Abstract
Background
Deletion of 3p is one of the most frequent genetic alterations in esophageal squamous cell carcinoma (ESCC), suggesting the existence of one or more tumor suppressor genes (TSGs) within these regions. In this study, one TSG, CACNA2D3 at 3p21.1, was characterized.
Methods
Expression of CACNA2D3 in ESCCs was tested by quantitative real-time PCR and tissue microarray. The mechanism of CACNA2D3 downregulation was investigated by methylation-specific polymerase chain reaction (MS-PCR). The tumor suppressive function of CACNA2D3 was characterized by both in vitro and in vivo tumorigenic assays, cell migration and invasion assays.
Results
CACNA2D3 was frequently downregulated in ESCCs (24/48, 50%), which was significantly associated with promoter methylation and allele loss (P<0.05). Tissue microarray result showed that downregulation of CACNA2D3 was detected in (127/224, 56.7%) ESCCs, which was significantly associated with lymph node metastasis (P = 0.01), TNM staging (P = 0.003) and poor outcome of ESCC patients (P<0.05). Functional studies demonstrated that CACNA2D3 could inhibit tumorigenicity, cell motility and induce apoptosis. Mechanism study found that CACNA2D3 could arrest cell cycle at G1/S checkpoint by increasing expressions of p21 and p53 and decreasing expression of CDK2. In addition, CACNA2D3 could upregulate intracellular free cytosolic Ca2+ and subsequently induce apoptosis.
Conclusion
CACNA2D3 is a novel TSG responsible to the 3p21 deletion event and plays a critical suppressing role in the development and progression of ESCC.
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most common cancers with a very poor outcome in China [1]. ESCC is characterized by its remarkable geographic distribution and high-risk areas include Northern China, Northern Iran and South Africa [2]. Although genetic alterations have been widely studied in ESCC, the precise mechanisms underlying esophageal carcinoma are poorly understood. Previous study in high-risk area suggested that genetic susceptibility might play a role in the pathogenesis of ESCC [3]. Like other solid tumors, the development of ESCC is also believed as a multi-stage process caused by the stepwise accumulation of genetic alterations. Comparative genomic hybridization and loss of heterozygosity studies found that deletion of 3p was one of the most frequent genetic alterations in ESCC [4]–[5], suggesting the existence of one or more tumor suppressor genes within these frequently deleted regions. Recently, single-nucleotide polymorphism (SNP)-mass array was applied to investigate the loss of heterozygosity at 3p in 100 primary ESCC cases, leading to the identification of four commonly deleted regions on 3p including 3p21 [6]. Two candidate TSGs, PLCD1 at 3p22 and PCAF at 3p24 have been characterized for their tumor suppressing functions and mechanisms [7]–[8].
In the present study, another candidate TSG, CACNA2D3 (calcium channel, voltage dependent, alpha-2/delta subunit 3) at 3p21.1, was characterized for its tumor suppressive function and mechanism. CACNA2D3 is an auxiliary member of the alpha-2/delta subunit family of the voltage-dependent calcium channel complex. Similar to CACNA2D2, it also regulates the influx of calcium ions entering the cell upon membrane polarization [9]. There are four calcium channel voltage-dependent alpha-2/delta subunit genes, CACNA2D1 to CACNA2D4 [10]. Frequent allele loss of CACNA2D2 has been reported in lung, breast and other cancers [11]. One report indicated that CACNA2D2 could mediate apoptosis in non-small cell lung cancer cells [12]. Another study found that promoter methylation of CACNA2D3 was frequently detected in gastric cancer, which was associated with poor prognosis of the disease [13]. Growing evidence showed that Ca2+ signaling regulates diverse cellular processes such as fertilization, development, proliferation, learning and memory, and cell death [9]. Although CACNA2D3 has been associated with the poor outcome of gastric cancer [13], the effect of CACNA2D3 on ESCC development is not clear. In the present study, expression of CACNA2D3 in ESCC was detected in primary ESCC and ESCC cell lines. Both in vitro and in vivo assays were used to characterize the potential tumor suppressive function of CACNA2D3.
Materials and Methods
Cell Lines and Primary Tumor Tissues
ESCC cell lines KYSE30, KYSE140, KYSE180, KYSE410, and KYSE510 were obtained from DSMZ, the German Resource Center for Biological Material [14]. Chinese ESCC cell line HKESC1 was kindly provided by Professor G Srivastava [15]. The cells were confirmed by cytogenetics as human origin in 2009 [16]. Patients with ESCC were selected consecutively from the surgical pathology archives of the Linzhou Cancer Hospital (Henan, China). None of the patients in the study had received preoperative radiation or chemotherapy. Tissue samples used in the study were approved by the Committees for Ethical Review of Research Involving Human Subjects in Zhengzhou University (Zhengzhou, China). Written informed consents for the original human work that produced the tissue samples were obtained.
Tissue Microarray and Immunohistochemistry
Tissue microarray containing 300 pairs of primary ESCC (tumor and non-tumor tissues) cases was constructed as described in previous report [16]. Corresponding matched non-tumor tissues were obtained about 3 cm away from the tumor tissues (on average). None of the patients in this study had received follow-up radiation or chemotherapy. The age of patients ranged from 40–80 years at the time of surgery (median age: 59 years) and the male/female ratio was 1.3∶1. Immunohistochemistry (IHC) was performed using the standard streptavidin-biotin-peroxidase complex method. A 1∶100 diluted anti-CACNA2D3 (Novus Biologicals, Littleton, CO) antibody was used for CACNA2D3 detection. CACNA2D3 expression was compared between tumor and paired non-tumor tissues.
Methylation Analysis
DNA extraction, bisulfate treatment, and MS-PCR was performed as described previously [7]. Briefly, FastStart Taq DNA Polymerase (Roche, IN) was utilized in the reaction, and the cycle number was 40 which was within the linear amplification range. The primers’ sequences of CACNA2D3 for methylation analyses were: CAC-M-F: 5′-TATTTCGAAATTTAGGGTGTTTTTC-3′; CAC-M-R: 5′-GATACTA CCACCACGACTTAAACG-3′; CAC-U-F: 5′-GTGGTGTGTTTGGAGTAGTAGAT ATT-3′; CAC-U-R: 5′-CCAAACTTAAACACAATAAATCACA-3′.
