Abstract
Ethnopharmacological relevance
Dangkwisoo-san (DS), an herbal medicinal formula, has long been used in Korea for the treatment of inflammatory complications caused by physical trauma. Although the therapeutic effect of DS is likely associated with anti-inflammatory activity, the precise underlying mechanisms are largely unknown. Here we sought to elucidate the possible mechanisms of anti-inflammatory activity of DS.
Materials and Methods
The water extract of DS was orally fed to C57BL/6 mice for 14 days prior to LPS intranasal instillation for lung inflammation. The effects of DS on lung inflammation were determined by differential cell counting, lung histology, and semi-quantitative RT-PCR of lung sections. The effects of DS on the activities of Nrf2 and NF-κB were assessed by western blotting, semi-quantitative RT-PCR, and luciferase reporter assays in RAW 264.7, an NF-κB reporter cell line, and HEK 293 transfected with an NF-κB reporter construct.
Results
Mice that were treated with a water extract of DS showed significant attenuation of lung inflammation induced by intranasal lipopolysaccharide (LPS) compared to control mice treated with vehicle. In vitro experiments show that DS activated Nrf2, an anti-oxidant transcription factor that protects from various inflammatory diseases, and inducedNrf2-regulated genes including GCLC, NQO-1 and HO-1. In addition, DS suppressed NF-κB activity and reduced the production of pro-inflammatory cytokines. Transfection experiment indicates that inhibition of NF-κB likely occurred upstream of IKK complex. Furthermore, DS enhanced the expression of HO-1 and suppressed that of IL-1β and TNF-α in inflamed mouse lungs.
Conclusions
These results suggest that the therapeutic effects of DS are related with suppression of inflammation, which is, at least in part, mediated by activation of anti-inflammatory factor Nrf2 and inhibition of pro-inflammatory factor NF-κB.
Keywords: acute lung inflammation, anti-inflammation, Nrf2, NF-κB, Dangkwisoo-san, lipopolysaccharide
1.Introduction
Physical trauma accompanied with hemorrhagic complications is the leading cause of morbidity and mortality in the United State (Minino, et al., 2006). Trauma is also an important cause of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). The prognosis of patients with ARDS continues to be abysmal with mortality rates ranging from 30% to 40% (Ware and Matthay, 2000). Hence, ARDS represents an unmet medical need and there is an urgent need to develop new therapies to treat patients with this condition. Regulation of inflammation can be a therapeutic target for the treatment of trauma and trauma-associated complications such as ALI and ARDS.
Macrophages play an essential role in regulation of inflammation. Using various Toll-like receptors (TLRs) on the cell surface, macrophages sense immunogens and initiate an inflammatory response. For example, lipopolysaccharide (LPS), a cell wall component of Gram negative bacteria, binds to TLR4 and triggers the release of pro-inflammatory mediators from macrophages. Engagement of LPS to TLR4 initiates a signaling that activates pro-inflammatory transcription factor NF-κB and induces transcription of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6, and IL-8(Beutler and Rietschel,2003). Macrophages also produce reactive oxygen species (ROS) that inflict collateral damage to tissue, exacerbating inflammation (Cathcart,2004). In response to ROS, cells express various proteins that scavenge ROS to lessen that damage incurred by ROS. The key factor for inducing these proteins is nuclear factor-E2-related factor 2 (Nrf2), a member of the cap’n’collar family of basic leucine zipper transcription factors (Johnson, et al. 2008). Normally, Nrf2 resides in the cytoplasm in low quantities (Zhang, et al. 2005). However, in oxidative environment generated by inflammation, Nrf2 accumulates in the nucleus to bind to the antioxidant responsive element (ARE), resulting in the expression of phase 2 detoxification and antioxidant enzymes such as glutamate-cysteine ligase catalytic subunit (GCLC), NAD(P)H:quinine oxidoreductase-1 (NQO1), and heme oxygenase-1 (HO-1)(Itoh, et al. 1997;Jaiswal, 2000).Accumulating evidence also shows that Nrf2 plays an essential role in regulation of inflammation (Cho, et al. 2002;Cho, et al. 2004;Rangasamy, et al. 2004;Thimmulappa, et al. 2006;Chan and Kan,1999;Mochizuki, et al. 2005;Rangasamy, et al. 2005). Thus, Nrf2 can be an excellent therapeutic target for regulation of various inflammatory diseases.
