 100, 180, 300 and 560 min after the injection to assess the time course of drug effects. The dose ranges tested were (+)-phenmetrazine (0.1-1.0 mg/kg), (−)-phenmetrazine (0.32 -10.0 mg/kg), (! )-phendimetrazine (1.0-10.0 mg/kg), (+)-phendimetrazine (0.32-10.0 mg/kg), and (−)-phendimetrazine (1.0-32.0 mg/kg). The time courses of the training stimuli (saline and 0.32 mg/kg cocaine) were also determined, and the N-methyl-D-aspartate glutamate receptor antagonist ketamine (0.32-3.2 mg/kg) was tested as a negative control. Test sessions were separated by at least 3 days and were usually conducted on Tuesdays and Fridays, with training sessions conducted on other weekdays. Test sessions were conducted only if performance during the previous two training sessions met the criteria for accurate discrimination described above. All test drug doses, except for cocaine and ketamine, were tested twice in each monkey. All doses of a given test drug were evaluated in a given monkey before testing the next drug, and vehicle (saline) test sessions were conducted before and after evaluation of each test drug. Drugs were tested in the following order in each monkey: cocaine, (+)-phenmetrazine, (−)-phenmetrazine, (! )-phendimetrazine, (+)-phendimetrazine, (−)-phendimetrazine, and ketamine. The order of drug doses was counterbalanced across different monkeys.
2.2.4 Data Analysis
The primary dependent variables for each component were (1) percent cocaine-appropriate responding (%CAR), defined as (total number of responses on the cocaine-associated key total number of responses on both the cocaine- and saline-associated keys)*100, and (2) response rate in responses per second. These variables were plotted as a function of time after test drug administration. Both %CAR and response rate data were analyzed using a two-way repeated-measures ANOVA with test drug dose and time as the main factors. A significant ANOVA was followed by the Dunnett multiple comparisons post-hoc test to compare test conditions with vehicle conditions. The criterion for significance was set a priori at the 95% level of confidence (p < 0.05).
2.3 Pharmacokinetic Procedures
2.3.1 Drug Dosing and Blood Collection
Once drug discrimination studies were completed, pharmacokinetic studies with the same compounds in the same monkeys were initiated. Every two weeks, monkeys were placed in a custom nonhuman primate restraint chair using the pole and collar technique, and a temporary venous catheter (24 gauge Exel safelet catheter, Fisher scientific, Pittsburg, PA) with an injection port was inserted into a saphenous vein. Immediately after a baseline blood sample (2-3 mLs) was collected using a 3 mL syringe, a dose of (+)-phenmetrazine (0.1-1.0 mg/kg), (−)-phenmetrazine (0.32-10.0 mg/kg), (+)-phendimetrazine (0.32-3.2 mg/kg), or (−)-phendimetrazine (1.0-10.0 mg/kg) was administered IM, and samples were collected 10, 30, 56, 100, 180, and 300 min post drug administration. Blood samples were immediately transferred to Vacutainer tubes containing K3-EDTA and stored on ice until centrifugation at 1000xg for 10 min. The plasma supernatant was then transferred into a labeled storage tube and frozen at −80C. Frozen samples were shipped overnight on dry ice to Research Triangle Institute (RTI) International for analysis of phenmetrazine and/or phendimetrazine levels. Racemic phendimetrazine was not tested in the pharmacokinetic studies, because analytic methods did not permit dissociation of the isomers.
2.3.2 Analytic Procedures
Plasma samples were thawed on ice, and 25μL of each was mixed with 150μL of acetonitrile containing internal standard (4-benzylpiperidine, 50 ng/mL) and 5μL of acetonitrile in a polypropylene 96 well plate. Standards were prepared similarly with a 5μL spike of phenmetrazine or phendimetrazine replacing the 5μL of acetonitrile. Plates were vortexed and centrifuged, and 30μL was mixed with 270μL of methanol:water (50:50) in a new 96 well plate. Samples were analyzed by liquid chromatography coupled with mass spectrometry/mass spectrometry on an Applied Biosystems API 4000 mass spectrometer coupled to Agilent 1100 binary pumps and autosampler. The column used was a Phenomenex Synergi Hydro-RP (50 × 2 mm, 4μm) with mobile phases consisting of A: 10 mM ammonium bicarbonate, pH 7.0, and B: Acetonitrile:Methanol (50:50) and a flow rate of 500 μL/min. Initial chromatography conditions consisted of 30% B held for 1 min and increased to 95% B over 1 min, and then held at 95% B for 2 min before returning to initial conditions. The first min of flow was diverted to waste while make-up solvent was pumped to the mass spectrometer. Phenmetrazine was monitored in positive electrospray mode with an MRM transition of 178.12 → 117.10 and phendimetrazine was monitored with a transition of 199.22 → 146.10. Mass spectrometer parameters were as follows: CUR=10, GS1=50, GS2=60, IS=2500, TEM=600°C, CAD=10.