LOH Detection in Tissue Samples and Fluorescence in situ Hybridization (FISH)
Genomic DNA was extracted from tumor samples using TIANamp genomic DNA kit (TIANGEN, China). SNP site (rs589281) within CACNA2D3 gene was PCR amplified and sequencing analyzed with primers (CAC-SNP-F1∶5′ TGTTGTGAT GATTAGGTGAG-3′; CAC-SNP-R1∶5′ CTGTGGAGAATCACCTAATTC-3′). The BAC probe was labeled and FISH was performed as previously described [17].
Establishment of Cell Lines with Ectopic CACNA2D3 Expression
CACNA2D3 was cloned into expression vector pcDNA3.1(+) and then transfected into KYSE30 and KYSE510 cells using lipofectamine™ 2000 (Invitrogen, Calsbad, CA). Empty vector was transfected into cell lines as negative controls. Stable colonies were screened by G418 at 500 µg/ml.
RNA Extraction and Quantitative Real-time PCR (qRT-PCR)
RNA was extracted from tissues and cultured cells using Trizol (Invitrogen, Calsbad, CA). Reverse transcription was performed using SuperScript III (Invitrogen, Calsbad, CA). qRT-PCR was processed using SYBR Green Supermix and ABI7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). (CAC-Fq: 5′-AGGGA TTCACGGTTATGCCTT-3′; CAC-Rq: 5′-GCCACACCTAAACCCTTTGTC-3′). Triplicate assays were done and values were normalized by the internal control (18S rRNA or GAPDH). PCR products were subjected to dissociation curve analysis to exclude amplification of nonspecific products. Quantitative of the PCR data was processed using the ΔCT method as described previously [18]. 2-fold was considered the cutoff for ascertaining CACNA2D3 downregulation.
Antibodies and Reagents
Antibodies used: CACNA2D3 (Novus Biologicals, Littleton, CO), GAPDH, p53, p21, Cyclin E, Cyclin A, CDK2, E-cadherin, Caspase 3, and Caspase 8 (Cell Signaling Technology, Danvers, MA). siRNA targeting CACNA2D3 was from Origene (MD).
In vitro Tumor Suppressive Assays
The effect of CACNA2D3 overexpression on cell proliferation was assessed by determining cell growth and viability with the use of CCK-8 (Dojindo, Japan). Foci formation assay and colony formation in soft agar was performed as described [16]. Triplicate independent assays were performed.
In vivo Tumor Suppressive Assay
The study was approved by Institutional Animal Care and Use Committee of Cancer Cancer, Sun Yat-sen University. Animal experiments were performed in compliance with the guidelines for the Welfare of Experimental Animals in Cancer Center, Sun Yat-sen University. The tumorigenicity of cells was assayed by tumor formation in nude mice. CACNA2D3- and vector-transfected cells (2×106) cells were subcutaneously injected into the flanks of 4-week-old male athymic BALB/c nu/nu mice (n = 5 for KYSE30 cells; n = 4 for KYSE510 cells), respectively. Tumor growth was checked twice a week. Following euthanasia, tumors were excised, fixed in 10% formalin and embedded in paraffin block for IHC study.
Cell Migration and Invasion Assays
Transwell Permeable Support (24-well plate) (Corning Incorpotated, NY) was used to assess the rate of cell migration. Briefly, 4×104 cells in 100 µl of serum-free medium were added to the upper chamber of the transwell insert. The lower chamber was filled with 600 µl medium with 10% fetal bovine serum. After 22 hr of incubation at 37°C, penetrated cells to the lower surface of the filter were fixed, stained with Crystal Violet, and counted under a microscope. The assay was repeated three times. For invasion assay, BioCoat™ Matrigel™ Invasion Chamber (24-well plate) (Becton Dickinson and company, Franklin Lakes, NJ) was used according to the manufacture’s protocol.
Cell Cycle Analysis
Tested cells (1×106) were fixed in 70% ethanol, stained with propidium iodide (Sigma-Aldrich, Germany) and DNA content was analyzed by Cytomics FC 500 (BECKMAN COULTER, Fullerton, CA). Cell cycle profile was then analyzed with Modfit LT 2.0. Three independent assays were performed.
Measurement of Cytosolic Free Calcium
The intracellular free Ca2+ was measured by FACS with free-Ca2+-sensitive Fluo3-AM green fluorescence probe (Sigma-Aldrich, Germany). Cells were washed and incubated with 5 µM probe diluted in medium without serum for 60 min at 37°C. Cells were then washed and incubated with PBS for 30 min at 37°C in the dark to allow cellular esterases to cleave the acetoxymethyl group of Fluo 3-AM. Cells were trypsinized, washed and gently resuspended in PBS. Fluorescence intensity was measured by FACS analysis at an excitation wavelength of 488 nM and an emission wavelength of 530 nM. Three independent experiments were repeated.
Apoptosis Assay
Cells were treated with Staurosporine (STS, Sigma-Aldrich, Germany) (0.25 µM for KYSE30, 0.1 µM for KYSE510), then collected and analyzed 15 hr later. Apoptosis was detected by Annexin-V-FLUOS Staining Kit (Roche, Germany) and in situ Cell Death Detection Kit (Roche, Germany) according to the manufacture’s protocol. Triplicate independent experiments were performed.
Statistical Analysis
Statistical analyses were done using statistical software package (Version 16.0; SPSS, Inc., USA). Pearson Chi-square test was used to analyze the relationship between CACNA2D3 expression and clinic-pathological features. Survival curves were generated according to the Kaplan-Meier method and statistical analysis was performed by Log-rank test. The Cox proportional hazards regression model was used to identify the independent prognostic factors. Students’ t-test was used to analyze data from function analysis, migration and invasion, TUNEL, and in vivo tumor formation. P<0.05 was considered statistically significant.
