Abstract
There is increasing interest in discovering mechanisms that mediate the effects of childhood stress on late-life disease morbidity and mortality. Previous studies have suggested one potential mechanism linking stress to cellular aging, disease and mortality in humans: telomere erosion. We examined telomere erosion in relation to children’s exposure to violence, a salient early-life stressor, which has known long-term consequences for well-being and is a major public-health and social-welfare problem. In the first prospective-longitudinal study with repeated telomere measurements in children while they experienced stress, we tested the hypothesis that childhood violence exposure would accelerate telomere erosion from age 5 to age 10 years. Violence was assessed as exposure to maternal domestic violence, frequent bullying victimization and physical maltreatment by an adult. Participants were 236 children (49% females; 42% with one or more violence exposures) recruited from the Environmental-Risk Longitudinal Twin Study, a nationally representative 1994–1995 birth cohort. Each child’s mean relative telomere length was measured simultaneously in baseline and follow-up DNA samples, using the quantitative PCR method for T/S ratio (the ratio of telomere repeat copy numbers to single-copy gene numbers). Compared with their counterparts, the children who experienced two or more kinds of violence exposure showed significantly more telomere erosion between age-5 baseline and age-10 follow-up measurements, even after adjusting for sex, socioeconomic status and body mass index (B = −0.052, s.e. = 0.021, P = 0.015). This finding provides support for a mechanism linking cumulative childhood stress to telomere maintenance, observed already at a young age, with potential impact for life-long health.
Keywords: childhood stress, cumulative violence exposure, erosion, longitudinal, telomere length
INTRODUCTION
Childhood stress is associated with later-life physical and mental-health problems.1 – 5 Interest in the etiological pathways that mediate the effect of early-life stress on physical and mental health has focused on key biological systems, including the sympathetic–nervous system, hypothalamus–pituitary–adrenal axis, immune system and the epigenome, leading to important insights about the systemic effects of stress.6 – 8 But the questions of how and when childhood stress gets ‘under the skin’ at the cellular level, specifically in humans, remain to be answered.
In the past decade, the length of telomeres, the repetitive TTAGGG sequence at the end of linear chromosomes, has emerged as a promising new biomarker of stress.9 In the 1960s, it was discovered that fibroblast cells have a built-in mechanism that limits their replicative capacity, later named the ‘Hayflick limit’.10 In the 1970s, it was suggested that telomeres are lost with each replication until a certain (Hayflick) limit, at which point the cell arrests and enters a state of senescence.11 Subsequent research confirmed that telomeres indeed get shorter with each division and established the utility of telomeres as a molecular clock for cellular replicative aging.12,13
The advent of high-throughput laboratory techniques for measuring telomere length (TL) has opened the gate to new studies linking TL with a broad range of risk factors that predict disease morbidity, including smoking,14 obesity,14 – 16 psychiatric disorders17,18 and psychosocial stress.19,20 It has thus been suggested that TL is a marker for biological aging (‘wear and tear’), rather than a clock for chronological aging.
Six recent studies have examined the possible association between childhood stress and TL. Four of these reported that adult cases who retrospectively recalled childhood adversity had shorter TL, as compared with adult controls.20 – 23 One study failed to replicate this association.24 In the only study of children, institutional care was associated with shorter TL in middle childhood.25
Although these studies advance understanding of the link between childhood stress and TL, their reliance on retrospective assessments of stress and cross-sectional designs raises important questions. Interpretation of findings from cross-sectional studies of TL is ambiguous in light of recent longitudinal analyses of repeated TL measurements. Longitudinal studies show that TL is highly variable across different age groups; that there is an inverse correlation between baseline TL and subsequent telomere erosion; and that, in some individuals, telomeres can lengthen over time.26 – 32 These recent findings indicate that the temporal process of telomere erosion is more complex than initially assumed, and that repeated measures (not just length at one time point) are needed to measure true telomere erosion in individuals who are experiencing stress.
Here, we used a longitudinal design to test the effects of violence exposure during childhood on telomere erosion in a cohort of young children. We assessed childhood adversity prospectively and measured TL at two time-points, at age-5 years and again at age-10 years. We assessed domestic violence, frequent bullying victimization and physical maltreatment. Based on evidence that the effects of stress are cumulative,33 we hypothesized that cumulative exposure to violence will be associated with accelerated TL erosion, already at a young age.
