Abstract
Resistance to E. coli L-asparaginase in canine lymphoma occurs frequently with repeated administration, a phenomenon often attributed, without substantiation, to the induction of neutralizing antibodies. To test the hypothesis that treated dogs develop antibodies against the drug, we created an ELISA to measure plasma anti-asparaginase IgG responses. Using samples from dogs that had received multiple doses, specific reactivity against L-asparaginase was demonstrated, while naïve patients’ samples were negative. The optimized ELISA appeared sensitive, with endpoint titers >1,600,000 in positive control dogs. Intra- and inter-assay coefficients of variation were 3.6 and 14.5%. The assay was supported by the observation that ELISA-positive plasma could immunoprecipitate asparaginase activity. When clinical patients were evaluated, 3/10 dogs developed titers after a single injection; with repeated administration, 4/7 dogs were positive. L-asparaginase antibodies showed reduced binding to the PEGylated drug formulation. The ELISA should prove useful in investigating the potential correlation of antibody responses with resistance.
Keywords: Antibodies, Canine, ELISA, L-asparaginase, lymphoma
Introduction
L-asparaginase, an enzyme that catalyzes the deamination of the amino acid asparagine (ASN), is a valuable chemotherapy agent for treating aggressive forms of lymphoid neoplasia in human and veterinary patients. By depriving malignant lymphoblasts, which lack asparagine synthetase (AS), of extracellular ASN, L-asparaginase leads to rapid inhibition of protein synthesis, causing apoptosis.1–3 Normal lymphocytes and other cells that generate ASN intracellularly are spared this effect, providing a wide therapeutic index for the drug. In dogs, the clinical utility of L-asparaginase in treating high-grade lymphoma was first shown in 1967.4 More than 20 years later, Valerius et al. demonstrated long-lasting, complete depletion of plasma ASN in lymphoma patients given 3 consecutive weekly doses of L-asparaginase.5 Because of its minimal toxicity and potency as monotherapy,6, 7 L-asparaginase is often administered to dogs as rescue therapy for relapsed lymphoma, and is commonly included in multi-drug protocols during the initial treatment phase, although a clear-cut benefit of the drug in prolonging remission duration or survival in the latter setting has not been established.8, 9
Despite the selective tumoricidal effect of L-asparaginase, its long-term usefulness in individual patients is limited, principally due to the development of drug resistance and hypersensitivity reactions. Because the enzyme is bacterial in origin, both phenomena have been attributed to the induction of antibody responses, which occurs frequently. In one large human trial, anti-asparaginase antibodies were documented in 70% of patients. Of these, was allergic reactions were observed in 57%, while the remainder were considered to have “silent hypersensitivity” – that is, antibodies without clinical signs .10 Whether such antibodies have functional or prognostic significance is controversial.11–15 Some studies have shown that high titers are associated with reduced serum asparaginase activity16, 17 and poorer outcome in higher-risk acute lymphoblastic leukemia (ALL).10 For human patients in which a significant clinical allergy develops, a switch from the frontline Escherichia coli-derived preparation is typically made, either to a PEGylated version with reduced antigenicity, or where available, to L-asparaginase from Erwinia chrysanthemi.11 When administered to such ALL patients previously treated with the E. coli-derived drug, E. chrysanthemi L-asparaginase dramatically lowered the risk for relapse or death when compared to patients that did not change therapy,10 which suggests that antibody responses are in fact a clinically significant limitation of L-asparaginase’s efficacy, and that monitoring of such responses could be used for optimal drug selection during treatment.
Similar to humans, dogs with lymphoma treated with multiple doses of L-asparaginase often develop resistance to the drug.18 While the mechanism is unknown, L-asparaginase antibodies frequently are cited as a cause.18, 19 Indeed, type 1 hypersensitivity reactions ranging in incidence from 6 to 40% have been observed in dogs treated with native E. coli L-asparaginase,6, 20 indicating that a humoral response is elicited; however, antibodies have not been formally demonstrated. In this study, we sought to develop an ELISA to measure the frequency, magnitude, and kinetics of circulating anti-asparaginase antibodies in dogs with lymphoma that were treated with one or more doses of native E. coli L-asparaginase.. This ELISA could be used to investigate the possible association between antibody titer and drug resistance, and should a correlation be established, might serve as helpful tool to assist in clinical decision making. Finding a patient titer associated with resistance, for example, could warrant a change to the PEGylated form of the drug. While effective,6, 20, 21 the prohibitive cost of pegaspargase precludes its routine use in dogs, but a positive assay result might constitute sufficient justification for the expense. Additionally, detection of anti-asparaginase antibodies could help predict hypersensitivity reactions. Using the ELISA developed in this study, we show that some dogs administered E. coli L-asparaginase generate strong immunoglobulin (Ig)G responses against this chemotherapy agent during the course of lymphoma treatment.
Materials and methods
Antibody reference pools
To generate a positive antibody control, EDTA-anticoagulated blood samples were obtained from dogs undergoing treatment for lymphoma at the North Carolina State University Veterinary Teaching Hospital that had previously received either three (dogs 1 and 3; Supplemental Table 1) or four (dog 2) doses of E. coli-derived L-asparaginase (Elspar®; Merck & Co., West Point, PA, USA) subcutaneously (SC). Plasma was separated by centrifugation (2350 g × 5 min). Equal volumes of the three samples were combined and stored in aliquots at −20°C. Pooled plasma obtained from lymphoma patients (dogs 4, 5 and 6) prior to beginning chemotherapy served as a negative control.
ELISA antigens and controls
Native and PEGylated pharmaceutical preparations of E. coli-derived L-asparaginase (Elspar®; Oncaspar® [pegaspargase], Enzon Pharmaceuticals, Bridgewater, NJ, USA) at varying dilutions served as target antigens. As an irrelevant control, E. coli-derived β-galactosidase (Sigma-Aldrich, St. Louis, MO, USA) was used at equimolar concentrations. The background optical density (OD) of the ELISA (i.e., non-specific binding of the detecting antibody) was determined in all experiments by incubating L-asparaginase-coated wells with diluent in place of plasma.