Results
CACNA2D3 is Frequently Downregulated in ESCC
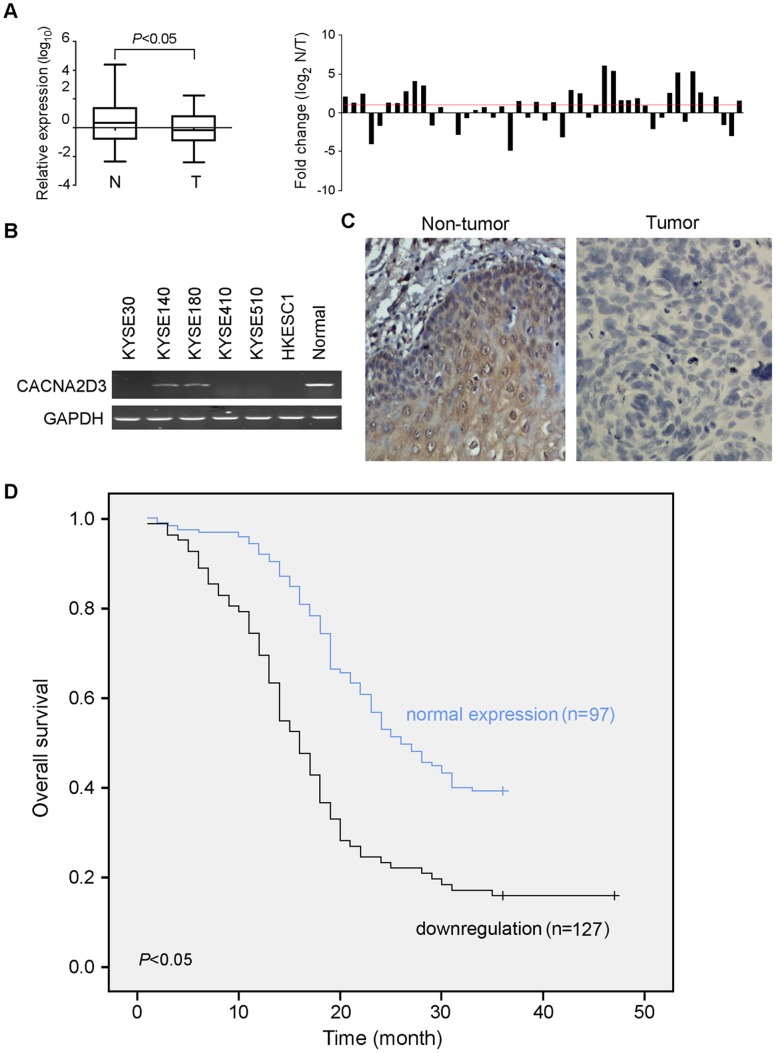
Expression of CACNA2D3 was compared between tumor and their paired non-tumor tissues in 48 ESCC patients by quantitative real-time PCR. The result showed that the downregulation of CACNA2D3 was detected in 24/48 (50%) of ESCC tumor tissues compared with their paired non-tumor tissues (Fig. 1A). The average expression level of CACNA2D3 was significantly reduced in tumor tissues compared with their paired non-tumor tissues (P<0.05). Downregulation of CACNA2D3 was also detected in 4 ESCC cell lines KYSE30, KYSE510, KYSE410 and HKESC1 (Fig. 1B).
Figure 1. Downregulation of CACNA2D3 in ESCC tumor tissues.
(A) qRT-PCR was used to compare CACNA2D3 expression between tumor (T) and non-tumor (N) tissues in 48 ESCCs. The result was normalized by GAPDH. Fold change (log2N/T) >1 (red line) was defined as “downregulation”. (B) Expression of CACNA2D3 in ESCC cell lines was detected by RT-PCR, compared to normal esophageal mucosa. (C) Representatives of CACNA2D3 expression (brown color) detected by IHC in a pair of ESCC tumor tissue and paired non-tumorous esophageal tissue (20×objective). (D) Kaplan-Meier analysis shows that CACNA2D3 downregulation is significantly associated with poor overall survival in 224 ESCC cases (P<0.05, Log-rank test).
Clinical Significance of CACNA2D3 Downregulation in ESCC
Expression of CACNA2D3 in protein level was studied by IHC using a tissue microarray containing 300 pairs (tumor and non-tumor tissues) of primary ESCCs. Informative results were obtained from 224 pairs ESCCs. Noninformative samples included lost or samples with too few tumor cells. Downregulation of CACNA2D3 was detected in 127/224 (56.7%) informative ESCC tumor tissues compared with their paired non-tumor tissues (Fig. 1C). Higher expression of CACNA2D3 in tumor tissue was observed in 13/224 (5.8%) of ESCCs. Clinical association analysis demonstrated that downregulation of CACNA2D3 was positively correlated with lymph node metastasis (P = 0.01) and advanced clinical staging (P = 0.003, Table 1). Univariate survival analysis revealed that downregulation of CACNA2D3 was significantly correlated with poor overall survival (P<0.05, Table 2 and Fig. 1D). CACNA2D3 downregulation as well as other clinicopathologic features which were significant in univariate analysis (e.g. tumor differentiation, lymph nodes metastasis and TNM stage) were examined in multivariate analysis (Table 2). The result showed that the downregulation of CACNA2D3 was not an independent risk factor for overall patient survival (P = 0.054).
Table 1. Association between CACNA2D3 and clinicopathologic features of ESCC.
| Clinicopathologic features | CACNA2D3 downregulation | P-value |
| Age (years old) | 0.686 | |
| ≤60 | 73/126 (57.9%) | |
| >60 | 54/98 (55.1%) | |
| Gender | 0.786 | |
| Male | 70/126 (55.6%) | |
| Female | 57/98 (58.2%) | |
| Tumor location | 0.273 | |
| Upper | 23/48 (47.9%) | |
| Middle | 90/149 (60.4%) | |
| Lower | 14/27 (51.9%) | |
| Differentiation | 0.068 | |
| Well | 15/26 (57.7%) | |
| Moderate | 93/147 (63.3%) | |
| Poor | 19/51 (37.3%) | |
| Tumor invasion | 0.409 | |
| T1+T2 | 13/27 (48.1%) | |
| T3+T4 | 114/197 (57.9%) | |
| Lymph nodes metastasis | 0.01 | |
| N0 | 47/100 (47%) | |
| N1 | 80/124 (64.5%) | |
| TNM stage | 0.003 | |
| I+IIa | 34/79 (43.0%) | |
| IIb+III+IV | 93/145 (64.1%) |
Table 2. Univariate and multivariate analysis of different prognostic variables in patients with ESCC.