2.3.3 Data Analysis
Plasma phenmetrazine and phendimetrazine levels in nM were plotted as a function of time after test drug administration. Data were analyzed using a two-way repeated-measures ANOVA with test drug dose and time as the main factors. A significant ANOVA was followed by the Holm-Sidak post-hoc test to compare plasma levels as a function of dose within a given time point and to compare changes in plasma levels as a function of time after drug administration. The criterion for significance was set a priori at the 95% level of confidence (p < 0.05). In addition, a non-compartmental model (200, WinNonlin, version 5.3) with an extravascular input was used to calculate the following pharmacokinetic parameters: maximum concentration, time to maximum concentration, half-life, and area under the curve from time zero to time last.
2.4 Correlation of Behavioral and Pharmacokinetic Data
Behavioral and pharmacokinetic data were correlated at each time point after drug administration (10, 30, 56, 100, 180, 300 min). In the case of the phendimetrazine isomers, separate correlations were conducted for both the parent phendimetrazine levels and the metabolite phenmetrazine levels. In the presence of a significant correlation between plasma levels and percent-cocaine appropriate responding, linear regression was used to calculate the effective concentration that produced 50% cocaine-appropriate responding (EC50).
2.5 Drugs
(−)-Cocaine HCl was obtained from the National Institute on Drug Abuse (National Institutes of Health, Bethesda, MD). (+)-Phenmetrazine fumarate, (−)-phenmetrazine fumarate, (! )-phendimetrazine hemifumarate, (+)-phendimetrazine hemifumarate and (−)-phendimetrazine hemifumarate were synthesized by BE Blough (RTI International, Research Triangle Park, NC). Ketamine HCl (100 mg/mL) was obtained from a commercial supplier (KetaVed, Vedco Inc, Saint Joseph, MO). All drugs were dissolved in sterile saline and administered intramuscularly in the thigh, and all doses were expressed as the salt forms listed above.
3. RESULTS
3.1 Time course of the discriminative stimulus effects of cocaine and ketamine
During the training days that preceded test days, monkeys responded almost exclusively on the saline-appropriate key during saline training components (mean SEM percent saline-appropriate responding: 99.82 0.09) and almost exclusively on the cocaine-appropriate key during cocaine training components (mean SEM percent cocaine-appropriate responding: 99.86 0.06). Mean response rates ( SEM) were 2.48 ( 0.17) and 2.64 ( 0.23) responses/s during saline and cocaine training cycles, respectively.
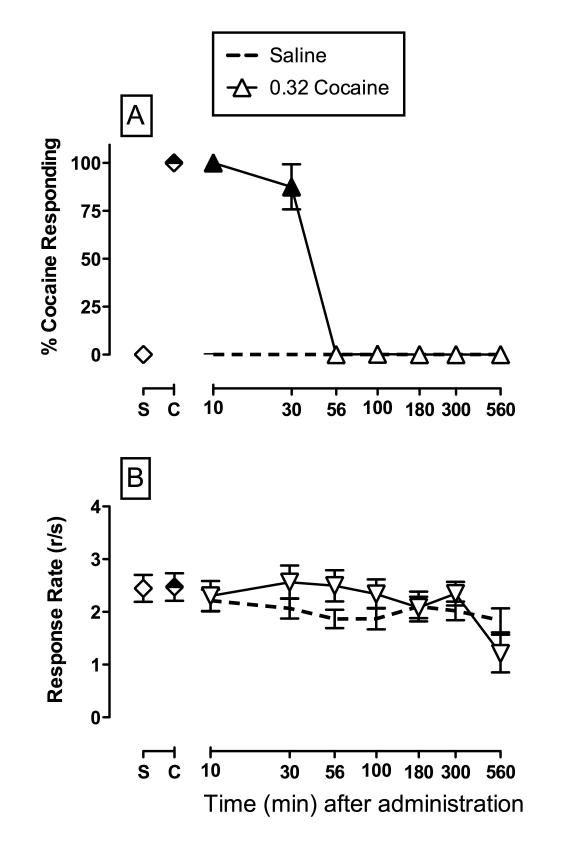
Figure 1A shows the time course for the discriminative stimulus effects of saline and the training dose (0.32 mg/kg, IM) of cocaine. In this and all subsequent tests with saline, saline administration produced 15%CAR at all time points and had no significant effect on rates of responding throughout the test period. In contrast, the training dose of cocaine dose produced full discriminative stimulus effects, defined as 85% CAR, at 10 and 30 min, and these effects were no longer apparent after 56 min. Rates of responding were not significantly altered by this cocaine dose (Panel B). Furthermore, rates of responding were stable across the 560 min test period. Ketamine failed to substitute for the discriminative stimulus effects of cocaine up to doses that eliminated responding (Supplemental Figure 1).
Figure 1.
Magnitude and time course of cocaine-like discriminative stimulus produced by the training dose of 0.32 mg/kg cocaine in rhesus monkeys (n=5). Effects of saline tests are also shown for comparison. Top Ordinate: percent cocaine-appropriate responding. Bottom Ordinate: rates of responding in responses per second. Abscissae: time in min after administration (log scale). Symbols above “S” and “C” represent the group averages SEM for all training sessions preceding test sessions when the saline- and cocaine-associated keys were correct, respectively. Filled symbols represent statistical significance (p < 0.05) within a given time point compared to experimental sessions when saline was administered (dotted line).