ELISA protocol
Ninety-six-well Nunc MaxiSorp plates (Nalge Nunc, Rochester, NY, USA) were coated with 50 μL/well L-asparaginase or β-galactosidase diluted in carbonate-bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3), pH 9.6, overnight at 4°C. Using a 12-channel Nunc Immuno-Wash, plates were washed three times with phosphate-buffered saline (PBS) containing 0.05% Tween-20 (PBS/Tween), and then blocked with 100 μL/well of 4% bovine serum albumin (BSA) in PBS for 1 h at room temperature. After washing, 50 μL of patient plasma diluted as indicated in PBS/Tween/1% BSA were pipetted into wells and incubated overnight at 4°C. Plasma samples were subsequently removed by aspiration, and plates were washed three times prior to the addition of 100 μL/well of sheep anti-dog IgG conjugated to horseradish peroxidase (HRP) (AbD Serotec, Raleigh, NC, USA) diluted in PBS/Tween/1% BSA. After 2 h incubation at room temperature, the plates again were washed three times, and all wells were then reacted with 100 μL of ABTS Single Solution chromogen/substrate (Invitrogen, San Diego, CA, USA) for 20 min in the dark. The reaction was halted by the addition of 100 μL/well of stop solution (0.01% sodium azide, 1% sodium dodecyl sulfate). The OD of each well was measured at 415 nm, with a reference negative control wavelength of 490 nm, using a Bio-Tek Universal microplate spectrophotometer (Bio-Tek, Winooski, VT, USA). All samples were assayed in duplicate.
L-asparaginase activity and neutralization assays
L-asparaginase activity was measured as the hydrolysis of L-aspartic beta-hydroxamate (AHA) in 96-well plate format, as previously described.22 To establish an activity curve, varying dilutions of L-asparaginase (10 μL) were mixed with 90 μL of 10 mM AHA in 0.015 M Tris with 0.015% BSA, pH 7.3, and incubated with shaking (100 rpm) for 12 min at 37°C. The reaction was stopped with 125 μL of 25% trichloroacetic acid. After centrifugation (600 g × 5 min), 10 μL of the supernatant was mixed with 40μL deionized water and 200μL 8-hydroxyquinoline solution (1 part 2% hydroxyquinoline in ethanol : 3 parts 1 M Na2CO3), heated to 95°C for 1 min, cooled to 22 °C, and read at 710 nm on a spectrophotometer. In immunoprecipitation experiments, plasma samples or fetal bovine serum (FBS) controls diluted in PBS (1:2.5) were mixed with L-asparaginase (3 units) in 100 μL total volume. After overnight incubation at 4°C, samples were subsequently divided, and reacted with PBS-washed protein A agarose beads (Millipore, Temecula, CA, USA), or PBS control, for 3 h at 4°C on a tube rotator. After pelleting the beads by brief centrifugation (21,130 g × 5 s), supernatants (10 μL) and PBS-washed beads (160 μL) were assayed in triplicate wells for AHA hydrolytic activity, as described above.
Data and statistical analyses
The sensitivity of the ELISA was defined at each sample dilution as the mean OD of wells containing pooled plasma from L-asparaginase-naïve patients plus two standard deviations. The intra- and inter-assay coefficients of variation (CV) were computed as the ratio of standard deviation to the mean, expressed as a percentage. Calculations of sensitivity and variance were performed using Excel (Microsoft, Bellevue, WA, USA). The in vitro activity of L-asparaginase was plotted by sigmoid curve-fitting, and the EC50 was determined by nonlinear regression analysis, using Prism 5.0 (GraphPad, San Diego, CA, USA). The differences between treatment groups were evaluated using Prism by either unpaired t test (drug dosages) or a one-way analysis of variance (ANOVA) procedure followed by the Dunnett multiple comparisons test (immunoprecipitation experiments), with the level of significance in all analyses set at a P value <0.05. All graphical results are shown as the arithmetic mean ± standard deviation; in some graphs, error bars are smaller than the data point symbol and are thus not visible.
Results
Optimization of ELISA reagents
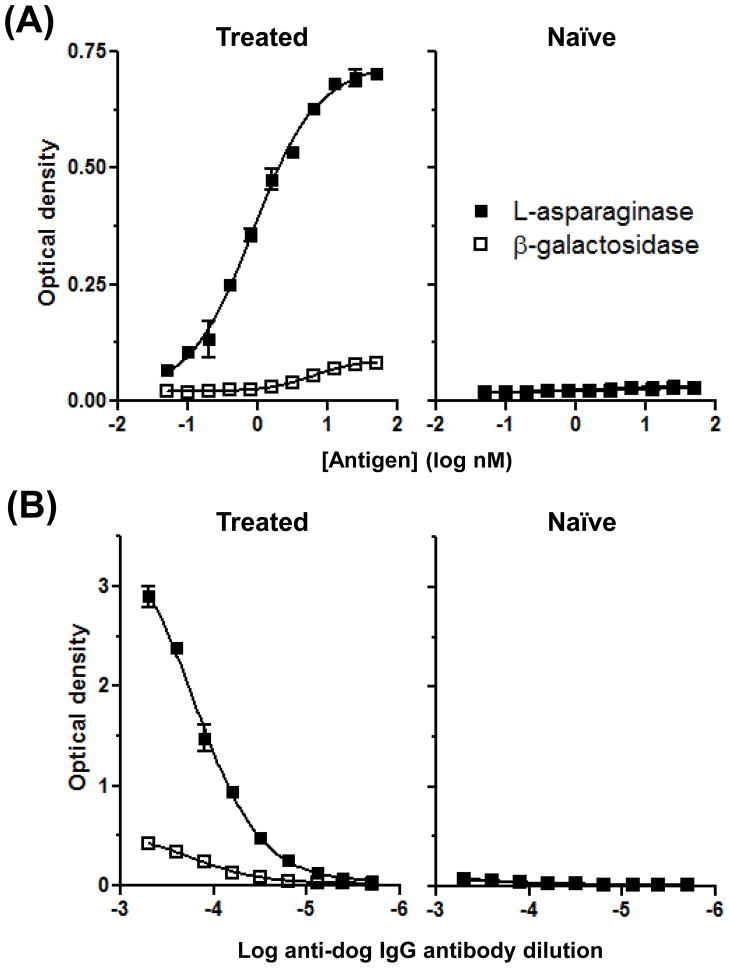
To determine whether dogs with lymphoma that had received one or more doses of native E. coli L-asparaginase develop circulating anti-asparaginase antibodies, we coated plates with the drug and used horseradish peroxidase (HRP)-conjugated sheep anti-dog IgG antibodies to detect possible reactivity in plasma. As no canine antibody against L-asparaginase has been established, we created a putative positive control by pooling plasma from three dogs that had been treated with multiple doses of L-asparaginase (dogs 1, 2 and 3); given the high incidence (>50%) of antibody formation in human ALL patients, we reasoned that at least one dog in this group was likely to have developed an analogous response. A negative control was generated by combining plasma from an equal number of antigen-naïve dogs with lymphoma (dogs 4, 5 and 6). The optimal antigen concentration first was determined using two-fold serial dilutions of L-asparaginase, or the control antigen β-galactosidase, from 50 to 0.049 nM, a protein concentration range typical for ELISAs.23 As seen in Figure 1A, plasma from the treated dogs had IgG reactivity that selectively bound to L-asparaginase-coated wells, similar to ELISA data generated from human ALL patients receiving multiple doses of the drug,24 and consistent with our hypothesis that dogs administered L-asparaginase develop circulating specific antibodies. This reactivity increased with increasing L-asparaginase concentrations. A plateau at approximately 25 nM was shown by non-linear curve fitting, and therefore, this concentration was used in all subsequent ELISAs. As anticipated, untreated dogs lacked antibody responses against either antigen. We then sought to determine the dilution of detecting antibody that would provide ideal assay performance. At 1:8,000 (Fig. 1B), the OD values of the positive signal fell in the middle of the optimal absorbance range of the spectrophotometer, with a low background in β-galactosidase-coated wells, and this dilution was used in succeeding experiments.