| Variables | Univariable analysis* | Multivariable analysis* | ||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.193 (0.915–1.554) | 0.192 | ||
| Gender | 1.132 (0.867–1.477) | 0.363 | ||
| Tumor location | 0.916 (0.730–1.151) | 0.452 | ||
| Tumor invasion | ||||
| T1+T2 | 1.00 | |||
| T3+T4 | 1.395 (0.937–2.076) | 0.101 | ||
| Differentiation | ||||
| Poor | 1.00 | |||
| Well or moderate | 0.682 (0.503–0.924) | 0.014 | 0.688(0.504–0.938) | 0.018 |
| Lymph nodes metastasis | ||||
| N0 | 1.00 | |||
| N1 | 1.868 (1.428–2.442) | <0.001 | 1.716(1.227–2.402) | 0.002 |
| TNM stage | ||||
| I+ II a | 1.00 | |||
| II b+III+IV | 1.158 (1.044–1.286) | 0.006 | 1.063(0.854–1.324) | 0.583 |
| CACNA2D3 downregulation | 1.393 (0.994–1.953) | 0.047 | 1.442(1.026–2.027) | 0.054 |
Cox regression model; HR, Hazards ratio; CI, confidence interval.
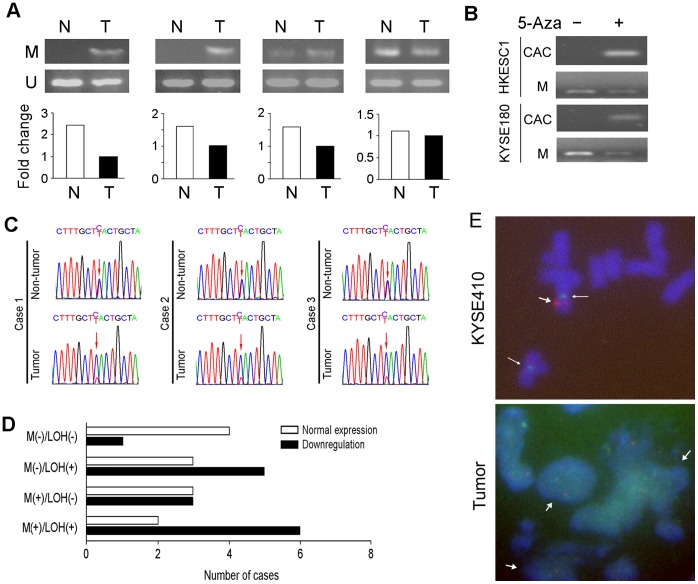
CACNA2D3 Downregulation is Associated with Promoter Methylation and Allele Loss
As a putative mechanism for the downregulation of CACNA2D3 in ESCCs, methylation status of a candidate CpG-rich promoter region was studied by methylation-specific PCR in 48 primary ESCCs. Promoter methylation was detected in 28/48 (58.3%) of tumor tissues (Fig. 2A). Downregulation of CACNA2D3 was detected in 17/28 (60.7%) methylated cases, which is higher than that in cases without promoter methylation (7/20, 35.0%). In addition, expression of CACNA2D3 could be restored in KYSE180 and HKESC1 cells after the treatment of the demethylation agent 5-AZA-dC (Fig. 2B). These data suggested that downregulation of CACNA2D3 was associated with hypermethylation in its promoter region.
Figure 2. Downregulation of CACNA2D3 is associated with promoter methylation and allele loss.
(A) Representatives of CACNA2D3 expression (bottom) and promoter methylation status (upper) in primary ESCC cases. (B) Restoration of CACNA2D3 mRNA expression could be observed in HKESC1 and KYSE180 after demethylating agent 5-AZA treatment. The promoter methylation status was also compared. (C) Representative of allele loss at SNP site rs589281 in three ESCC tumors. The SNP site was indicated by red arrows. (D) Pattern of CACNA2D3 expression, promoter methylation and allele loss in 27 informative ESCC cases. (E) 2 centrimere signals (green, indicated by narrow arrows) and 1 CACNA2D3 signal (red, indicated by wide arrow) were detected in KYSE410 cells; Tumor cells with CACNA2D3 deletion are indicated by arrows (100×objective).
In our previous study, LOH at the SNP site rs589281 within CACNA2D3 gene was detected in about 50% of primary ESCC cases [6]. LOH at rs589281 site was also investigated in 48 ESCC samples and LOH was detected in 16/27 (59.3%) of informative cases with heterozygosity at the SNP site (Fig. 2C). Downregulation of CACNA2D3 was detected in 11/16 (68.8%) LOH cases, which is higher than that in cases without allele loss (4/11, 36.4%). We next investigated the correlation of CACNA2D3 downregulation with its allelic loss and hypermethylation in 27 informative cases. In 15 ESCCs with CACNA2D3 downregulation, inactivation of CACNA2D3 was significantly associated with either methylation (n = 9) or LOH (n = 11), or both methylation and LOH (n = 6) (P<0.05, Fisher’s exact test, Fig. 2D). To validate qRT-PCR result, Fluorescence in situ hybridization (FISH) using BAC probe containing CACNA2D3 was performed to check the deletion of 3p21 in 3 ESCC cell lines and 2 primary ESCC cases. The FISH result was consistent with qRT-PCR result. Loss of CACNA2D3 allele could be observed in 2 cell lines (KYSE410 and KYSE510) and 1 primary ESCC tumor with the downregulation of CACNA2D3. (Fig. 2E).