3.2 Time course of the discriminative stimulus effects of phenmetrazine
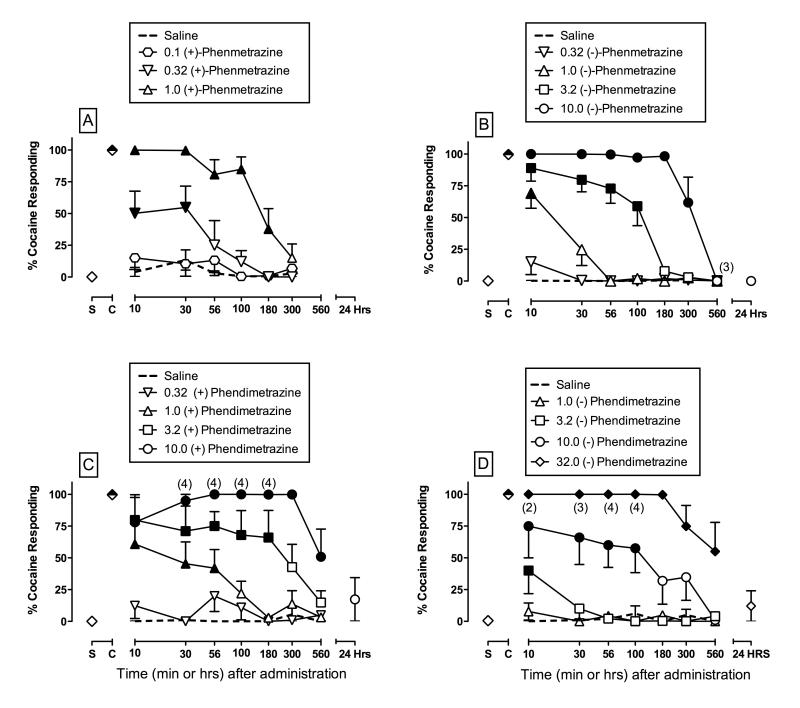
Both (+)-phenmetrazine (Figure 2A) and (−)-phenmetrazine (Figure 2B) produced dose- and time-dependent increases in cocaine-appropriate responding. For (+)-phenmetrazine, there was a significant main effect of dose (F3,12=37.3, p<0.05), time (F5,20=12.3, p<0.05) and a significant interaction (F15,60=6.0, p<0.05) for %CAR (Figure 2A). Post-hoc analysis indicated that 0.32 mg/kg (+)-phenmetrazine significantly increased %CAR relative to saline at 10 and 30 min, and 1.0 mg/kg significantly increased %CAR from 10 to 180 min. Full substitution (≥85% CAR) was produced in one monkey by 0.32 mg/kg and in all five monkeys by 1.0 mg/kg (+)-phenmetrazine. Effects of (+)-phenmetrazine on rates of responding are shown in Supplemental Figure 2.
Figure 2.
Time course of the cocaine-like discriminative stimulus of (+)-phenmetrazine (0.1 – 1.0 mg/kg, Panel A), (−)-phenmetrazine (0.32 – 10.0 mg/kg, Panel B), (+)-phendimetrazine (0.32 – 10.0 mg/kg, Panel C), and (−)-phendimetrazine (1.0 – 32.0 mg/kg, Panel D) in rhesus monkeys (n=5). All points represent the mean SEM of 5 monkeys except where indicated by numbers in parentheses in the top panels. In these cases, responding was eliminated in some monkeys, and the parenthetic number indicates the number of subjects responding at levels sufficient to contribute to the data point. Other details as in Figure 1.
For (−)-phenmetrazine, there was a significant main effect of dose (F4,8=136.6, p<0.05), time (F6,12=22.0, p<0.05), and a significant interaction (F24,46=12.6, p<0.05) for %CAR (Figure 2B). Post-hoc analysis indicated that 1.0 mg/kg (−)-phenmetrazine significantly increased %CAR at 10 min only, 3.2 mg/kg significantly increased %CAR from 10 to 100 min, and 10.0 mg/kg significantly increased %CAR from 10 to 300 min. Full substitution was produced in two monkeys by 1.0 mg/kg, in four monkeys by 3.2 mg/kg, and in five monkeys by 10.0 mg/kg (−)-phenmetrazine. Rates of responding were not significantly altered by (−)-phenmetrazine (Supplemental Figure 2).
3.3 Time course of the discriminative stimulus effects of phendimetrazine
Figure 2 also shows that (+)-phendimetrazine (Panel C), and (−)-phendimetrazine (Panel D) produced dose- and time-dependent increases in cocaine-appropriate responding. For (+)-phendimetrazine, there was a significant main effect of dose (F4,16=32.3, p<0.05), time (F6,24=5.6, p<0.05), and a significant interaction (F24,91=2.6, p<0.05) on %CAR (Figure 2C). Post-hoc analysis indicated that 1.0 mg/kg significantly increased %CAR from 10-56 min, 3.2 mg/kg significantly increased %CAR from 10-180 min, and 10.0 mg/kg significantly increased %CAR from 10-560 min. Full substitution was produced in two monkeys by 1.0 mg/kg, in four monkeys by 3.2 mg/kg, and in all five monkeys by 10.0 mg/kg (+)-phendimetrazine. Effects of (+)-phendimetrazine on rates of responding are shown in Supplement Figure 2.