PARTICIPANTS AND METHODS
Participants
Participants were members of the Environmental-Risk (E-Risk) Study, which tracks the development of a birth cohort of 2232 British children. The sample was drawn from a larger birth register of twins born in England and Wales in 1994 – 1995.34 Full details about the sample are reported elsewhere.35 Briefly, the E-Risk sample was constructed in 1999 – 2000, when 1116 families with same-sex 5-year-old twins (93% of those eligible) participated in home-visit assessments. Families were recruited to represent the UK population of families with newborns in the 1990s, based on (a) residential location throughout England and Wales and (b) mother’s age (that is, older mothers having twins via assisted reproduction were under-selected and teenage mothers with twins were over-selected). We used this sampling (a) to replace high-risk families who were selectively lost to the register via nonresponse and (b) to ensure sufficient numbers of children growing up in high-risk environments. Home visits were conducted when the children were aged 5, 7, 10 and, most recently, 12 years (96% participation). DNA samples were collected via buccal swabs at ages 5 and 10 years. The Joint South London and Maudsley and the Institute of Psychiatry NHS Ethics Committee (UK) approved each phase of the study.
As costs of measuring TL in all cohort children nationwide exceeded study resources, a subsample of 118 Caucasian families (236 children) was used for this study, comprising families who: (a) lived proximal to London (the city of our research lab), (b) had MZ twins, and (c) had violence exposure in one or both twins or (d) were lacking any violence exposure but matched on socioeconomic status and child sex.36,37 The subsample is compared with the original cohort on Table 1.
Table 1.
Characteristics of the sample and of children who were victimized
| N | % Females | % Socioeconomic deprivationa | BMIb | Mean TL (s.e.) at age 5c | Mean TL (s.e.) at age 10c | % Telomere lengtheningd | |
|---|---|---|---|---|---|---|---|
| Cumulative exposure | |||||||
| None | 128 | 46.9 | 30.5 | 17.0 | 1.11 (0.04) | 0.99 (0.03) | 16.4 |
| 1 | 69 | 53.6 | 36.2 | 18.4 | 1.04 (0.06) | 0.95 (0.03) | 17.4 |
| 2+ | 39 | 48.7 | 66.7 | 17.6 | 1.04 (0.07) | 0.84 (0.05) | 17.9 |
| Domestic violence | 40 | 60 | 65 | 18.3 | 1.09 (0.07) | 0.91 (0.05) | 27.5 |
| Bullying victimization | 57 | 50.9 | 50.9 | 17.9 | 1.02 (0.06) | 0.90 (0.04) | 17.5 |
| Physical maltreatment | 63 | 49.2 | 47.6 | 17.9 | 1.03 (0.06) | 0.88 (0.04) | 14.3 |
| Total sample | 236 | 49.2 | 38.1 | 17.5 | 1.08 (0.03) | 0.96 (0.02) | 16.9 |
| Original cohort | 2232 | 51.1 | 33.2 | 17.4 | Not applicable | Not applicable | Not applicable |
Abbreviations: BMI, body mass index; TL, telomere length.
Socioeconomic deprivation was constructed from a standardized composite of household income, parents’ highest education and parents’ highest occupational grade. Deprivation was defined as the lowest tertile of the distribution in the entire cohort.
BMI was constructed from parents’ reports of children’s height and weight, and computed as weight (kg)/(height (m2)).
Mean TL at ages 5 and 10 years adjusted for sex, socioeconomic deprivation and BMI at age 10 years.
Defined as >15% increase in TL.
Measurement of mean relative TL
DNA was extracted using standard procedures.38 – 40 Age-5 and age-10 DNA were stored at −80 °C until assayed, to prevent degradation of the samples. All DNA samples were assayed for TL at the same time, independently of caseness and all operations were carried out by a laboratory technician blind to violence exposure.
TL was measured using a validated quantitative-PCR method,41 which determines mean TL across all chromosomes for all cells sampled (Supplementary Information). Briefly, the method involves two quantitative PCR reactions for each subject; one for a single-copy gene (S) and the other in the telomeric repeat region (T). Given each of these reactions is compared with a standard reference sample before the (T)/(S) ratio is calculated, the ratio will equal 1 when the experimental sample is identical to the reference sample. All DNA samples were run in triplicate for telomere and single-copy reactions at both age-5 and age-10, that is, 12 reactions per study subject.