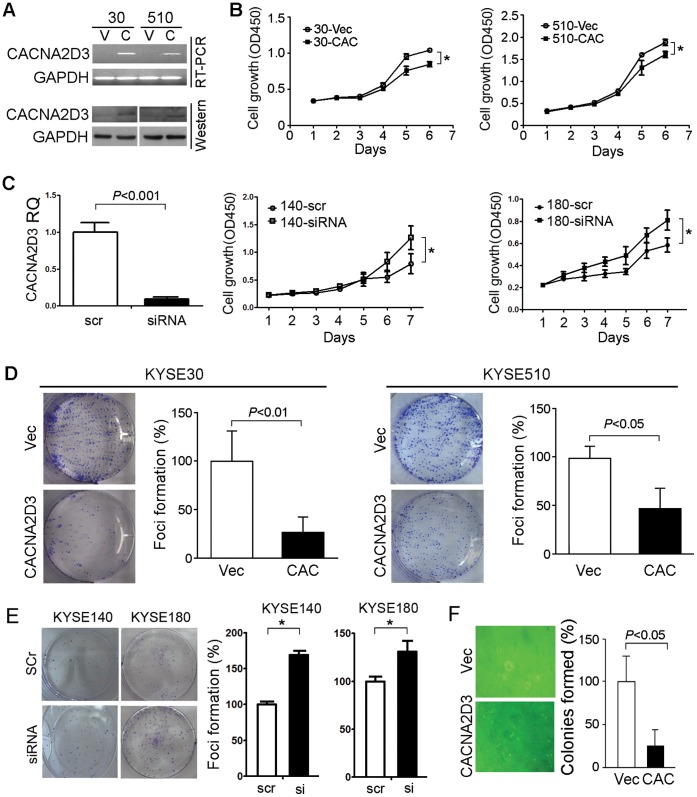
Tumor Suppressive Function of CACNA2D3
To characterize its tumor suppressive function, CACNA2D3 was stably transfected into KYSE30 (30-CAC) and KYSE510 (510-CAC). Empty vector-transfected cells (30-Vec and 510-Vec) were used as controls. Expression of CACNA2D3 was confirmed by RT-PCR and western blotting (Fig. 3A). Cell growth assay found that CACNA2D3 could significantly inhibit cell growth in both tested cell lines (P<0.05, Fig. 3B). Similarly, CACNA2D3 could significantly reduce focus formation (P<0.05, Fig. 3D) and colony formation in soft agar (P<0.05, Fig. 3F) in both tested cell lines, compared with vector-transfected cells. Silencing CACNA2D3 could also reverse the results in KYSE140 and KYSE180 cells (Fig. 3C, 3E).
Figure 3. CACNA2D3 inhibits tumorigenicity.
(A) Detection of CACNA2D3 expression in CACNA2D3-transfected cells (30-CAC or 510-CAC) compared with vector-transfected cells (30-Vec or 510-Vec). GAPDH was used as loading control. (B) Cell growth rate was significantly inhibited in CACNA2D3-transfected cells compared with vector-transfected cells. (C) qRTPCR result of KYSE140 and cell growth rate was elevated in siRNA treated cells. (D, E) Ability of focus formation was decreased by CACNA2D3 overexpression (D) and increased by silencing CACNA2D3 in cells (E). (F) Ability to form colony in soft agar decreased significantly in CACNA2D3-transfected cells compared with vector cells. *, P<0.05.
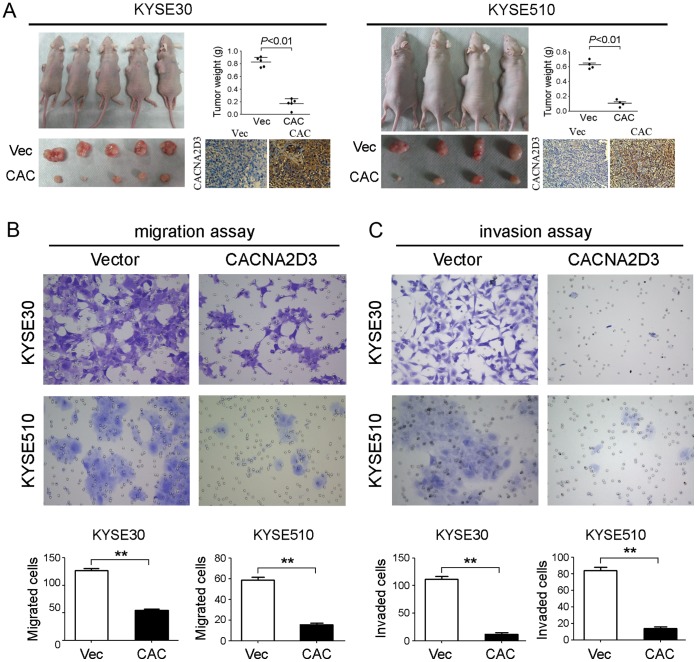
To further explore the in vivo tumor suppressive ability of CACNA2D3, tumor formation in nude mice was performed. Empty vector- and CACNA2D3-transfected cells were injected into the left and right flanks of nude mice, respectively. Twenty-four days after injection, mice were sacrificed and xenografts were excised for further analysis. The average weight of tumors induced by 30-CAC cells (0.168±0.080 g) was significantly decreased compared to the tumors induced by 30-Vec cells (0.825±0.072 g) (P<0.01, Fig. 4A). Similar result was also observed in KYSE510 cells (P<0.01, Fig. 4A). The IHC result showed that CACNA2D3 expression in CACNA2D3- transfected cells was much stronger than the vector controls (Fig. 4A).
Figure 4. CACNA2D3 inhibits tumorigenicity in vivo and cell motility in vitro.
(A) Tumor formation in nude mice was inhibited by CACNA2D3 in 30-CAC (left) and 510-CAC cells (right). Representatives of CACNA2D3 expression were detected by IHC in xenograft (20×objective). (B, C) Representative and summary of cell migration assay (B) and cell invasion assay (C) performed with CACNA2D3 forced expression cells or vector cells. The results show that CACNA2D3 inhibits cell migration and cell invasion (20×objective). **, P<0.001.
CACNA2D3 Inhibits Cell Motility
Cell migration assay showed that 56.76% and 76.19% decrease in cells migrated through transwell were observed in the 30-CAC and 510-CAC cells, respectively, compared with control cells (Fig. 4B). Cell invasion assay found that cells invaded through Matrigel were also significantly decreased in 30-CAC (91.03%) and 510-CAC (75.07%) cells, compared with that in 30-Vec and 510-Vec cells, respectively (Fig. 4C).