For (−)-phendimetrazine, there was a significant main effect of dose (F4,16=27.8, p<0.05), time (F6,24=6.9, p<0.05), and a significant interaction (F24,88=2.2, p<0.05) on %CAR (Figure 2D). Post-hoc analysis indicated that 3.2 mg/kg significantly increased %CAR at only 10 min, 10.0 mg/kg significantly increased %CAR from 10-100 min, and 32.0 mg/kg significantly increased %CAR from 10-560 min. Full substitution was produced in one monkey by 3.2 mg/kg, in three monkeys by 10.0 mg/kg and in all five monkeys by 32.0 mg/kg (−)-phendimetrazine. Effects of (−)-phendimetrazine on rates of responding are shown in Supplement Figure 2.
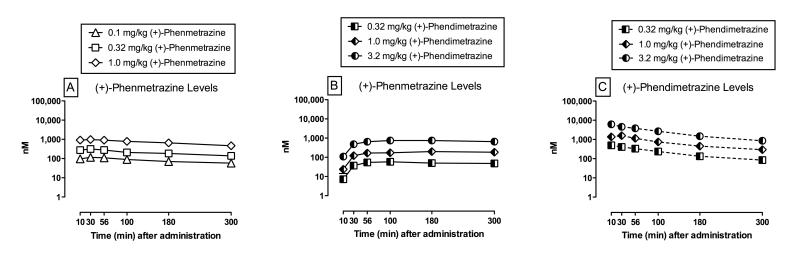
3.4 Pharmacokinetics of phenmetrazine
Figure 3 (Panel A) shows plasma phenmetrazine levels as a function of time after (+)-phenmetrazine administration; mean pharmacokinetic parameters, such as maximum concentration, time to maximum concentration, and half life, are reported in Supplemental Table 1. For (+)-phenmetrazine, two-way repeated-measures ANOVA demonstrated a significant main effect of dose (F2,8=23.2, p<0.05) and time (F5,20=16.1, p<0.05). Post-hoc analysis using the Holm-Sidak method indicated each (+)-phenmetrazine dose produced phenmetrazine levels that were significantly different from each other at each time point. Furthermore, post-hoc analysis indicated that plasma (+)-phenmetrazine levels peaked between 10 – 30 min and then decreased over time such that plasma levels were significantly higher at 10 and 30 min vs. 300 min. Plasma phenmetrazine levels as a function of time after (−)-phenmetrazine are shown in Supplemental Figure 4.
Figure 3.
Plasma levels (nM) of phenmetrazine and phendimetrazine as a function of time after administration of (+)-phenmetrazine (0.1 – 1.0 mg/kg; Panel A), (+)-phendimetrazine (0.32 – 3.2 mg/kg; Panels B, C) in rhesus monkeys (n=5). Ordinates: plasma levels in nM (log scale). Abscissae: time in min after drug administration (linear scale).
3.5 Pharmacokinetics of phendimetrazine
Figure 3 also shows plasma levels of phenmetrazine (Panel B) and phendimetrazine (Panel C) after (+)-phendimetrazine administration; mean pharmacokinetic parameters, such as maximum concentration, time to maximum concentration, and half life, for both phenmetrazine and phendimetrazine are also listed in Supplemental Table 1. For (+)-phenmetrazine plasma levels after (+)-phendimetrazine administration (Fig. 3B), two-way repeated-measures ANOVA for phendimetrazine levels demonstrated a significant main effect of dose (F2,8=321.8, p<0.05) and time (F5,20=56.0, p<0.05). Post-hoc analysis indicated each (+)-phendimetrazine dose produced phenmetrazine plasma levels that were significantly different from each other at each time point. Furthermore, post-hoc analysis indicated that plasma (+)-phenmetrazine levels were lowest at 10 min after (+)-phendimetrazine and were significantly increased from 30 – 300 min. For (+)-phendimetrazine, two-way repeated-measures ANOVA on phendimetrazine plasma levels (Fig. 3C) demonstrated a significant main effect of dose (F2,8=184.4, p<0.05) and time (F5,20=50.8, p<0.05). Post-hoc analysis indicated each (+)-phendimetrazine dose produced phendimetrazine plasma levels that were significantly different from each other at each time point. Furthermore, post-hoc analysis indicated that plasma (+)-phendimetrazine levels peaked between 10 – 30 min and then decreased over time such that plasma levels were significantly higher at 10 and 30 min vs. 300 min. Overall, these results demonstrate that as plasma (+)-phendimetrazine levels decreased over time, plasma (+)-phenmetrazine levels increased over the same time period. Similar to results with (+)-phendimetrazine, as (−)-phendimetrazine plasma levels decreased, plasma (−)-phenmetrazine levels increased over the same time period (Supplemental Figure 4).