As part of traditional Korean medicine, herbal extracts have long been prescribed and often effectively treated a variety of inflammatory diseases(Lim, et al. 2009). However, paucity of data regarding the underlying mechanisms has precludeda broad usage of these agents along with mainstream therapies. Because of potentially significant health benefits and minimal toxicity, herbal remedies warrant further studies as an alternative and complementary therapies. Dangkwisoo-san (DS) is an herbal formula that has been traditionally used for the treatment of pain and blood stagnation caused by physical trauma in Korea (Kim, et al. 2011). Thus, we speculated that the therapeutic effect of DS is associated with anti-inflammatory activity. In this study, we sought to examine this possibility. We prepared the water extract of DS, and investigated its anti-inflammatory effects in a murine model of acute lung inflammation induced by administration of LPS. Further we defined the molecular mechanisms of DS in vitro in macrophages. Results show that DS suppressed LPS-induced acute lung inflammation, which could be attributed to activation of Nrf2, an anti-inflammatory factor, and inhibition of NF-κB, a key pro-inflammatory molecule.
2. Materials and Methods
2. 1. Water Extraction of Dangkwisoo-san (DS-WE)
Dangkwisoo-san (DS) is composed of 9 species of herbal plants, each of which were purchased from Kwangmyungdang Natural Pharmaceutical Co., Ulsan, Korea. The herbal components were identified by one of the authors (Dr. Su-In Cho). The formula of DS is described in Table 1, and a voucher specimen (number:pnukh002) is kept in the School of Korean Medicine, Pusan National University. Sixty gram of DS was boiled in 1 L of distilled water in an Herb Extractor (Dae-Woong Co, Korea) for 2 h, yielding final 200 ml of DS extract. The supernatant was harvested in sterile condition by centrifugation and lyophilized through evaporation at −80°C, yielding final 4.6 g. The lyophilized DS extract was dissolved in an appropriate volume of sterile PBS prior to administrating to mice and to cells.
Table 1.
Prescription of DS
| Scientific name | Herbal name | (g) |
|---|---|---|
| Angelica gigasNakai | Angelicaegigantis Radix | 5.625 |
| PaeonialactifloraPall | Paeoniae Radix | 3.75 |
| LinderastrychnifoliaFernandez-Villar | Linderae Radix | 3.75 |
| CaesalpiniasappanL. | Sappan Lignum | 3.75 |
| CyperusrotundusL. | CyperiRhizoma | 3.75 |
| CarthamustinctoriusL. | CarthamiFlos | 3.0 |
| PrunuspersicaBatsch | Persicae Semen | 2.655 |
| Cinnamomum cassia Presl | Cinnamomi Cortex | 2.25 |
| GlycyrrhizauralensisFisch | Glycyrrhizae Radix et Rhizoma | 1.875 |
|
| ||
| Total | 30.405 | |
2. 2. Reagents and Antibodies
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Sulforaphane were purchased from Sigma Chemical Co. (St. Louis, MO, USA). TLR4-specific Escherichia coli LPS was purchased from Alexis Biochemical (San Diego, CA, USA). All antibodies used in this study were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2. 3. Animals
Male C57BL/6 mice, inbred in a specific pathogen-free (SPF) facility, were purchased from the Samtaco Bio Korea, Ltd. (Osan, Korea). Animals were housed in certified, standard laboratory cages, and fed with food and water ad libitum prior to experiment. All experimental procedures followed the NIH of Korea Guidelines for the Care and Use of Laboratory Animals, and all the experiments were approved by the Institutional Animal Care and Use Committee of Pusan National University, Pusan, Korea.
2. 4. Evaluations of Acute Lung Inflammation
Gram-negative Escherichia coli LPS (serotype 055:B5) was obtained from Sigma. Prior to LPS administration, three different doses were orally given to mice administered orally to mice: 0.1, 0.5, and 1 gram of DS per kilogram of a mouse for 15 days. The daily dose,1g of DS/kg of a mouse, was approximated by accounting for the dose given to patients three times per day in Korean medicine clinic and a higher metabolism of mice, which was equivalent to about 8 times more than a single dose for patients. At day 15,mice were anesthetized by Zoletil (Virbac), and received a single dose of 10 mg LPS/kg body weight intranasally while control mice received sterile saline. At 24 h after the administration of LPS, mice were euthanized by CO2 gas. The trachea was exposed through midline incision and cannulated with a sterile 24-gauge intravascular catheter. Bilateral bronchoalveolar lavage (BAL) was performed by two consecutive instillations of 1.0 ml of PBS. Total cell numbers in BAL fluid were counted with hemocytometer, and the cells in BAL fluid were prepared by a cytospin and stained for the differentiation of macrophages, lymphocytes, or neutrophils by Hemacolor (Merck, Darmstadt, Germany). Three hundred cells in total were counted, and one hundred of the cells in each microscopic field were scored. The mean number of cells per field was reported.