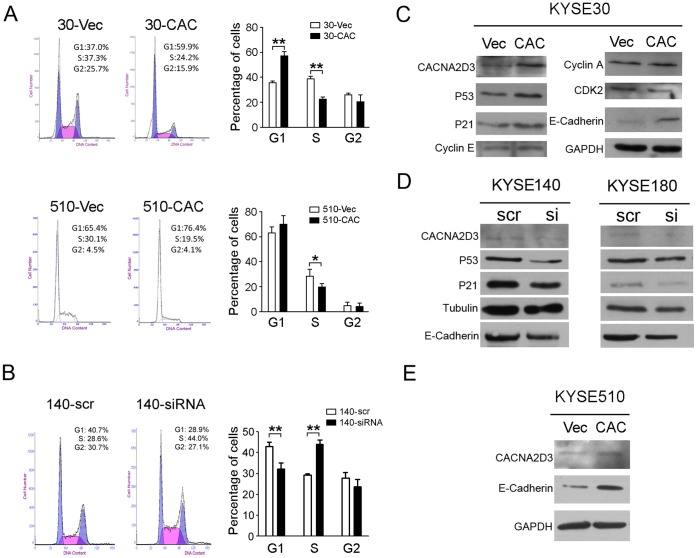
CACNA2D3 Arrests Cell Cycle at G1/S Checkpoint
The cell cycle distribution between 30-CAC and 30-Vec cells was compared by flow cytometry. The result found that 30-CAC cells were arrested at G1/S checkpoint, manifested as an accumulation of cells in G1 phase (average 56.4%) and a decrease in S-phase cells (average 22.2%) compared to 30-Vec cells (G1 phase: average 36.9%; S-phase: average 37.2%) (Fig. 5A). Similar G1/S checkpoint arrest was also detected in 510-CAC cells (Fig. 5A). Silencing CACNA2D3 in KYSE140 cells caused a decrease in G1 phase cells (140-siRNA: average 32.1%) and increase in S phase cells (140-siRNA: average 44.1%) compared with 140-scr control (G1: average 42.9%; S: average 29.2%). (Fig. 5B). To investigate the potential mechanism of CACNA2D3 in cell cycle arrest, expression of several key cell cycle regulators including p21, p53, CDK2, Cyclin A and Cyclin E were tested by western blotting. Increased expressions of p21 and p53, and decreased expression of CDK2 were detected in 30-CAC cells compared with 30-Vec cells (Fig. 5C). Silencing endogenous CACNA2D3 with siRNA in KYSE140 and KYSE180 cells also decreased p21 and p53 (Fig. 5D). In addition, the expression of E-cadherin was increased in CACNA2D3 overexpressed cells compared with vector cells (Fig. 5E), silencing CACNA2D3 induced the decrease of E-cadherin expression (Fig. 5D).
Figure 5. CACNA2D3 arrests cell cycle at G1/S checkpoint.
(A) Representatives and summary of DNA content of cells detected by flow cytometry. The results are expressed as mean±SD of three independent experiments. *, P<0.05, **, P<0.01. (B) Representatives and summary of DNA content in CACNA2D3 silenced cells. Scramble siRNA was used as a control. **, P<0.01. (C, D, E) Expression of proteins was detected in CACNA2D3 overexpressed cells (C, E) and silenced cells (D). GAPDH and tubulin were used as loading controls.
CACNA2D3 Upregulates Intracellular Free Cytosolic Ca2+
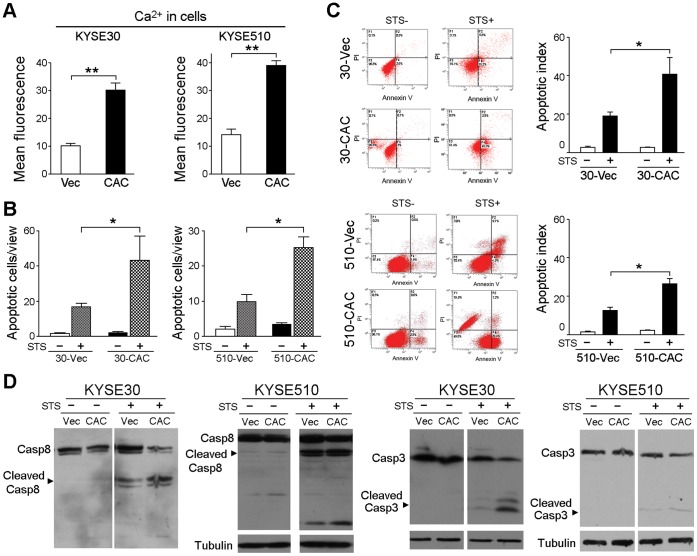
A previous study has reported that CACNA2D2 could elevate intracellular Ca2+ level in non-small cell lung cancer cells [14]. In the present study, intracellular Ca2+ level in the KYSE30 cells after CACNA2D3 transfection was measured by FACS. A significant increase in fluorescence emission was observed in 30-CAC cells compared with 30-Vec cells (P<0.01) (Fig. 6A), indicating that intracellular free cytosolic Ca2+ could be upregulated by CACNA2D3. Similar results were also observed in 510-CAC cells (Fig. 6A).
Figure 6. CACNA2D3 induces apoptosis.
(A) Intracellular Ca2+ level was compared between CACNA2D3-transfected and vector-transfected cells by FACS with Fluo 3-AM. A significant increase in fluorescence emission was observed in CACNA2D3-transfected cells compared with vector-transfected cells. **, P<0.001. (B) Summary of TUNEL assay performed with CACNA2D3-transfected cells or vector-transfected cells treated with or without STS. The results are expressed as mean±SD of three independent experiments. *, P<0.01. (C) Representative and summary of apoptotic index detected by flow cytometry in CACNA2D3-transfected cells or vector-transfected cells treated with or without STS. The results are expressed as mean±SD of three independent experiments. *, P<0.05. (D) Cleaved caspase-8 and caspase-3 were compared between CACNA2D3- and vector-transfected cells by western blot analysis. Tubulin was used as a loading control.
CACNA2D3 Induces Apoptosis
Since Ca2+ is able to mediate mitochondrial permeability transition and trigger apoptosis [19], FACS and TUNEL assays were used to compare apoptotic indexes between 30-CAC and 30-Vec cells. The TUNEL results showed that the number of apoptotic cells was similar between CACNA2D3-transfected and vector-transfected cells (Fig. 6B). However, the number of apoptotic cells was significantly increased in CACNA2D3-transfected cells (P<0.05) after STS treatment, compared to empty vector-transfected cells (Fig. 6B). Similar results were found by using FACS assay. After STS treatment, the apoptotic index, defined as the percentage of apoptotic cells (F2+F4), was significantly higher in CACNA2D3 transfectants (P<0.05) compared with empty vector-transfected cells (Fig. 6C). Western blot analysis demonstrated that caspase-8 and caspase-3 were activated by the detection of cleaved forms of caspase-8 and caspase-3 (Fig. 6D).