3.6 Correlation between behavioral and pharmacokinetic measures
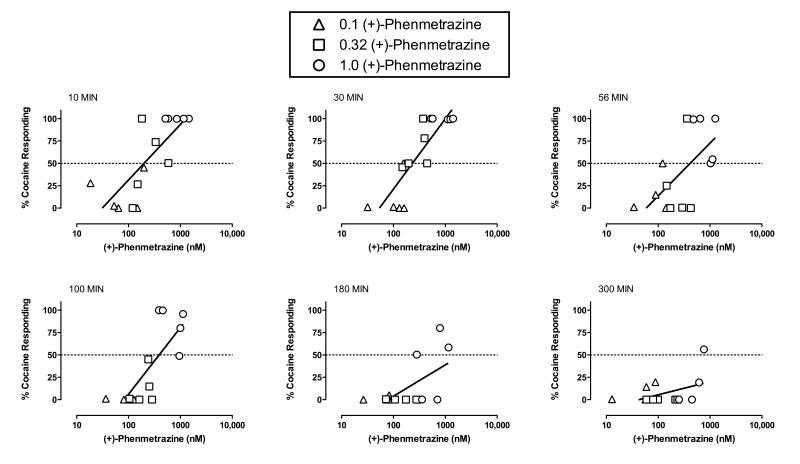
Figure 4 shows the correlation between plasma (+)-phenmetrazine levels and percent cocaine-appropriate responding as a function of time after (+)-phenmetrazine administration. (+)-Phenmetrazine plasma levels significantly correlated with the cocaine-like discriminative stimulus effects of phenmetrazine from 10 to 180 min. In the presence of a significant correlation, linear regression was used to calculate the effective concentration that produced 50% cocaine-appropriate responding (EC50) and 95% confidence limits. In general, EC50 values increased as a function of time as indicated by non-overlapping 95% confidence limits for the EC50 values at 10 vs. 180 min (Supplemental Table 4). The correlation between plasma (+)-phenmetrazine levels and cocaine-like discriminative stimulus effects was no longer significant at 300 min. Similar correlations and regression analyses between plasma drug levels and cocaine-appropriate responding for: (−)-phenmetrazine levels after (−)-phenmetrazine administration, (+)-phenmetrazine levels after (+)-phendimetrazine administration, (+)-phendimetrazine levels after (+)-phendimetrazine administration, (−)-phenmetrazine levels after (−)-phendimetrazine administration, and (−)-phendimetrazine levels after (−)-phendimetrazine administration are shown in the supplemental materials.
Figure 4.
Correlation between plasma (+)-phenmetrazine levels and percent cocaine-appropriate responding as a function of time after (+)-phenmetrazine administration in rhesus monkeys (n=5). Ordinates: percent cocaine-appropriate responding. Abscissae: plasma (+)-phenmetrazine levels in nM (log scale). Different symbols represent different doses of (+)-phenmetrazine in individual monkeys. In the presence of a significant correlation, the effective concentration, in nM, that produced 50% cocaine-appropriate responding (EC50) value was calculated using linear regression. Each panel shows data for a different time after (+)-phenmetrazine administration, and this time is shown in the upper left corner of each panel.
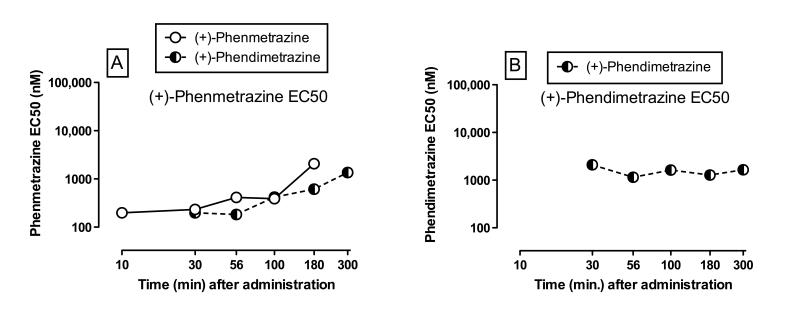
Figure 5A shows (+)-phenmetrazine EC50 values as a function of time after administration of (+)-phenmetrazine or (+)-phendimetrazine. Points are shown only for times at which the correlation was significant between plasma (+)-phenmetrazine levels and cocaine-appropriate responding. In general, EC50 values for plasma (+)-phenmetrazine levels across time were similar or slightly lower after (+)-phendimetrazine than after (+)-phenmetrazine administration, and EC50 values increased over time after treatment with either drug. However, after (+)-phendimetrazine administration, plasma (+)-phenmetrazine levels failed to correlate with cocaine-appropriate responding at the earliest time point (10 min), but did correlate at the latest time point (300 min). Figure 5B shows that plasma (+)-phendimetrazine levels also correlated with cocaine-appropriate responding from 30-300 min after (+)-phendimetrazine administration, and (+)-phendimetrazine EC50 values were relatively stable over that time and higher than (+)-phenmetrazine EC50s.
Figure 5.
EC50 values for (+)-phenmetrazine (Panel A) and (+)-phendimetrazine (Panel B) as a function of time (min) after phenmetrazine or phendimetrazine administration in rhesus monkeys. EC50 value in nM (log scale). Abscissae: time in min after administration of phenmetrazine (open circles) or phendimetrazine (half-filled circles) (log scale). EC50 values were only calculated in the presence of a significant correlation; see Supplemental Tables 2 and 3 for correlation results. All points show mean data from 5 monkeys.