For collecting lung tissue, mice were perfused with saline and the whole lung was inflated with fixatives. After paraffin embedding, 5 μm sections were cut and placed on charged slides, and stained with hematoxylin and eosin (H&E) staining method. Three separate H&E-stained sections were evaluated in 200X microscopic magnifications per mouse.
2. 5. Cell Culture
RAW 264.7 cells and HEK 293 cells were obtained from ATCC (American Type Culture Collection, Rockville, MD, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing L-glutamine (200 mg/L) (Hyclone; Logan, UT, USA) supplemented with 10 % (v/v) heat-inactivated fetal bovine serum (FBS) and 100 U/ml penicillin and 100 μg/ml streptomycin(Invitrogen; Carlsbad, CA, USA). The cells were cultured and maintained in a humidified incubator at 37 °C and 5 % CO2 prior to experiment.
2. 6. Assessment of Cytotoxicity
The cytotoxicity of DS was indirectly assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)-based colorimetric assay (Skehan, 1998). The percentage of metabolically active cells was calculated against untreated cells. All experiments were performed three times independently.
2. 7. Isolation of Total RNA from Cells and RT-PCR
Total RNA from cells or tissue was isolated by using Trizol reagent and the manufacturer’s instructions (Invitrogen). After the concentration of RNA was determined by spectrophotometer, 3μg of RNAs were reverse-transcribed by M-MLV reverse transcriptase (Promega, Madison, WI, USA). Resultant single-stranded cDNA was amplified by PCR with a set of specific primers: the forward and the reverse primers for NQO-1 were 5′ - ACTACGCCATGAAGGAGGCT - 3′ and 5′ - TTCCAGCTTCTTGTGTTCGG - 3′, respectively; the primers for HO-1 were 5′-TGAAG GAGGCCACCA AGGAGG - 3′ and 5′-AGAGGTCACCCAGGTAGCGGG - 3′, respectively; the primers for GCLC were 5′-CACTGCCAGAACACAGACCC - 3′ and 5′-ATGGTCTGGCTGAGAAGCCT - 3′, respectively; the primers for GAPDH were 5′-GGAGCCAAAAGGGTCATCAT- 3′ and 5′-GTGATGGCATGGACTGTGGT- 3′, respectively; the primers for TNF-α were 5′-CTACTCCTCAGAGCCCCCAG- 3′ and 5′-AGGCAACCTGACCACTCTCC - 3′, respectively; and the primers for IL-1β were 5′-GTGTCTTTCCCGTGGACCTT- 3′ and 5′-TCGTTGCTTGGTTCTCCTTG- 3′, respectively;
For PCR amplification, TaqPCRx DNA polymerase, Recombinant (Invitrogen) and the manufacturer’s protocol were used. The reaction conditions were as follows: an initial denaturation at 95 °C for 5 min followed by 28 cycles of denaturation for 30 sec at 95 °C, annealing for 30 sec at 58 °C (NQO1: 55 °C) and extension for 40 sec at 72 °C with a final extension for 7 min at 72 °C. Amplicons were separated in 1.5 % agarose gels in 1× TBE buffer at 100 V for 30 min, stained with ethidium bromide and visualized under UV light. GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) was used as internal controls to evaluate relative expressions of GCLC, HO-1, and NQO1.Relative expression of each gene over GAPDH was determined by densitometric analysis software ImageJ (WayneRasband, Research Services Branch, National Institute of Mental Health, Bethesda, Maryland, USA).
2. 8. Western Blot Analysis
Total cell extracts of 5×106 cells were prepared as described previously(Joo, et al. 2007). Nuclear proteins were isolated by NE-PER nuclear extraction kit and the manufacture’s protocol (Thermo Scientific, IL, USA). The amounts of proteins were measured by Bradford (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of proteins were fractionated by SDS-PAGE and then transferred to PVDF membrane (Bio-Rad Laboratories). Blots were blocked for at least 1 h with 5 % non-fat dry milk prior to incubation with polyclonal antibodies for Nrf2, β-actin, and lamin A/C at 4 °C overnight. After incubation with secondary antibodies conjugated with HRP for 1 h at room temperature, the bands of interest were revealed by chemiluminescence (SuperSignal® West Femto, Thermo Scientific).