Discussion
In this study, we studied the downregulation of CACNA2D3 and its tumor suppressive function and mechanism in ESCC. CACNA2D3 is located at 3p21.1, a chromosomal region frequently deleted in lung [20], esophageal [6], [7], nasopharyngeal [21] and renal cell [22] cancers, suggesting that the existence of tumor suppressor gene(s) within the region that plays a critical role in the development and progression in various solid malignancies including ESCC. TSGs within 3p21 such as RAR-β, RASSF1A [23], DLC1 [24] have been studied in ESCC. Here we described the characterization of another candidate TSG CACNA2D3 at 3p21.
Downregulation of CACNA2D3 was detected in 50% and 56.7% of primary ESCCs in mRNA and protein levels, respectively. Further study found that downregulation of CACNA2D3 was significantly correlated with allele loss and promoter hypermethylation (P<0.05), indicating that DNA copy-number loss combined with promoter methylation played an important role in CACNA2D3 downregulation. We noted that 2/8 of ESCC cases with both promoter methylation and allele loss retained expression of CACNA2D3 (Fig. 2D). We believe that this issue could be either caused by the heterogenicity of cancer or normal cell contamination (e.g. endothelial cells and lymphocytes). Actually, a small peak of lost allele could be observed in all tumor samples. We also noted that the promoter hypermethylation could be detected in 12/28 (42.8%) of methylation-positive ESCCs matched non-tumor tissues, which might be promoted by local environment. For example, Vasavi et al. found that patients with gastroesophageal reflux disease showed a high degree of hMLH1 hypermethylation, suggesting that local environment due to reflux might promote hypermethylation [25]. We believe “epigenetic filed defect” exists, which means slight methylation in the normal tissue but also a pre-cancerous change. Because intraepitherial neoplasia is frequently seen prior to ESCC, CACNA2D3 could be a feasible biomarker to detect early change of the normal mucosa. Clinical significance study indicated that CACNA2D3 could significantly inhibit lymph nodes metastasis (P = 0.01) in ESCC. Kaplain-Meier analysis showed that overall survival rate of ESCC patients decreased as CACNA2D3 was downregulated in tumor tissues.
To explore the tumor suppressive function of CACNA2D3 in ESCC, functional analysis of CACNA2D3 was performed by ectopic expression of CACNA2D3 in ESCC cell lines KYSE30 and KYSE510. The results found that CACNA2D3 could inhibit cell growth, focus formation, colony formation in soft agar and tumor formation in nude mice. Further study showed that CACNA2D3 could arrest cell cycle at G1/S checkpoint by upregulating p53 and p21 expression and downregulating CDK2 expression. In addition, an elevated intracellular Ca2+ level was detected when CACNA2D3 was introduced into cells, which was similar to the results of CACNA2D3 in gastric cancer cells [13] and CACNA2D2 in non-small cell lung cancer cells [12]. It has been reported that Ca2+ influx could promote activation of the transcription factor CREB (cAMP response element binding protein) leading to the cell cycle arrest in G1 phase via transactivation of p53/p21 signaling pathways [26]. Moreover, Ca2+ regulates the cell cycle through various signaling pathways including Ras [27], PTEN [28] and Rb [29] signaling pathways. It has been found that Ca2+ could mediate mitochondrial permeability transition and trigger apoptosis [19]. In this study, we found that CACNA2D3 could significantly increase apoptotic index after STS treatment (P<0.05).
E-cadherin is the prototypic cadherin which is often lost partially or completely in epithelial tumors when they progress toward malignancy [30]. In this process, neoplastic cells have lost many of the epithelial characteristics and exhibit a highly invasive pattern [31]. In diffuse gastric cancer, E-cadherin is inactivated mainly through LOH, somatic mutations and promoter hypermethylation [32]. It has been reported that calcium signals regulate cell to cell adhesion through recruitment of cadherins and β-catenin into intracellular junction in fibroblasts [33]. Studies also found that reducing E-cadherin could induce disruption of the E-cadherin adhesion complex and correlated with elevated cell migration and invasion of different carcinoma cells [34]. In this study, we found that CACNA2D3 could effectively inhibit cell motility in KYSE30 and KYSE510 cells, which might be associated with the upregulation of E-cadherin caused by the influx of Ca2+. In summary, our data indicate for the first time that downregulation of CACNA2D3 is frequently detected in ESCC and associated with poor prognosis. Both promoter hypermethylation and allele loss contribute to the downregulation. We also demonstrate that CACNA2D3 has strong tumor suppressive function through the cell cycle arrest and induction of apoptosis.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81172338, 30971606), Sun Yat-Sen University “Hundred Talents Program” (85000-3171311), Hong Kong Research Grant Council Central Allocation (HKUST 2/06C), Sun Yat-sen University Young Talent Teachers Plan (11ykpy58). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wei WQ, Yang J, Zhang SW, Chen WQ, Qiao YL (2010) Analysis of the esophageal cancer mortality in 2004–2005 and its trends during last 30 years in China. Zhonghua Yu Fang Yi Xue Za Zhi 44: 398–402. [PubMed] [Google Scholar]
- 2. Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer stastistics, 2002. Ca-A Cancer Journal for Clinicians 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 3. Hu N, Wang C, Ng D, Clifford R, Yang HH, et al. (2009) Genomic characterization of esophageal squamous cell carcinoma from a high-risk population in China. Cancer Res 69: 5908–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwong D, Lam A, Guan XY, Law S, Tai A, et al. (2004) Chromosomal aberrations in esophageal squamous cell carcinoma among Chinese: Gain of 12p predicts poor prognosis after surgery. Human Pathol 35: 309–316. [DOI] [PubMed] [Google Scholar]