Relative quantities of samples for each of the reactions were calculated as follows: first, reaction efficiencies (E) were calculated from the standard curve slope using E = 10(1/−slope), where at 100% efficiency E = 2. Next, relative quantities (RQs) were calculated using RQ = EΔCt where ΔCt is the difference between the average triplicate Ct value of the within-plate mean Ct, and the Ct value of the individual sample. Sample RQ values were calculated for each reaction separately (T and S). Finally, the TL relative to the amount of single-copy transcript was calculated using the ratio RQ(T)/RQ(S). The coefficient of variation (CV) for the triplicate samples was 0.97% for the telomere (T) reaction and 0.49% for the single-copy gene (S) reaction, indicating low measurement error.
Exposure to violence
We assessed three kinds of violence experiences between ages 5 and 10 years: exposure to domestic violence between the mother and her partner, frequent bullying victimization and physical maltreatment by an adult. We interviewed mothers (or the primary caregiver) about each exposure when the children were 5, 7 and 10 years of age and compiled a cumulative record of each child’s exposure to violence. We have previously reported evidence on reliability and validity in this research for the measures of domestic violence,42 bullying victimization43,44 and physical maltreatment by an adult.45 – 47
Domestic violence was assessed with the Conflict Tactics Scale,48 inquiring about 12 acts of physical violence (for example, kicking a partner, threatening a partner with a knife). The physical violence acts reported at three ages were summed and children who grew up in families who scored in the top 10% of the distribution of partner violence were coded as exposed to domestic violence. Of the children studied here, 17.0% (N = 40) were exposed to domestic violence.
Bullying victimization was assessed by explaining to mothers that ‘someone is being bullied when another child (1) says mean and hurtful things, makes fun, or calls a person mean and hurtful names; (2) completely ignores or excludes someone from their group of friends or leaves them out of things on purpose; (3) hits, kicks, or shoves a person, or locks them in a room; (4) tells lies or spreads rumors about them; or (5) does other hurtful things like these. We call it bullying when these things happen often and it is difficult for the person being bullied to stop it happening. We do not call it bullying when it is done in a friendly or playful way.’ Narratives recorded by interviewers were later checked by an independent rater to verify that the events described were instances of bullying, operationally defined as evidence of repeated harmful actions between children where there was a power differential between the bully and the victim.43,44 Of the children studied here, 24.2% (N = 57) were frequently bullied.
Physical maltreatment by an adult was assessed using a standardized clinical interview protocol49 designed to enhance mothers’ comfort with reporting valid child maltreatment information, while also meeting researchers’ responsibilities for referral under the UK Children Act. No family has left the study following intervention. When mothers reported any maltreatment, interviewers followed with standardized probes (for example, accidental harm was ruled out, and sexual abuse was queried directly and was included in the measure). Over the years of data collection, the study maintained a cumulative dossier for each child, comprising recorded debriefings with interviewers who had coded any indication of maltreatment at any of the three successive home visits, recorded narratives of the three successive caregiver interviews and information from clinicians whenever the study made a referral. Based on review of each child’s cumulative dossier, two clinical psychologists (TEM and the project coordinator) reached consensus for whether physical maltreatment had occurred. Examples of maltreatment in E-risk children included: the mother smacked the child weekly leaving marks or bruises; child was repeatedly beaten by a young adult step-sibling; child was routinely smacked by father when drunk ‘just to humiliate him’; child was fondled sexually and often slapped by the mothers’ boyfriend. Many, but not all cases identified in the course of our research were under investigation by police or social services, already on the child-protection register, or in foster care at follow-up, having been removed from their parents because of abuse. Of the children studied here, 26.7% (N = 63) were maltreated by an adult.
Finally, we summed each type of violence exposure to create an index of cumulative violence exposure: 54.2% (N = 128) of the children were not violence exposed, 29.2% (N = 69) were exposed to one type of violence (domestic violence, N = 14; bullying victimization, N = 28; physical maltreatment, N = 27), and 16.5% (N = 39) were exposed to two or more types of violence (domestic violence, N = 26; bullying victimization, N = 29; physical maltreatment, N = 36).
Table 1 summarizes information about the characteristics of the sample and about groups of children who were victimized.
Covariates
Socioeconomic deprivation was constructed from a standardized composite of household income, parents’ highest education and parents’ highest occupational grade. Deprivation was defined as the lowest tertile of the distribution in the entire cohort.
Body mass index was constructed from parents’ reports of children’s height and weight, and computed as weight (kg)/height (m2).