Figure 1.
Determination of optimal reagent concentrations for a canine anti-asparaginase ELISA. (A) Titration of antigen. Plates were coated overnight with L-asparaginase or β-galactosidase diluted in carbonate-bicarbonate buffer. The anti-dog IgG antibody was used at 1:15,000. (B) Titration of the detecting antibody. The anti-dog IgG antibody was serially diluted two-fold from 1:2,000 to 1:512,000, extending below and above the manufacturer’s suggested range of 1:10,000 to 1:100,000. In (A) and (B), plasma samples were used at 1:12,500; the data represent two independent repetitions of each experiment.
Sensitivity and reproducibility of the ELISA
Using the reagents at these validated concentrations, we then investigated the assay sensitivity by testing the reactivity of the individual treated patient samples (from dogs 1, 2 and 3) that together had constituted the positive control in preceding experiments. An endpoint titer was approached, but not reached, for each dog (Supplemental Fig. 1). In this analysis, the sensitivity cutoff was defined at each dilution as the mean OD of the separate naïve patient samples plus two standard deviations (dotted line). The same result was obtained when the assay cutoff was computed using the more stringent statistical method described by Frey25 at the 95% confidence level. To assess the relative variability of the ELISA, we measured the OD of L-asparaginase-coated wells incubated with pooled positive plasma (1:50,000), and calculated the coefficient of variation (CV) both within a single assay (data from 10 wells), and between multiple repetitions of the ELISA (data from 3 assays, conducted weeks apart). The intra-assay CV was 3.6%, and the inter-assay CV was 14.5%.
Validation of the ELISA
As additional confirmation that the IgG reactivity detected by ELISA was indeed directed against L-asparaginase, we sought to determine whether positive plasma had an inhibitory effect on enzyme function. First, an assay for measuring L-asparaginase activity in vitro was established (Fig. 2A). Next, we incubated individual positive plasma samples (dogs 1, 2 and 3) at varying dilutions with L-asparaginase for 2 h, but no inhibition of substrate hydrolysis was observed (not shown). When diluted plasma from one of these patients (dog 3) was incubated overnight with L-asparaginase and subsequently mixed with protein A-coated agarose beads, however, the supernatant was significantly depleted of enzyme activity (Fig. 2B), compared to ELISA-negative control plasma (dog 23). Further, the L-asparaginase activity removed by positive plasma was now bound to the agarose beads (Fig. 2C). The same results were obtained with a separate pair of ELISA-positive (2) and –negative (22) dogs (not shown). These data provide a functional correlate to the ELISA, demonstrating that positive, but not negative, plasma contains antibodies capable of binding and immunoprecipitating soluble L-asparaginase.
Figure 2.
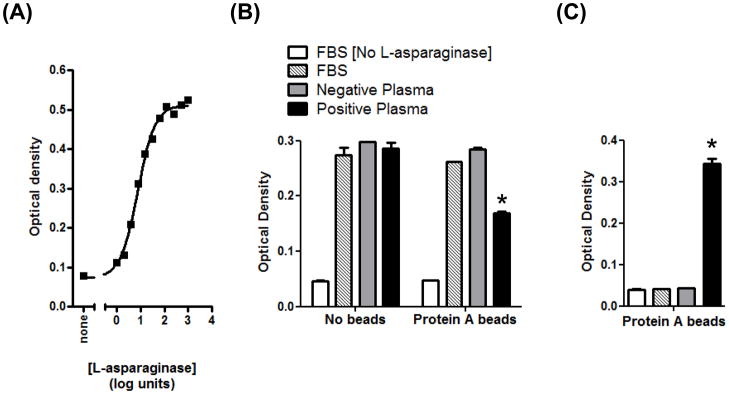
Canine anti-asparaginase antibodies immunoprecipitate, but do not directly inhibit, enzyme function. (A) Titration of enzyme activity. Dilutions of L-asparaginase were incubated with the substrate AHA at 37°C, and hydrolysis was measured as indooxine production, as described in the Materials and Methods. The EC50 is 7.5 units mL−1 (95% confidence intervals are 5.5–10.3). The data represent two independent repetitions of the experiment. (B) Positive plasma binds soluble L-asparaginase, and subsequent removal of total IgG with protein A reduces asparaginase activity in the supernatant. Plasma from ELISA-positive and –negative dogs, or FBS control, were incubated with L-asparaginase overnight, prior to the addition of a protein A agarose bead slurry, or PBS, for 3 h. The final concentration of L-asparaginase was 6 units mL−1. When all treatments containing L-asparaginase were tested against the control sample (FBS without beads), only the activity in the starred group was significantly decreased (P<0.001). (C). Soluble L-asparaginase activity removed by immunoprecipitation with plasma and protein A is retained on the agarose beads. Beads from (B) were washed five times with PBS, and then incubated with the AHA substrate. Only the starred group possessed significant enzyme activity (P<0.001) when compared to the control lacking L-asparaginase.