4. DISCUSSION
Phendimetrazine is a Schedule III drug under consideration as a candidate “agonist” medication for treatment of cocaine dependence. This study compared behavioral and pharmacokinetic data in rhesus monkeys to test the hypothesis that phendimetrazine serves as an inactive prodrug for the active N-demethylated metabolite phenmetrazine, which is a potent releaser of dopamine and norepinephrine. There were three main findings. First, both isomers of phenmetrazine, and the racemate and both isomers of phendimetrazine, produced dose- and time-dependent substitution for the discriminative stimulus effects of cocaine. Second, pharmacokinetic data demonstrated that phendimetrazine administration was associated with a gradual emergence of phenmetrazine levels as phendimetrazine levels declined. Finally, substitution for cocaine was often associated with similar plasma phenmetrazine levels (i.e., similar EC50 values) whether monkeys were treated with phenmetrazine or phendimetrazine. However, the onset of cocaine-like discriminative stimulus effects produced by phendimetrazine was quicker than would have been predicted by generation of the phenmetrazine metabolite and quicker than may be desirable to reduce the abuse liability of a candidate agonist medication. Overall, these results support the hypothesis that metabolism to phenmetrazine contributes to the cocaine-like behavioral effects of phendimetrazine, but other mechanisms (e.g., direct effects of the parent compound) may also play a role and may complicate the use of phendimetrazine to treat cocaine abuse.
4.1 Effects of phenmetrazine
The behavioral results of the present study are consistent with previous findings that phenmetrazine produced cocaine-like discriminative stimulus effects in rats (Wood and Emmett-Oglesby, 1988) and rhesus monkeys (Negus et al., 2009). Phenmetrazine has also produced amphetamine-like discriminative stimulus effects in pigeons (Evans and Johanson, 1987), monkeys (de la Garza and Johanson, 1987), and humans (Chait et al., 1986) suggesting that phenmetrazine shares subjective effects with two commonly abused psychostimulants. This is the first study to examine the stereoselectivity of discriminative stimulus effects produced by phenmetrazine, and the (+) isomer of phenmetrazine was approximately five times more potent than the (−) isomer in producing cocaine-like discriminative stimulus effects. These behavioral results are consistent with neurochemical studies demonstrating that (+)-phenmetrazine is approximately five-fold more potent than (−)-phenmetrazine to promote release of dopamine, a neurotransmitter thought to be related to cocaine-like discriminative stimulus effects (Rothman et al., 2002). Moreover, the absolute EC50 values for the phenmetrazine isomers to produce cocaine-like discriminative stimulus effects at the early 10-min time point in this study [198.6 and 990.8 nM for (+) and (–)-phenmetrazine at 10 min, respectively] are similar to their EC50s to release dopamine in vitro [87.4 and 415 nM for (+) and (–)-phenmetrazine, respectively (Rothman et al., 2002)]. Over the 300 min time course of each experiment, the correlations between plasma phenmetrazine levels and cocaine-appropriate responding weakened for both isomers, and calculated EC50 values increased. The declining potencies of the phenmetrazine isomers over time provide evidence for acute tolerance to their cocaine-like discriminative stimulus effects, and these findings are similar to apparent acute tolerance to the discriminative stimulus effects of cocaine itself in rhesus monkeys (Lamas et al., 1995). Overall, these results demonstrate a strong correlation between plasma phenmetrazine levels and the behavioral effects of phenmetrazine in the discrimination assay and provide a foundation for comparison to the phendimetrazine studies.
4.2 Effects of phendimetrazine
A major goal of this study was to test the hypothesis that phendimetrazine functions as prodrug for its N-demethylated analog phenmetrazine in rhesus monkeys, and three findings supported this hypothesis. First, like phenmetrazine, phendimetrazine produced cocaine-like discriminative stimulus effects. These results confirm and extend previous studies examining the discriminative stimulus effects of phendimetrazine in subjects trained to discriminate d-amphetamine. In both pigeons (Evans and Johanson, 1987) and monkeys (de la Garza and Johanson, 1987), phendimetrazine fully substituted for amphetamine, and as in the present study with cocaine-trained subjects, phendimetrazine was less potent than phenmetrazine. Second, the stereoselectivity of phendimetrazine matched the stereoselectivity of phenmetrazine to produce cocaine-like discriminative stimulus effects. Thus, as with phenmetrazine, the (+) isomer of phendimetrazine was approximately five times more potent than the (−) isomer in producing cocaine-like discriminative stimulus effects. Finally and most importantly, administration of phendimetrazine produced a gradual emergence of plasma phenmetrazine, and especially with the (+)-isomers, plasma phenmetrazine EC50 levels were similar after administration of either phenmetrazine or phendimetrazine at most time points. Taken together, these data suggest that behaviorally active doses of phendimetrazine generated sufficiently high levels of plasma phenmetrazine to support phendimetrazine’s cocaine-like discriminative stimulus effects.