Children’s health
Mothers reported about their children’s health, on a four-point scale (0 = poor; 3 = very good); 8.9% (N = 21) of the children were rated as 0 or 1. In addition, we collected information from mothers about each child’s asthma, using an event history calendar for recording children’s history of chronic health conditions; 12.3% (N = 29) of the children were asthmatic.
Statistics
We tested the main hypothesis that violence exposure would be associated with telomere erosion between ages 5 and 10 years using ordinary least squares multiple regression analysis. The outcome variable was age-10 TL, controlling for baseline TL at age-5 years and sex, socioeconomic deprivation and body mass index at age-10 years as covariates. As each study family contains two children, all statistical analyses were corrected conservatively for the non-independence of the two twin observations per family, by using tests based on the sandwich or Huber/White variance estimator50 in SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
Descriptive statistics about TL during childhood
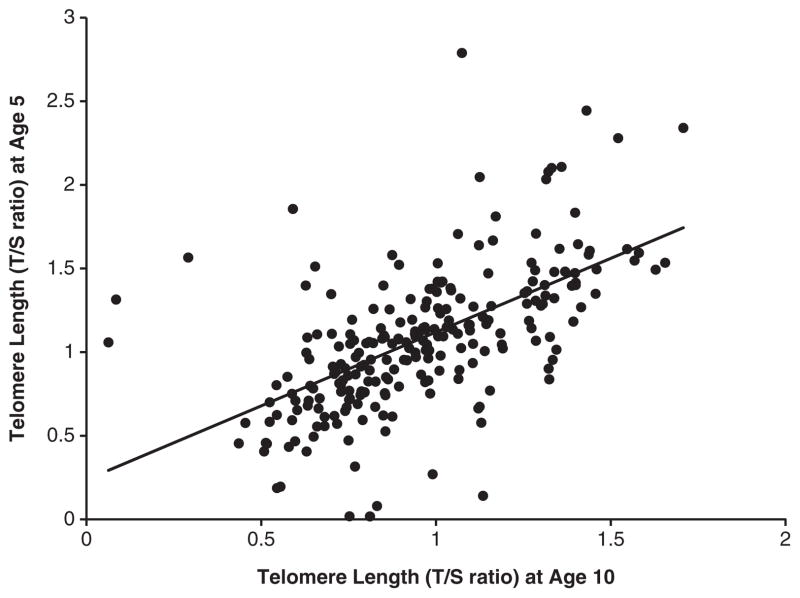
TL at age-5 years was significantly and positively correlated with TL at age-10 years (r = 0.57, P<0.001; Figure 1). Despite this significant rank-order stability, there was also significant change. There was a significant decline in mean TL from age-5 to age-10 for the whole sample (General Linear Model repeated measures: F(353,1) = 18.49, P<0.001). The average baseline TL at age-5 was 1.08 T/S (s.d. = 0.44), and ranged from 0.02 to 3.07 T/S. Follow-up TL at age-10 averaged 0.96 T/S (s.d. = 0.28) and ranged from 0.06 to 1.71 T/S. These means are similar to those reported by the two other studies of TL in children.25,51
Figure 1.
Association between telomere length at 5 and 10 years of age.
Does exposure to violence accelerate telomere erosion?
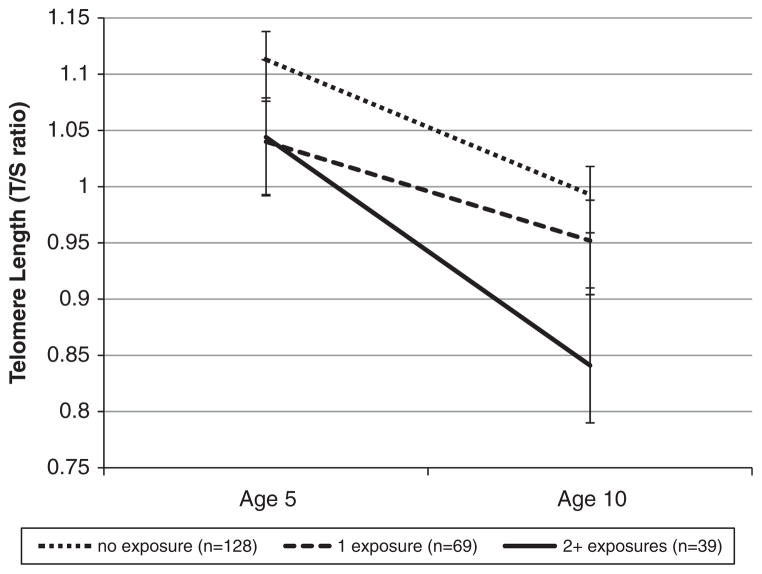
We tested our main hypothesis that cumulative violence exposure was associated with TL change during childhood. Children who experienced two or more types of violence exposure showed significantly accelerated TL erosion from baseline to follow-up measurement compared with children who had one type of violence exposure or who were not exposed to violence (B = −0.052, s.e. = 0.021, P = 0.015) (Figure 2). This association remained significant when we added controls for poor health and asthma to the analysis (B = −0.047, s.e. = 0.021, P = 0.028).