Anti-asparaginase antibody responses in dogs following one or two doses of L-asparaginase
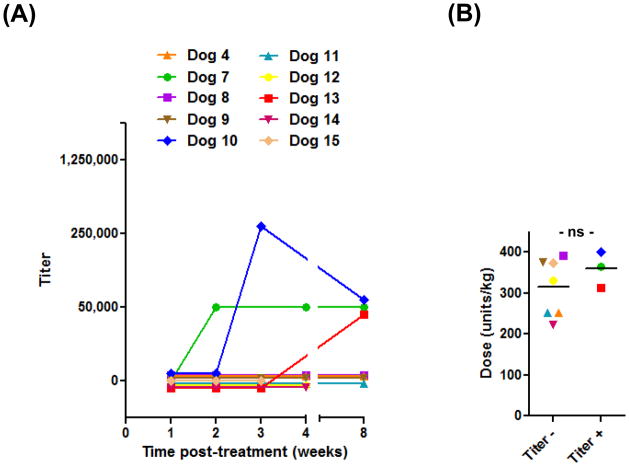
In our hospital, dogs with multicentric, high-grade lymphoma or ALL routinely receive a single dose of E. coli L-asparaginase as part of their first treatment. Using the optimized ELISA, we sought to examine whether anti-asparaginase antibodies developed in such patients, and if so, to determine the frequency and kinetics of such responses. To accomplish this objective, plasma samples were obtained from 10 randomly selected lymphoma patients (dogs 4, 7 – 15; Supplemental Table 1) immediately prior to and 1, 2, 3, 4 and 8 weeks after injection of the drug. As seen in Figure 3A, three dogs (7, 10 and 13) developed strong anti-asparaginase IgG reactivity following their initial exposure, with the earliest response detected at 2 weeks post-treatment. Because we may have failed to identify antibody-positive dogs with lower titers, samples were re-tested at dilutions of 1:3,125, 1:12,500 and 1:50,000, but no additional positive dogs were found. Titers were maintained through the last sample collection time point, 8 weeks after L-asparaginase administration. The failure of some dogs to develop antibody responses conceivably could have resulted from receiving a lesser amount of drug. While our standard L-asparaginase prescription is 400 units kg−1, the administered dose typically is restricted to a maximum of 104 units (1 vial) per dog, and consequently, most dogs that exceed 25 kg receive lower dosages. However, when the mean dosages of these 10 ELISA-positive and –negative dogs treated with a single dose of L-asparaginase were compared, no difference was observed (Fig. 3B).
Figure 3.
The incidence, magnitude and time course of anti-asparaginase IgG responses after an initial dose of L-asparaginase. (A) Plasma samples were collected from 10 dogs immediately prior to and at the indicated time points following L-asparaginase injection, stored at −20°C, and assayed at dilutions of 1:50,000, 1:250,000 and 1:1,250,000 in a single ELISA run. The titer is the reciprocal of the highest dilution of plasma yielding a mean OD above the sensitivity cutoff. Absent symbols indicate time points for which a sample was not available for that patient. (B) The dosages of L-asparaginase administered to ELISA-positive and –negative dogs were not significantly different (ns; two-tailed P=0.34).
We also concurrently examined anti-asparaginase IgM responses in a subset of these first-dose patients (dogs 4, 7, 8, 9 and 10). Of these five dogs, one (7) developed IgG reactivity without ever having detectable IgM responses, while another (10) first mounted only an IgM response (3,125) at week 2 post-administration, followed by equivalent IgM and IgG responses (50,000) by the subsequent week.
Lastly, we investigated the incidence of anti-asparaginase IgG responses in seven randomly selected dogs with lymphoma that had received a second injection of L-asparaginase as part of their chemotherapy protocol, typically for re-induction or rescue at the time of relapse (dogs 9, 16 – 21, Supplemental Table 1; one of these patients, dog 9, had been additionally evaluated for antibody development after the first dose of L-asparaginase). Four of these dogs had anti-asparaginase antibodies, while three remained negative (Table 1).
Table 1.
The magnitude of anti-asparaginase IgG responses after administration of two doses of L-asparaginase*
| Dog | Elapsed time (days) from 1st and 2nd doses | Titer |
|---|---|---|
| 9 | 172, 14 | ND† |
| 16 | 68, 18 | 12,500 |
| 17 | 462, 25 | 3,125 |
| 18 | 150, 28 | ≥50,000 |
| 19 | 575, 22 | ≥50,000 |
| 20 | 87, 12 | ND |
| 21 | 54, 19 | ND |
Plasma samples were obtained 12 to 28 days following a second L-asparaginase injection from dogs with lymphoma undergoing chemotherapy. All samples were stored at −20°C, and diluted 1:3125, 1:12,500 and 1:50,000 for assay in a single ELISA run. The titer is determined as described in Fig. 3.
ND: Not detected.