- 5. Hu N, Clifford RJ, Yang HH, Wang C, Goldstein AM, et al. (2010) Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genomics 11: 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qin YR, Fu L, Sham PC, Kwong DL, Zhu CL, et al. (2008) Single-nucleotide polymorphism-mass array reveals commonly deleted regions at 3p22 and 3p14.2 associate with poor clinical outcome in esophageal squamous cell carcinoma. Int J Cancer 123: 826–830. [DOI] [PubMed] [Google Scholar]
- 7. Fu L, Qin YR, Xie D, Hu L, Kwong DL, et al. (2007) Characterization of a novel tumor-suppressor gene PLC delta 1 at 3p22 in esophageal squamous cell carcinoma. Cancer Res 67: 10720–10726. [DOI] [PubMed] [Google Scholar]
- 8. Zhu C, Qin YR, Xie D, Chua DT, Fung JM, et al. (2009) Characterization of tumor suppressive function of P300/CBP-associated factor at frequently deleted region 3p24 in esophageal squamous cell carcinoma. Oncogene 28: 2821–2828. [DOI] [PubMed] [Google Scholar]
- 9. Gong HC, Hang J, Kohler W, Li L, Su TZ (2001) Tissue-specific expression and gabapentin-binding properties of calcium channel alpha2delta subunit subtypes. J Membr Biol 184: 35–43. [DOI] [PubMed] [Google Scholar]
- 10. Qin N, Yagel S, Momplaisir ML, Codd EE, D'Andrea MR (2002) Molecular cloning and characterization of the human voltage-gated calcium channel alpha(2)delta-4 subunit. Mol Pharmacol 62: 485–496. [DOI] [PubMed] [Google Scholar]
- 11. Gao B, Sekido Y, Maximov A, Saad M, Forgacs E, et al. (2000) Functional properties of a new voltage-dependent calcium channel alpha(2)delta auxiliary subunit gene (CACNA2D2). J Biol Chem 275: 12237–12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carboni GL, Gao B, Nishizaki M, Xu K, Minna JD, et al. (2003) CACNA2D2-mediated apoptosis in NSCLC cells is associated with alterations of the intracellular calcium signaling and disruption of mitochondria membrane integrity. Oncogene 22: 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wanajo A, Sasaki A, Nagasaki H, Shimada S, Otsubo T, et al. (2008) Methylation of the calcium channel-related gene, CACNA2D3, is frequent and a poor prognostic factor in gastric cancer. Gastroenterology 135: 580–590. [DOI] [PubMed] [Google Scholar]
- 14. Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T (1992) Characterization of 21 newly established esophageal cancer cell lines. Cancer 69: 277–284. [DOI] [PubMed] [Google Scholar]
- 15. Wong ML, Tao Q, Fu L, Wong KY, Qiu GH, et al. (2006) Aberrant promoter hypermethylation and silencing of the critical 3p21 tumour suppressor gene, RASSF1A, in Chinese oesophageal squamous cell carcinoma. Int J Oncol 28: 767–773. [PubMed] [Google Scholar]
- 16. Li Y, Chen L, Nie CJ, Zeng TT, Liu H, et al. (2011) Downregulation of RBMS3 Is Associated with Poor Prognosis in Esophageal Squamous Cell Carcinoma. Cancer Res 71: 6106–6115. [DOI] [PubMed] [Google Scholar]
- 17. Guan XY, Sham JS, Tang TC, Fang Y, Huo KK, et al. (2001) Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res 61: 3806–3809. [PubMed] [Google Scholar]
- 18. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 19. Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565. [DOI] [PubMed] [Google Scholar]
- 20. Tai AL, Mak W, Ng PK, Chua DT, Ng MY, et al. (2006) High-throughput loss-of-heterozygosity study of chromosome 3p in lung cancer using single-nucleotide polymorphism markers. Cancer Res 66: 4133–4138. [DOI] [PubMed] [Google Scholar]
- 21. Fang Y, Guan XY, Guo Y, Sham J, Deng M, et al. (2001) Analysis of genetic alterations in primary nasopharyngeal carcinoma by comparative genomic hybridization. Genes Chromosomes Cancer. 30: 254–260. [DOI] [PubMed] [Google Scholar]
- 22. Alimov A, Kost-Alimova M, Liu J, Li C, Bergerheim U, et al. (2000) Combined LOH/CGH analysis proves the existence of interstitial 3p deletions in renal cell carcinoma. Oncogene 19: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 23. Kuroki T, Trapasso F, Yendamuri S, Matsuyama A, Alder H, et al. (2003) Allele loss and promoter hypermethylation of VHL, RAR-beta, RASSF1A, and FHIT tumor suppressor genes on chromosome 3p in esophageal squamous cell carcinoma. Cancer Res 63: 3724–3728. [PubMed] [Google Scholar]
- 24. Daigo Y, Nishiwaki T, Kawasoe T, Tamari M, Tsuchiya E, et al. (1999) Molecular cloning of a candidate tumor suppressor gene, DLC1, from chromosome 3p21.3. Cancer Res 59: 1966–1972. [PubMed] [Google Scholar]
- 25. Vasavi M, Ponnala S, Gujjari K, Boddu P, Bharatula RS, et al. (2006) DNA methylation in esophageal diseases including cancer: special reference to hMLH1 gene promoter status. Tumori. 92: 155–162. [DOI] [PubMed] [Google Scholar]
- 26. Lipskaia L, Lompre AM (2004) Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell 96: 55–68. [DOI] [PubMed] [Google Scholar]
- 27. Cook SJ, Lockyer PJ (2006) Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium 39: 101–112. [DOI] [PubMed] [Google Scholar]
- 28. Minaguchi T, Waite KA, Eng C (2006) Nuclear localization of PTEN is regulated by Ca(2+) through a tyrosil phosphorylation-independent conformational modification in major vault protein. Cancer Res 66: 11677–11682. [DOI] [PubMed] [Google Scholar]
- 29. Rey O, Young SH, Jacamo R, Moyer MP, Rozengurt E (2010) Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J Cell Physiol 225: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berx G, van Roy F (2009) Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 1: a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guarino M, Rubino B, Ballabio G (2007) The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39: 305–318. [DOI] [PubMed] [Google Scholar]
- 32. Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, et al. (1994) E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 54: 3845–3852. [PubMed] [Google Scholar]
- 33. Ko KS, Arora PD, Bhide V, Chen A, McCulloch CA (2001) Cell-cell adhesion in human fibroblasts requires calcium signaling. J Cell Sci 114: 1155–1167. [DOI] [PubMed] [Google Scholar]
- 34. Imamichi Y, Menke A (2007) Signaling pathways involved in collagen-induced disruption of the E-cadherin complex during epithelial-mesenchymal transition. Cells Tissues Organs 185: 180–190. [DOI] [PubMed] [Google Scholar]