Figure 2.
Association between cumulative violence exposure and telomere length at 5 and 10 years of age.
We also tested the association between TL erosion and each violence exposure separately (Table 1). Consistent with the analysis of cumulative stress, the individual violence exposures all predicted more rapid telomere erosion, but this acceleration was statistically significant only in the case of exposure to physical maltreatment (for domestic violence, B = −0.056, s.e. = 0.043, P = 0.196; for bullying victimization, B = −0.037, s.e. = 0.037 P = 0.311; for physical maltreatment, B = −0.084, s.e. = 0.035, P = 0.018). In a supplementary analysis, we compared non-exposed children (N = 128), children exposed only to physical maltreatment (N = 27), and children with two or more exposures (including physical maltreatment and another violence exposure, N = 36). The results showed that cumulative violence exposure was associated with TL change in childhood (B = −0.056, s.e. = 0.024, P = 0.020), implicating cumulative exposure rather than physical maltreatment alone.
Telomere lengthening?
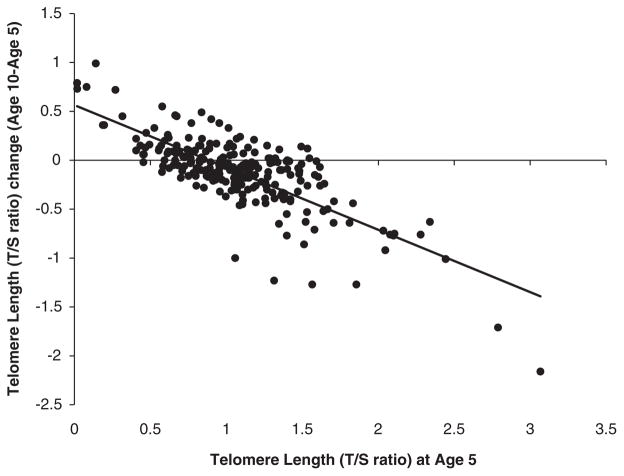
Consistent with previous repeated-measures longitudinal studies,26 – 31 we observed telomere lengthening in a minority of children (Table 1). In addition, consistent with previous repeated-measures studies,26,28,31 shorter TL at baseline predicted more lengthening from age-5 to age-10 years (Figure 3, r = −0.77, P<0.001). Given uncertainty about the interpretation of telomere lengthening,29,50 and the possibility that different mechanisms are likely to govern telomere shortening versus lengthening, we retested our main hypothesis of association between cumulative violence exposure and TL in individuals whose telomeres did not lengthen during the follow-up period. Following previous research,29 we defined lengthened telomeres as >15% increase in TL between measurements (16.9% of the sample). After excluding these children from the analysis, cumulative violence exposure remained significantly associated with accelerated telomere erosion at the same effect size (B = −0.057, s.e. = 0.024, P = 0.019).
Figure 3.
Association between baseline telomere length (TL) at age 5 years and TL change from 5 to 10 years of age.
DISCUSSION
This study shows for the first time that cumulative violence exposure is associated with accelerated TL erosion in children, from baseline to follow-up, for children who experienced violence at a young age. Adjusting for sex, socioeconomic deprivation and body mass index, as well as children’s health, children who were exposed to multiple forms of violence had the fastest TL erosion rate.