Cross-reactivity of anti-asparaginase antibodies with PEGylated L-asparaginase
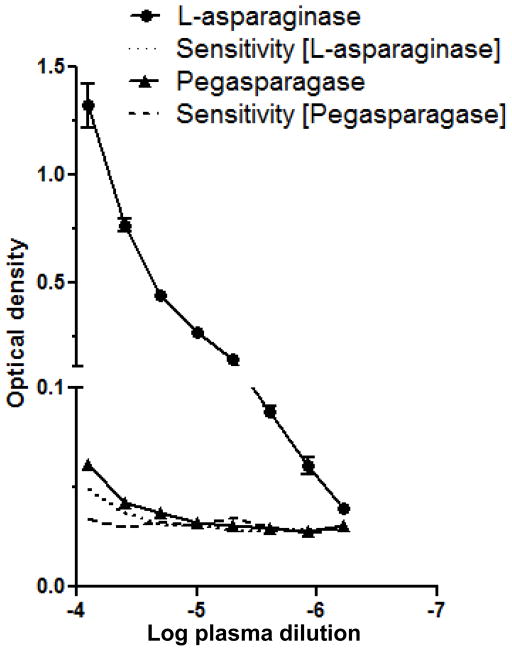
Immunization of mice with E. coli L-asparaginase in adjuvant induces specific antibodies that inhibit the anti-lymphoma properties of the native, but not the PEGylated, form of the drug, demonstrating the reduced antigenicity of the latter preparation.26 Analogously, canine lymphoma patients with clinical resistance to native L-asparaginase have been reported to maintain responsiveness to pegaspargase.21 Conceivably, this phenomenon could be due to decreased binding of antibodies to the PEGylated drug. To test this hypothesis, we performed the ELISA with native and PEGylated L-asparaginase as antigens. Using pooled plasma from the same treated dogs as in Fig. 1, IgG reactivity against pegaspargase is observed, but as expected, is markedly reduced when compared to reactivity against the unmodified form of the antigen (Fig. 4). The endpoint titer against pegaspargase was 100,000, but as in previous experiments with native L-asparaginase, was not reached at a plasma dilution of 1:1,600,000.
Figure 4.
The reactivity of antibody-positive plasma to PEGylated vs. native L-asparaginase is greatly diminished. Because the molecular weights of the two forms are different, plate wells were coated with identical drug concentrations, calculated as enzyme activity (0.79 units mL−1, equivalent to 25 nM native L-asparaginase). Pooled plasma samples from treated (1, 2 and 3) and naïve (4, 5 and 6) dogs were serially diluted two-fold from 1:12,500 to 1:1,600,000. The assay sensitivity curves for each antigen are indicated by dashed and dotted lines, and were calculated as in Figure 2. The data represent two independent repetitions of the experiment.
Discussion
The objective of this study was to develop an ELISA to determine whether dogs with lymphoma that are treated with L-asparaginase develop antibody responses against this valuable anti-neoplastic agent, a frequently raised question that has not been answered previously. In humans, a number of studies have reported the use of ELISA to demonstrate circulating anti-asparaginase antibodies.10–12, 15, 17, 24 Other methodologies, such as passive hemagglutination,27 solid-phase radioimmunoassay,13 and surface plasmon resonance,28 have also been employed. The detection of such antibodies has value in predicting clinical allergy, and potentially, acquired resistance to L-asparaginase, which is an important limitation of the drug that occurs with repeated administration in both human and canine patient populations.18, 29
While anti-asparaginase antibodies have been incriminated as a cause of resistance, other mechanisms have been demonstrated, including 1) upregulation of AS in leukemic cells;30 2) ASN secretion by bone marrow-derived mesenchymal cells;31 and 3) degradation of bacterial asparaginases by lysosomal endopeptidases in malignant lymphoblasts.32 Importantly, however, antibody titers in human ALL patients have been correlated with decreased asparaginase activity in vivo and poorer clinical outcome.10, 16, 17 Moreover, the loss of responsiveness to native E. coli L-asparaginase in humans and dogs can be overcome by the use of alternate preparations (PEGylated or E. chrysanthemi asparaginases),10, 21 suggesting that specific antibody formation, rather than tumor cell insensitivity, contributes significantly to resistance. Hence, identification of patients with anti-asparaginase antibodies – particularly those without clinical signs – can be important in providing optimal therapy for patients with ALL or lymphoma.
Our data show that the ELISA can detect anti-asparaginase IgG (and IgM) in canine plasma. While antibodies often are measured in serum, plasma is convenient to assay, because most dogs undergoing lymphoma chemotherapy have anticoagulated blood samples drawn repeatedly for hemogram analysis. Plasma has also been used in L-asparaginase ELISAs in human patients.15, 24 In human ELISAs, positive controls often consist of blood samples pooled from multiple individuals with clinical drug allergy; however, the incidence of this adverse effect in our hospital is very low (<1%). Given the unavailability of such samples, we instead obtained plasma from canine patients that had received three or more injections of the drug over many months, as increasing treatment time and dose number are associated with a higher incidence of hypersensitivity in humans.33, 34 The validity of the assay was then established, in part, by identifying antibody responses in these dogs, while finding no reactivity in naïve dogs, and no responses in either set of patients against the control antigen. We examined IgG with our ELISA, because this isotype has been associated with hypersensitivity and resistance in humans;13 IgM reactivity generally mirrors that of IgG.16. Interestingly, despite the fact that the majority of allergic reactions to L-asparaginase are Type 1 (urticaria, anaphylaxis), circulating IgE reactivity to the drug is sporadic and weak, and does not correlate with hypersensitivity;13, 35 hence, we did not evaluate IgE. The sensitivity of the ELISA appeared surprisingly high, as an endpoint titer was not reached in some patients even with plasma dilutions greater than 10−6. Lastly, assay reproducibility was acceptable, with intra- and inter-assay coefficients of variation meeting published standards (<10% and <25%, respectively).36
Circulating antibodies potentially could reduce or neutralize asparaginase activity in several ways, including 1) increased clearance by opsonization and binding of Ig-drug complexes to Fc receptors; 2) sequestration of L-asparaginase at the injection site; or 3) direct inhibition, by steric hindrance or binding to the catalytic site proper. Consistent with the last mechanism, Killander et al. found an in vitro reduction in asparaginase activity that varied directly with the concentration of anti-asparaginase antibodies in human plasma.35 On the other hand, Peterson et al. reported that hyperimmune rabbit antiserum could only reduce L-asparaginase activity by 50%, even at antibody excess. Direct inhibition of the same magnitude has been found in immunized mice, however, increased clearance is the major mechanism by which antibodies decrease asparaginase activity in vivo in that species.37 In our study, no evidence of direct inhibition was found, as there was no difference in L-asparaginase activity when incubated with antibody-positive or –negative plasma (Fig. 2B, samples without beads). This observation presumably reflects a dominant immunogenic B-cell epitope in dogs that is distant from the catalytic site. While Peterson et al. observed only modest inhibition with rabbit or patient serum, L-asparaginase incubated with sera from antibody-positive individuals could be precipitated with ammonium sulfate, and these insoluble complexes retained enzymatic activity. Similarly, in our study, L-asparaginase in solution could be bound by antibodies and immunoprecipitated by protein A, which subsequently was seen as AHA hydrolytic activity on the agarose beads. This phenomenon was observed in both of the ELISA-positive dogs that were evaluated in these experiments. Thus, while canine anti-asparaginase antibodies may not directly interfere with enzyme function, L-asparaginase activity in positive dogs might be reduced substantially by IgG-mediated clearance or sequestration, leading to clinical resistance.