Despite these data supportive of a role for phenmetrazine as an active metabolite of phendimetrazine, the onset of cocaine-like discriminative stimulus effects was faster than would have been predicted based exclusively on metabolism to phenmetrazine. More specifically, the phendimetrazine isomers produced high levels of cocaine-appropriate responding at the earliest experimental time point (10 min), when plasma phenmetrazine levels were low and did not correlate with discriminative stimulus effects. One possible explanation for this apparent discrepancy is that pharmacokinetic studies, which sampled plasma at 10 min after phendimetrazine administration, underestimated phenmetrazine levels that affected discrimination performance, which was sampled from 10-15 min after phendimetrazine administration. During this period of behavioral sampling, phenmetrazine levels were rising rapidly. A second possibility is that phendimetrazine is not entirely inactive as a prodrug. Although in vitro studies found that (±)-phendimetrazine was inactive as a monoamine releaser at concentrations up to 10 μM, it did have low but measureable potency to block uptake of dopamine (IC50=19 μM) and norepinephrine (IC50 = 8.3 μM) (Rothman et al., 2002). In the present study, administration of behaviorally active phendimetrazine doses produced plasma phendimetrazine levels that approached or exceeded these IC50 values at 10 min after administration, suggesting that phendimetrazine effects on monoamine uptake may have contributed to early cocaine-like effects. Moreover, sustained phendimetrazine levels may also have contributed to cocaine-like discriminative stimulus effects at later times, and residual phendimetrazine effects provide one possible explanation for the finding that phenmetrazine EC50 values in this study tended to be lower after administration of phendimetrazine than after phenmetrazine. Overall, behavioral effects of phendimetrazine may have reflected a summation of effects both by the parent drug and by phenmetrazine as its active metabolite.
4.3 Implications for phendimetrazine as a candidate “agonist” medication
Regardless of the reason for the rapid onset of phendimetrazine effects, the occurrence of these rapid effects may undermine one rationale for use of phendimetrazine as a candidate agonist medication. Specifically, a hypothesized advantage of phendimetrazine is that it might have a slower onset and thereby a lower abuse liability than phenmetrazine; however, the present results suggest similar rates of onset for the two drugs. A caveat to this conclusion is that the earliest time examined in this study was 10 min after drug administration, and dissociation of onset rate may have required assessment of earlier time points. Moreover, rate of onset is also influenced by route of administration, and results obtained here with IM administration may differ from results with oral or intravenous routes more likely to be encountered clinically. Nonetheless, the present results raise the possibility that phendimetrazine may be distinguished from phenmetrazine more by its longer duration of action than its slower onset. This longer duration of action may in turn be consistent with previously published data from self-administration studies that directly compared reinforcing effects of phendimetrazine and phenmetrazine. Thus, phendimetrazine maintained self-administration in a smaller proportion of monkeys (1/4) than phenmetrazine (2/2) under a self-administration procedure that used relatively short behavioral sessions (2hr) and short timeouts after completion of each response requirement (10 sec, the time required for delivery of the self-administered injection; Corwin et al., 1987). However, poor self-administration under these conditions can reflect not only weak reinforcing effects, but also long durations of drug action that delay behavioral recovery from each self-administered injection and thereby limit maximal self-administration rates relative to shorter acting drugs. By contrast, phendimetrazine maintained more reliable self-administration (3/3 baboons) in a procedure with longer behavioral sessions (24 hr/day) and longer timeouts after each drug injection (3hr) that permitted dissipation of effects between self-administration opportunities (Griffiths et al., 1979). These latter data suggest that rate of onset of phendimetrazine effects is adequate to maintain self-administration, at least after intravenous administration. Overall, then, the clinical availability and Schedule III status of phendimetrazine may lower administrative barriers to its use as a candidate agonist medication for treatment of cocaine dependence; however, the present results do not provide evidence for slower onset of phendimetrazine vs. phenmetrazine effects, and phendimetrazine should still be regarded as drug with substantial abuse liability.
The use of phendimetrazine as a prodrug for phenmetrazine may also introduce other complications. For example, the mechanism of phendimetrazine N-demethylation to phenmetrazine remains to be established, but involvement of cytochrome P450 enzymes is likely, and genetic variability in these enzymes can be expected to result in variability across individuals in rate and extent of metabolism and corresponding variability in clinical effect (Huttunen et al., 2011). Additionally, as with other prodrugs, off-target effects may be produced by the parent drug or by metabolites other than the desired active metabolite. The full metabolic profile of phendimetrazine has not been described (Beckett and Salami, 1972; Rothman et al., 2002), but the present study provided evidence for qualitative differences in effects of phendimetrazine and phenmetrazine unrelated to potency or time course. In particular, doses of (+)-phenmetrazine that produced cocaine-like discriminative stimulus effects also produced small but dose- and time-dependent increases in response rates. By contrast, the highest doses of phendimetrazine required to produce full substitution for cocaine also tended to reduce response rates, and these rate-decreasing effects were statistically significant for 32 mg/kg (−)-phendimetrazine. The precise mechanisms and clinical implications of this effect remains to be determined.
Supplementary Material
Acknowledgements
We appreciate the technical assistance of Crystal Reyns and Jennifer Gough and the statistical assistance of Leroy Thacker.