The methodological strengths of this study include a longitudinal design with reliable and valid prospective assessments of multiple violence exposures during childhood and repeated measurements of TL during this same developmental period. Previous studies that have documented the association between childhood stress and TL have relied on adult measures of TL and retrospective recall of stress years after the stress was experienced.20 – 23 In cross-sectional studies with a single measurement point for TL, it has not been possible to assess the association between stress exposure and change in TL over time. Moreover, given the elapsed time between the putative stress exposure and the measurement of TL, it has not been clear whether telomeres began eroding during stress exposure or whether erosion occurred years later, possibly promoted by the sequelae of childhood stress or other intervening variables. For example, given that maltreated children often grow up to be in poor physical health as adults52,53 telomere erosion could be a consequence of later health problems, as opposed to a proximal effect of maltreatment itself. This study provides evidence that childhood stress is related to telomere erosion over time, and while children are experiencing stress.
The results of this study underscore some of the complexities involved in research on psychosocial stress and telomere erosion. First, although each kind of violence exposure was associated with telomere erosion in the predicted direction, the most significant effect was observed between multiple violence exposures and telomere erosion. These findings are consistent with theory that the effect of stress is seen most clearly when stress is measured in a cumulative way.8,33 As more studies accumulate, it will be possible to interrogate the specific features of a stressor that matter most in relation to telomere erosion (for example, duration, severity, physical harm, perceived threat). Second, some of the complexities revealed in recent reports using repeated measurements of TL were confirmed here, including that in some individuals telomeres can lengthen.26 – 31 Interestingly, most ‘lengtheners’ in our and others’ studies have shorter TL at baseline.26,28,31 Observed lengthening could result from (a) measurement error, (b) regression to the mean, (c) differences in cells sampled at different ages (even if the tissue that is sampled is held constant, as in our study) or (d) meaningful telomere dynamics involving telomerase activity or alternative lengthening of telomeres in cells that lack telomerase activity.54,55 As TL can predict health outcomes, the mechanisms that regulate TL are of great interest and demand more investigation.
The results of this study raise the question of what biological processes explain the association between psychosocial stress and telomere erosion. Most of the insights about mechanisms associated with telomere erosion originate from research on oxidative stress and inflammation, indicating both as important influences on TL.56 – 59 Telomeres are sensitive to damage by oxidative stress, as demonstrated by experiments showing increased erosion under conditions of high reactive oxygen species in vitro.56 Inflammation is associated with increased proliferation of immune cells and, as a consequence, with more telomere erosion.60 Childhood maltreatment predicts elevated inflammation,61 suggesting a possible cause for the increased telomere erosion observed in this study. A study of Alzheimer’s disease patients’ caregivers demonstrated association between TL and altered T-cell function, as well as immune markers.62 Research is needed to test whether effects of psychosocial stress on telomere erosion are mediated by oxidative stress and immune-system changes in children.
This study has several limitations. First, there are tradeoffs regarding the best ways to measure TL. The two main methods are southern blot analysis of the terminal restriction fragments63 and quantitative reverse transcriptase-PCR to measure the ratio between a single-copy gene and telomeric repeat region (T/S ratio).41 The T/S ratio was used in this study. Its principal advantage is that it can be performed at high-throughput and low-cost. However, the T/S ratio can suffer measurement error; prior studies denote a CV range between 0.9 and 28%. In this study, we observed low measurement error; CV was <1% in all assays. Moreover, measurement errors should primarily affect assays among individuals with a slower rate of telomere erosion,26 – 28 whereas slower erosion is more common in adults than children.32
A second limitation concerns the measurement of TL in different tissues.64 As a result of ethical difficulties obtaining blood from children in the community, this study and two others of children have used buccal cells,25,51 instead of the peripheral blood cells more commonly used in studies of adults. Buccal swabs may not only yield buccal cells, and it is possible that infiltration of immune cells because of poor oral hygiene or infection could alter oral cell composition, which has different telomere dynamics than buccal cells. Positive correlations have been reported between TL from buccal cells and TL from blood.65 In addition, buccal cell TL has been associated with age-related disease,66 although one report showed no significant correlation between TL from buccal cells and white blood cells.67 Our and others’ studies suggest that estimating TL from buccal cells can detect effects of stress, although validation in larger studies that measure TL from multiple tissues is needed.
Third, our sample comprised MZ twins. However, to our knowledge there is no evidence that MZ twins react differently to violence or have different TL dynamics than singletons. Unfortunately, we were unable to compare TL in twin siblings discordant for violence exposure, as there were too few discordant pairs for adequate statistical power. We intend to attempt a discordant twin comparison in our larger cohort in the future. Fourth, our first TL measure was taken at age-5 years, but some mothers reported their children experienced violence before age-5 as well as after. Figure 2 hints at this possibility by showing that children with violence exposure already had shorter TL at age-5 than children without violence exposure. Nevertheless, children with multiple violence exposures showed subsequent accelerated erosion between ages 5 to 10 years.