After a single SC administration of L-asparaginase, 30% of dogs had demonstrable antibodies. With two injections, the percentage increased, although the number of patients examined in both groups is too small to accurately predict the incidence in the larger population of L-asparaginase-treated dogs. There is a report of a human patient developing antibodies after only two doses of L-asparaginase.27 Nonetheless, it is difficult to compare the incidence of antibodies in dogs to humans undergoing intensive L-asparaginase therapy, as most ALL patients initially receive a total of eight or nine doses, given at 3 day intervals. While it might be predicted that greater drug exposure would increase the frequency of responses, in one study, none of 13 patients had anti-asparaginase IgG at the end of the induction period,38 and in a follow-up study, only 10 of 47 (21.3%) patients had antibodies.16 When later time points are examined, however, data compiled from several studies indicate an average antibody prevalence of 54% (range: 15–61%) in humans treated with a regimen of native E. coli L-asparaginase,10, 13, 15–17, 27, 38 which is similar to what we observed.
In addition to the number and schedule of L-asparaginase doses, other factors, such as the route of administration or concurrent drug therapy, could affect the frequency of antibody formation. Thus, 30% of dogs with lymphoma receiving biweekly intraperitoneal injections of E. coli L-asparaginase developed anaphylaxis,20 while none of 44 that received multiple (range: 2–10) doses intramuscularly (IM) did.39 Similarly, no signs of anaphylaxis were observed in 26 and 23 dogs injected IM or SC, respectively, with 3 doses of L-asparaginase. Both routes were equally effective in depleting ASN and inducing remission.5 The dogs in our study were administered L-asparaginase SC. Despite the presence of circulating antibodies, no allergic reactions were noted in ELISA-positive patients, analogous to the silent hypersensitivity noted in humans;40 however, the premedication with diphenhydramine and dexamethasone that is routinely prescribed in our hospital could have masked such signs. Identifying a route of administration in dogs that is less immunogenic may be important not only in preventing clinical hypersensitivity, but in preserving tumor responsiveness to the drug by diminishing antibody formation. It is also important to note that, in dogs and humans, L-asparaginase typically is given as part of a multi-drug protocol, and concomitantly administered corticosteroids or chemotherapy agents may modulate the immune response to the drug. For example, the likelihood of an anaphylactic reaction to L-asparaginase was lower in human patients receiving concurrent vincristine and prednisone,34 a combination that was also administered to the 10 dogs in this study that were given a single dose of L-asparaginase. Whether the incidence of anti-asparaginase IgG formation would have been higher without this therapy is unknown, however, multi-drug chemotherapy has been shown to not suppress de novo antibody responses to the model immunogen keyhole limpet hemocyanin in dogs.41 Nonetheless, it may be possible to optimize canine protocols to effectively treat lymphoma and decrease the incidence of anti-asparaginase antibodies. For example, Cheung et al. observed that the administration of cyclophosphamide early in the treatment regimen significantly reduced the percentage of ALL patients that developed IgG reactivity against L-asparaginase,13 and hence, inclusion of this drug at induction could help to delay or prevent the acquisition of resistance to L-asparaginase in dogs. If resistance does develop, use of pegaspargase may be warranted, as some canine lymphoma patients can be rescued by this substitution. Consistent with this clinical observation, and the lowered antigenicity of this preparation,26 we found that canine anti-asparaginase IgG induced by treatment with native E. coli L-asparaginase cross-reacts only relatively weakly with the PEGylated drug (Fig. 4), similar to what is seen in human patients.12 A more extensive survey of cross-reactivity in ELISA-positive dogs treated with native L-asparaginase will be useful in determining the antigenicity of the PEGylated form in this species.
In summary, anti-asparaginase IgG antibodies can be detected in canine patients undergoing multi-drug chemotherapy for high-grade lymphoma by ELISA. Whether such humoral responses can predict clinical resistance to this valuable chemotherapy drug is the next essential question. We have evaluated responsiveness to L-asparaginase alone in a small number of dogs with measurable disease that had previously received one or more doses of the drug (not shown). Of the 10 patients with detectable titers (≥3125), there was one that experienced a complete remission, five with partial remissions, and four that exhibited no response. While very preliminary, these data suggest that, as in humans, a large patient data set, as well as correlation with functional consequences (i.e., decreased serum asparaginase activity), will likely be needed to make such a critical determination. Should an association between titer and resistance ultimately be established, the ELISA may also prove useful in designing and comparing treatment protocols that effectively minimize the immunogenicity of E. coli L-asparaginase.
Supplementary Material
Anti-asparaginase IgG responses are detectable at a plasma dilution of at least 1:1,600,000. Individual samples from treated (n=3) and naïve (n=3) dogs were serially diluted two-fold from 1:12,500 to 1:1,600,000; OD values from naïve dogs were used to calculate the assay sensitivity curve (dotted line). The data represent two independent repetitions of the experiment.
Acknowledgments
We thank Elise Grover and the Tonkonogy laboratory for their advice and technical assistance. This work was supported by a National Institutes of Health grant K08 DK082264 (to PRH).
Abbreviations
- AHA
L-aspartic beta-hydroxamate
- ALL
acute lymphoblastic leukemia
- AS
asparagine synthetase
- ASN
asparagine
- CLL
chronic lymphocytic leukemia
- CV
coefficient of variation
- FBS
fetal bovine serum
- HRP
horseradish peroxidase
- Ig
immunoglobulin
- IM
intramuscularly
- OD
optical density
- SC
subcutaneously
Footnotes
This research was presented, in part, at the Veterinary Cancer Society Annual Meeting, 2009.