Role of Funding Source: Funding for this study was provided by National Institutes of Heath grants R01-DA026946 and R01-DA012790 from the National Institute on Drug Abuse, National Institutes of Health. The research project also utilized services supported by CTSA award UL1TR000058 from the National Center for Advancing Translational Sciences. NIDA and NCATS had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Banks, Blough, and Negus designed the study. Blough synthesized the phenmetrazine and phendimetrazine isomers. Fennell and Snyder developed the methods for analysis of the plasma samples and calculated the pharmacokinetic parameters. Banks, Blough, Snyder, and Negus wrote the manuscript. All authors have contributed to and have approved the final manuscript.
Conflict of Interest: None of the authors have any conflicts of interest to declare.
REFERENCES
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J. Exp. Anal. Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Interaction between behavioral and pharmacological treatment strategies to decrease cocaine choice in rhesus monkeys. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.193. doi: 10.1038/npp.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav. Pharmacol. 2011;22:824–836. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Sokoloff P, Balster RL, Schwartz JC. The D3R partial agonist, BP 897, attenuates the discriminative stimulus effects of cocaine and D-amphetamine and is not self-administered. Behav. Pharmacol. 2001;12:1–11. doi: 10.1097/00008877-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Beckett AH, Salami MA. A note on the identification of N-hydroxyphenmetrazine as a metabolic product of phendimetrazine and phenmetrazine. J. Pharm. Pharmacol. 1972;24:900–902. doi: 10.1111/j.2042-7158.1972.tb08910.x. [DOI] [PubMed] [Google Scholar]
- Chait LD, Uhlenhuth EH, Johanson CE. The discriminative stimulus and subjective effects of d-amphetamine, phenmetrazine and fenfluramine in humans. Psychopharmacology. 1986;89:301–306. doi: 10.1007/BF00174364. [DOI] [PubMed] [Google Scholar]
- Chait LD, Uhlenhuth EH, Johanson CE. Reinforcing and subjective effects of several anorectics in normal human volunteers. J. Pharmacol. Exp. Ther. 1987;242:777–783. [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, Johanson CE. Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res. 1987;7:351–361. [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. Discriminative stimulus properties of cocaine in pigeons. Psychopharmacology. 1985;85:23–30. doi: 10.1007/BF00427317. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. Discriminative stimulus properties of intragastrically administered d-amphetamine and pentobarbital in rhesus monkeys. J. Pharmacol. Exp. Ther. 1987;243:955–962. [PubMed] [Google Scholar]
- Evans SM, Johanson CE. Amphetamine-like effects of anorectics and related compounds in pigeons. J. Pharmacol. Exp. Ther. 1987;241:817–825. [PubMed] [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology. 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J. Clin. Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004a;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict. Behav. 2004b;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Bradford LD. Predicting the abuse liability of drugs with animal drug self-administration procedures: psychomotor stimulants and hallucinogens. In: Thompson T, Dews PB, editors. Advances in Behavioral Pharmacology. Academic Press; New York: 1979. pp. 164–208. [Google Scholar]
- Griffiths RR, Winger G, Brady JV, Snell JD. Comparison of behavior maintained by infusions of eight phenylethylamines in baboons. Psychopharmacology. 1976;50:251–258. doi: 10.1007/BF00426841. [DOI] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann. NY Acad. Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Huttunen KM, Raunio H, Rautio J. Prodrugs--from serendipity to rational design. Pharmacol. Rev. 2011;63:750–771. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- Kampman KM. What’s new in the treatment of cocaine addiction? Curr. Psychiatry Rep. 2010;12:441–447. doi: 10.1007/s11920-010-0143-5. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat. Rev. Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Hall E, Mello NK. Relationship between the discriminative stimulus effects and plasma concentrations of intramuscular cocaine in rhesus monkeys. Psychopharmacology. 1995;121:331–338. doi: 10.1007/BF02246072. [DOI] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective suppression of cocaine-versus food-maintained responding by monoamine releasers in rhesus monkeys: benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine. J. Pharmacol. Exp. Ther. 2009;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyswander M. The Drug Addict as a Patient. Grune and Stratton; New York: 1956. [Google Scholar]
- Oliveto AH, McCance-Katz E, Singha A, Hameedi F, Kosten TR. Effects of d-amphetamine and caffeine in humans under a cocaine discrimination procedure. Behav. Pharmacol. 1998;9:207–217. [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J. Pharmacol. Exp. Ther. 2005;313:1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Katsnelson M, Vu N, Partilla JS, Dersch CM, Blough BE, Baumann MH. Interaction of the anorectic medication, phendimetrazine, and its metabolites with monoamine transporters in rat brain. Eur. J. Pharmacol. 2002;447:51–57. doi: 10.1016/s0014-2999(02)01830-7. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Thorndike EB. Effect of rate of delivery of intravenous cocaine on self-administration in rats. Pharmacol. Biochem. Behav. 2009;93:375–381. doi: 10.1016/j.pbb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J, Wodak A, Van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Vocci F, Ling W. Medications development: successes and challenges. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wood DM, Emmett-Oglesby MW. Substitution and cross-tolerance profiles of anorectic drugs in rats trained to detect the discriminative stimulus properties of cocaine. Psychopharmacology. 1988;95:364–368. doi: 10.1007/BF00181948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.