In conclusion, our findings provide evidence that stress-related accelerated telomere erosion can be observed already in childhood. This suggests the importance of integrating telomeres as stress markers in research to evaluate the effects of maltreatment on young victims. TL measurement is now offered to adults as a diagnostic tool to monitor health and predict disease risk.68 It is conceivable that research may eventually implicate TL measurement in clinical pediatrics. However, extreme caution should be taken as more research is needed to uncover mechanisms that govern TL dynamics. Meanwhile, our findings help to address the basic-science puzzle of how and when childhood stress gets ‘under the skin’ at the cellular level.
Supplementary Material
Acknowledgments
The first author is supported by NICHD Grant HD061298 and by the Jacobs Foundation. Dr Andrea Danese was supported by the 2009 NARSAD/Brain and Behaviour Research Foundation Young Investigator Award. The E-Risk Study is funded by the Medical Research Council (UKMRC Grants G1002190 and G9806489). Additional support was provided by NIA Grant AG032282, ESRC Grant RES-177-25-0013, NICHD Grant HD061298, and by funds from the Jacobs Foundation, the British Academy, the Nuffield Foundation and NIMH Grant MH077874. We are grateful to the study mothers, fathers and the twins for their participation, and to members of the E-Risk team for their dedication, hard work and insights.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med. 2009;71:805– 812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- 2.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun HJ, Todd TJ, Kawachi I, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med. 2010;39:529– 536. doi: 10.1016/j.amepre.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. J Epidemiol Community Health. 2010;64:413– 418. doi: 10.1136/jech.2009.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761– 1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 5.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry. 2012;169:141– 151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 6.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959– 997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resilience in children. Ann NY Acad Sci; Proceedings of a conference sponsored by the New York Academy of Sciences and Brown Medical School; Arlington, VA, USA . 2006. pp. 1– 368. [PubMed] [Google Scholar]
- 8.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29– 39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Epel ES. Telomeres in a life-span perspective: a new “psychobiomarker”? Curr Dir Psychol Sci. 2009;18:6– 10. [Google Scholar]
- 10.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585– 621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 11.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181– 190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 12.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458– 460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 14.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662– 664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 15.Buxton JL, Walters RG, Visvikis-Siest S, Meyre D, Froguel P, Blakemore AI. Childhood obesity is associated with shorter leukocyte telomere length. J Clin Endocrinol Metabol. 2011;96:1500– 1505. doi: 10.1210/jc.2010-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordfjall K, Eliasson M, Stegmayr B, Melander O, Nilsson P, Roos G. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008;16:2682– 2689. doi: 10.1038/oby.2008.413. [DOI] [PubMed] [Google Scholar]
- 17.Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci. 2008;33:244– 247. [PMC free article] [PubMed] [Google Scholar]
- 18.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432– 435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312– 17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16– 22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010;67:531– 534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PloS One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465– 471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biol Psychiatry. 2010;68:e21–e22. doi: 10.1016/j.biopsych.2010.02.026. author reply e23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry. 2011 May 17; doi: 10.1038/mp.2011.53. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323– 329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol Ser A Biol Sci Med Sci. 2011;66:312– 319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, et al. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38:1725– 1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- 29.Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging. 2009;1:81– 88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PloS One. 2010;5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordfjall K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5:e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeichner SL, Palumbo P, Feng Y, Xiao X, Gee D, Sleasman J, et al. Rapid telomere shortening in children. Blood. 1999;93:2824– 2830. [PubMed] [Google Scholar]
- 33.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245– 258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 34.Trouton A, Spinath FM, Plomin R. Twins early development study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Res Off J In Soc Twin Stud. 2002;5:444– 448. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- 35.Moffitt TE. Teen-aged mothers in contemporary Britain. J Child Psychol Psychiatry Allied Disciplines. 2002;43:727– 742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- 36.Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, et al. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol Psychiatry. 2011;70:1016– 1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry. 2011;16:244– 246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowtell DD. Rapid isolation of eukaryotic DNA. Anal Biochem. 1987;162:463– 465. doi: 10.1016/0003-2697(87)90421-0. [DOI] [PubMed] [Google Scholar]