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Asselin BL, Ryan D, Frantz CN, Bernal SD, Leavitt P, Sallan SE, Cohen HJ. In vitro and in vivo killing of acute lymphoblastic leukemia cells by L-asparaginase. Cancer Res. 1989;49 (15):4363–8. [PubMed] [Google Scholar]
- 2.Capizzi RL, Bertino JR, Skeel RT, Creasey WA, Zanes R, Olayon C, Peterson RG, Handschumacher RE. L-asparaginase: clinical, biochemical, pharmacological, and immunological studies. Ann Intern Med. 1971;74(6):893–901. doi: 10.7326/0003-4819-74-6-893. [DOI] [PubMed] [Google Scholar]
- 3.Story MD, Voehringer DW, Stephens LC, Meyn RE. L-asparaginase kills lymphoma cells by apoptosis. Cancer Chemother Pharmacol. 1993;32(2):129–33. doi: 10.1007/BF00685615. [DOI] [PubMed] [Google Scholar]
- 4.Old LJ, Boyse EA, Campbell HA, Brodey RS, Fidler J, Teller JD. Treatment of lymphosarcoma in the dog with L-asparaginase. Cancer. 1967;20(7):1066–70. doi: 10.1002/1097-0142(196707)20:7<1066::aid-cncr2820200709>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Valerius KD, Ogilvie GK, Fettman MJ, Walton JA, Richardson K, Powers BE, McNiel EA, Rogers QR. Comparison of the effects of asparaginase administered subcutaneously versus intramuscularly for treatment of multicentric lymphoma in dogs receiving doxorubicin. J Am Vet Med Assoc. 1999;214(3):353–6. [PubMed] [Google Scholar]
- 6.MacEwen EG, Rosenthal RC, Fox LE, Loar AS, Kurzman ID. Evaluation of L-asparaginase: polyethylene glycol conjugate versus native L-asparaginase combined with chemotherapy. A randomized double-blind study in canine lymphoma. J Vet Intern Med. 1992;6(4):230–4. doi: 10.1111/j.1939-1676.1992.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 7.Hardy WD, Jr, Old LJ. L-asparaginase in the treatment of neoplastic diseases of the dog, cat and cow. Recent Results Cancer Res. 1970;33:131–9. doi: 10.1007/978-3-642-99984-0_15. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald VS, Thamm DH, Kurzman ID, Turek MM, Vail DM. Does L-asparaginase influence efficacy or toxicity when added to a standard CHOP protocol for dogs with lymphoma? J Vet Intern Med. 2005;19(5):732–6. doi: 10.1892/0891-6640(2005)19[732:dlieot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Jeffreys AB, Knapp DW, Carlton WW, Thomas RM, Bonney PL, Degortari A, Lucroy MD. Influence of asparaginase on a combination chemotherapy protocol for canine multicentric lymphoma. J Am Anim Hosp Assoc. 2005;41(4):221–6. doi: 10.5326/0410221. [DOI] [PubMed] [Google Scholar]
- 10.Panosyan EH, Seibel NL, Martin-Aragon S, Gaynon PS, Avramis IA, Sather H, Franklin J, Nachman J, Ettinger LJ, La M, Steinherz P, Cohen LJ, Siegel SE, Avramis VI. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004;26(4):217–26. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Woo MH, Hak LJ, Storm MC, Evans WE, Sandlund JT, Rivera GK, Wang B, Pui CH, Relling MV. Anti-asparaginase antibodies following E. coli asparaginase therapy in pediatric acute lymphoblastic leukemia. Leukemia. 1998;12(10):1527–33. doi: 10.1038/sj.leu.2401162. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Relling MV, Storm MC, Woo MH, Ribeiro R, Pui CH, Hak LJ. Evaluation of immunologic crossreaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia. 2003;17(8):1583–8. doi: 10.1038/sj.leu.2403011. [DOI] [PubMed] [Google Scholar]
- 13.Cheung NK, Chau IY, Coccia PF. Antibody response to Escherichia coli L-asparaginase. Prognostic significance and clinical utility of antibody measurement. Am J Pediatr Hematol Oncol. 1986;8(2):99–104. [PubMed] [Google Scholar]
- 14.Klug Albertsen B, Schmiegelow K, Schroder H, Carlsen NT, Rosthoj S, Avramis VI, Jakobsen P. Anti-Erwinia asparaginase antibodies during treatment of childhood acute lymphoblastic leukemia and their relationship to outcome: a case-control study. Cancer Chemother Pharmacol. 2002;50(2):117–20. doi: 10.1007/s00280-002-0466-y. [DOI] [PubMed] [Google Scholar]
- 15.Woo MH, Hak LJ, Storm MC, Sandlund JT, Ribeiro RC, Rivera GK, Rubnitz JE, Harrison PL, Wang B, Evans WE, Pui CH, Relling MV. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000;18(7):1525–32. doi: 10.1200/JCO.2000.18.7.1525. [DOI] [PubMed] [Google Scholar]
- 16.Zalewska-Szewczyk B, Andrzejewski W, Mlynarski W, Jedrychowska-Danska K, Witas H, Bodalski J. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48(5):931–6. doi: 10.1080/10428190701292049. [DOI] [PubMed] [Google Scholar]
- 17.Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, Ettinger AG, Ettinger LJ, Franklin J, Gaynon PS, Hilden JM, Lange B, Majlessipour F, Mathew P, Needle M, Neglia J, Reaman G, Holcenberg JS, Stork L. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99(6):1986–94. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 18.Rogers KS. L-asparaginase for treatment of lymphoid neoplasia in dogs. J Am Vet Med Assoc. 1989;194(11):1626–30. [PubMed] [Google Scholar]
- 19.Chun R, Garrett LD, Vail DM. Cancer Chemotherapy. In: Withrow SJ, Vail DM, editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 4. St. Louis, MO: Saunders, Elsevier; 2007. [Google Scholar]
- 20.Teske E, Rutteman GR, van Heerde P, Misdorp W. Polyethylene glycol-L-asparaginase versus native L-asparaginase in canine non-Hodgkin’s lymphoma. Eur J Cancer. 1990;26(8):891–5. doi: 10.1016/0277-5379(90)90193-w. [DOI] [PubMed] [Google Scholar]