- 39.Jeanpierre M. A rapid method for the purification of DNA from blood. Nucleic Acids Res. 1987;15:9611. doi: 10.1093/nar/15.22.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet. 2003;33:67– 72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- 41.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moffitt TE, Caspi A, Krueger RF, Magdol L, Margolin G, Silva PA, et al. Do partners agree about abuse in their relationship? A psychometric evaluation of interpartner agreement. Psychol Assessment. 1997;9:47– 56. [Google Scholar]
- 43.Arseneault L, Walsh E, Trzesniewski K, Newcombe R, Caspi A, Moffitt TE. Bullying victimization uniquely contributes to adjustment problems in young children: a nationally representative cohort study. Pediatrics. 2006;118:130– 138. doi: 10.1542/peds.2005-2388. [DOI] [PubMed] [Google Scholar]
- 44.Shakoor S, Jaffee SR, Andreou P, Bowes L, Ambler AP, Caspi A, et al. Mothers and children as informants of bullying victimization: results from an epidemiological cohort of children. J Abnormal Child Psychol. 2011;39:379– 387. doi: 10.1007/s10802-010-9463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaffee SR, Caspi A, Moffitt TE, Taylor A. Physical maltreatment victim to antisocial child: evidence of an environmentally mediated process. J Abnormal Psychol. 2004;113:44– 55. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- 46.Jaffee SR, Caspi A, Moffitt TE, Polo-Tomas M, Price TS, Taylor A. The limits of child effects: evidence for genetically mediated child effects on corporal punishment but not on physical maltreatment. Dev Psychol. 2004;40:1047– 1058. doi: 10.1037/0012-1649.40.6.1047. [DOI] [PubMed] [Google Scholar]
- 47.Jaffee SR, Caspi A, Moffitt TE, Polo-Tomas M, Taylor A. Individual, family, and neighborhood factors distinguish resilient from non-resilient maltreated children: a cumulative stressors model. Child Abuse Neglect. 2007;31:231– 253. doi: 10.1016/j.chiabu.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the Parent-Child Conflict Tactics Scales: development and psychometric data for a national sample of American parents. Child Abuse Neglect. 1998;22:249– 270. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- 49.Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250:1678– 1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- 50.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645– 646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 51.Kroenke CH, Epel E, Adler N, Bush NR, Obradovic J, Lin J, et al. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom Med. 2011;73:533– 540. doi: 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135– 1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental health consequences of childhood physical abuse: results from a large population-based sample of men and women. Child Abuse Neglect. 2007;31:517– 530. doi: 10.1016/j.chiabu.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slatter TL, Tan X, Yuen YC, Gunningham S, Ma SS, Daly E, et al. The alternative lengthening of telomeres pathway may operate in non-neoplastic human cells. J Pathol. 2012;226:509– 518. doi: 10.1002/path.2981. [DOI] [PubMed] [Google Scholar]
- 55.Svenson U, Nordfjall K, Baird D, Roger L, Osterman P, Hellenius ML, et al. Blood cell telomere length is a dynamic feature. PloS One. 2011;6:e21485. doi: 10.1371/journal.pone.0021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339– 344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 57.O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PloS One. 2011;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiels PG, McGlynn LM, MacIntyre A, Johnson PC, Batty GD, Burns H, et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PloS One. 2011;6:e22521. doi: 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilmonen P, Kotrschal A, Penn DJ. Telomere attrition due to infection. PloS One. 2008;3:e2143. doi: 10.1371/journal.pone.0002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goronzy JJ, Fujii H, Weyand CM. Telomeres, immune aging and autoimmunity. Exp Gerontol. 2006;41:246– 251. doi: 10.1016/j.exger.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319– 1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249– 4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allshire RC, Dempster M, Hastie ND. Human telomeres contain at least three types of G-rich repeat distributed non-randomly. Nucleic Acids Res. 1989;17:4611– 4627. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hewakapuge S, van Oorschot RA, Lewandowski P, Baindur-Hudson S. Investigation of telomere lengths measurement by quantitative real-time PCR to predict age. Leg Med (Tokyo) 2008;10:236– 242. doi: 10.1016/j.legalmed.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging. 2010;2:867– 874. doi: 10.18632/aging.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26:1263– 1271. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- 67.Thomas P, NJOC, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev. 2008;129:183– 190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Wolinsky H. Testing time for telomeres. Telomere length can tell us something about disease susceptibility and ageing, but are commercial tests ready for prime time? EMBO Rep. 2011;12:897– 900. doi: 10.1038/embor.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.