- 21.MacEwen EG, Rosenthal R, Matus R, Viau AT, Abuchowski A. A preliminary study on the evaluation of asparaginase. Polyethylene glycol conjugate against canine malignant lymphoma. Cancer. 1987;59(12):2011–5. doi: 10.1002/1097-0142(19870615)59:12<2011::aid-cncr2820591207>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Lanvers C, Vieira Pinheiro JP, Hempel G, Wuerthwein G, Boos J. Analytical validation of a microplate reader-based method for the therapeutic drug monitoring of L-asparaginase in human serum. Anal Biochem. 2002;309(1):117–26. doi: 10.1016/s0003-2697(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 23.Crowther JR. The ELISA guidebook. Methods Mol Biol. 2000;149:III–IV. 1–413. doi: 10.1385/1592590497. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Hak LJ, Relling MV, Pui CH, Woo MH, Storm MC. ELISA to evaluate plasma anti-asparaginase IgG concentrations in patients with acute lymphoblastic leukemia. J Immunol Methods. 2000;239(1–2):75–83. doi: 10.1016/s0022-1759(00)00182-4. [DOI] [PubMed] [Google Scholar]
- 25.Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221(1–2):35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 26.Wada H, Imamura I, Sako M, Katagiri S, Tarui S, Nishimura H, Inada Y. Antitumor enzyme: polyethylene glycol-modified asparaginase. Ann N Y Acad Sci. 1990;613:95–108. doi: 10.1111/j.1749-6632.1990.tb18151.x. [DOI] [PubMed] [Google Scholar]
- 27.Peterson RG, Handschumacher RE, Mitchell MS. Immunological responses to L-asparaginase. J Clin Invest. 1971;50(5):1080–90. doi: 10.1172/JCI106579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avramis VI, Avramis EV, Hunter W, Long MC. Immunogenicity of native or pegylated E. coli and Erwinia asparaginases assessed by ELISA and surface plasmon resonance (SPR-biacore) assays of IgG antibodies (Ab) in sera from patients with acute lymphoblastic leukemia (ALL) Anticancer Res. 2009;29(1):299–302. [PubMed] [Google Scholar]
- 29.Goldberg AI, Cooney DA, Glynn JP, Homan ER, Gaston MR, Milman HA. The effects of immunization to L-asparaginase on antitumor and enzymatic activity. Cancer Res. 1973;33(2):256–61. [PubMed] [Google Scholar]
- 30.Hutson RG, Kitoh T, Moraga Amador DA, Cosic S, Schuster SM, Kilberg MS. Amino acid control of asparagine synthetase: relation to asparaginase resistance in human leukemia cells. Am J Physiol. 1997;272(5 Pt 1):C1691–9. doi: 10.1152/ajpcell.1997.272.5.C1691. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest. 2007;117(4):1049–57. doi: 10.1172/JCI30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel N, Krishnan S, Offman MN, Krol M, Moss CX, Leighton C, van Delft FW, Holland M, Liu J, Alexander S, Dempsey C, Ariffin H, Essink M, Eden TO, Watts C, Bates PA, Saha V. A dyad of lymphoblastic lysosomal cysteine proteases degrades the antileukemic drug L-asparaginase. J Clin Invest. 2009;119(7):1964–73. doi: 10.1172/JCI37977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dellinger CT, Miale TD. Comparison of anaphylactic reactions to asparaginase derived from Escherichia coli and from Erwinia cultures. Cancer. 1976;38(4):1843–6. doi: 10.1002/1097-0142(197610)38:4<1843::aid-cncr2820380463>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 34.Evans WE, Tsiatis A, Rivera G, Murphy SB, Dahl GV, Denison M, Crom WR, Barker LF, Mauer AM. Anaphylactoid reactions to Escherichia coli and Erwinia asparaginase in children with leukemia and lymphoma. Cancer. 1982;49(7):1378–83. doi: 10.1002/1097-0142(19820401)49:7<1378::aid-cncr2820490713>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Killander D, Dohlwitz A, Engstedt L, Franzen S, Gahrton G, Gullbring B, Holm G, Holmgren A, Hoglund S, Killander A, Lockner D, Mellstedt H, Moe PJ, Palmblad J, Reizenstein P, Skarberg KO, Swedberg B, Uden AM, Wadman B, Wide L, Ahstrom L. Hypersensitive reactions and antibody formation during L-asparaginase treatment of children and adults with acute leukemia. Cancer. 1976;37(1):220–8. doi: 10.1002/1097-0142(197601)37:1<220::aid-cncr2820370132>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 36.DeSilva B, Smith W, Weiner R, Kelley M, Smolec J, Lee B, Khan M, Tacey R, Hill H, Celniker A. Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm Res. 2003;20(11):1885–900. doi: 10.1023/b:pham.0000003390.51761.3d. [DOI] [PubMed] [Google Scholar]
- 37.Baechtel S, Prager MD. Basis for loss of therapeutic effectiveness of L-asparaginase in sensitized mice. Cancer Res. 1973;33(8):1966–9. [PubMed] [Google Scholar]
- 38.Zalewska-Szewczyk B, Andrzejewski W, Bodalski J. Development of anti-asparaginase antibodies in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;43(5):600–2. doi: 10.1002/pbc.20064. [DOI] [PubMed] [Google Scholar]
- 39.Ogilvie GK, Atwater SW, Ciekot PA, Bergman PJ, Henkel S, Walters LM. Prevalence of anaphylaxis associated with the intramuscular administration of L-asparaginase to 81 dogs with cancer: 1989–1991. J Am Anim Hosp Assoc. 1994:30. [Google Scholar]
- 40.Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol. 1999;457:621–9. [PubMed] [Google Scholar]
- 41.Walter CU, Biller BJ, Lana SE, Bachand AM, Dow SW. Effects of chemotherapy on immune responses in dogs with cancer. J Vet Intern Med. 2006;20(2):342–7. doi: 10.1892/0891-6640(2006)20[342:eocoir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-asparaginase IgG responses are detectable at a plasma dilution of at least 1:1,600,000. Individual samples from treated (n=3) and naïve (n=3) dogs were serially diluted two-fold from 1:12,500 to 1:1,600,000; OD values from naïve dogs were used to calculate the assay sensitivity curve (dotted line). The data represent two independent repetitions of the experiment.