2. 9. Reporter Constructs, Reporter Cell Line, and Luciferase Assay
To measure NF-κB transcriptional activity, we created 1×106of RAW 264.7 cells that were stably transfected with an NF-κB reporter construct. The reporter construct harbors four tandem copies of a 36-base enhancer from the 5′ HIV-long terminal repeat (containing two NF-κB binding site, GGGACTTTCC) placed upstream of the HSV minimal thymidine kinase promoter, which were cloned into pEGFPLuc (BD Biosciences, San Jose, CA, USA). The reporter construct was introduced to RAW 264.7 cells by transfection with Lipofectimine (Invitrogen), and the transfected cells were selected under G418 (600 μg/ml, Invitrogen). Candidate cell lines harboring the NF-κB reporter construct were tested for the introduction of luciferase activity. HEK 293(1×106 cells) was transiently transfected with the NF-κB reporter construct along with tk-Renilla luciferase construct and an IKK-β expressing plasmid (a gift from Dr. Michael Karin at University of California, San Diego, USA). Luciferase assay was performed with a dual luciferase assay kit and the manual of the manufacturer (Promega). NF-κB mediated luciferase activity was normalized by the amounts of proteins in total cell lysate of the stable cell line, which was measured by Bradford (Bio-Rad Laboratories),or by the tk-Renilla luciferase activity in HEK293 cells.
2. 10. Measurement of Intracellular Reactive Oxygen Species (ROS) Generation
Intracellular ROS generation in RAW 264.7 cells was determined by carboxy-H2DCFDA (5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (Molecular Probes, Eugene, OR, USA). Briefly, after various pharmacological treatments, 1×106of RAW 264.7 cells were treated with 100 μM carboxy-H2DCFDA in cultured medium and incubated at 37 °C for 30 min. After incubation, the cells were washed with PBS and then fluorescence was measured using BD FACSCanto II (BD Biosciences).
2. 11. Measurement of Pro-inflammatory Cytokines
Each cytokines were measured by ELISA kits and the manufacture’s protocol (BD Biosciences).
2. 12. Statistical Analysis
Data are presented as ± SEM (standard error of the mean) of at least three separate experiments. For comparison among groups, paired or unpaired T tests and one-way analysis of variance (ANOVA) tests were used (with the assistance of InStat, Graphpad Software, Inc., San Diego, CA).P values less than 0.05 were considered statistically significant.
3. Results
3. 1. The water extract of DS ameliorates acute lung inflammation caused by LPS
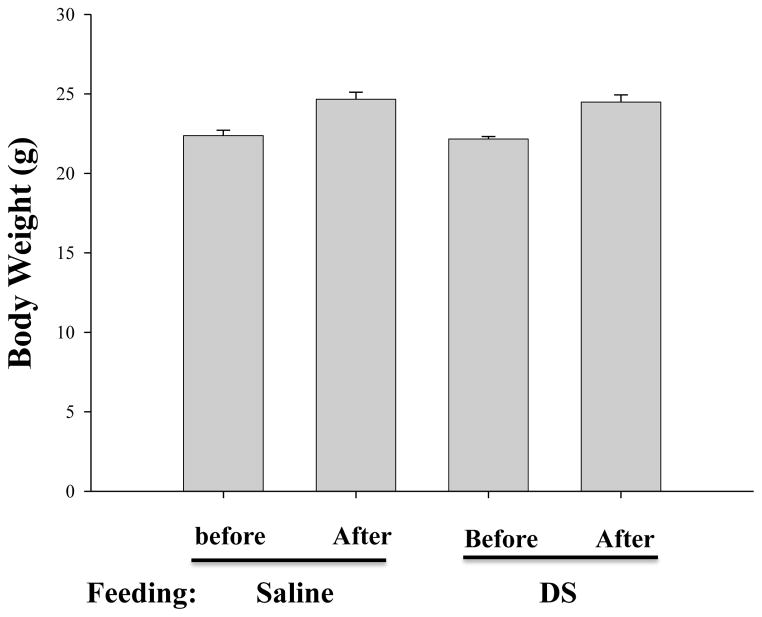
To investigate whether DS regulates inflammation in vivo, we prepared the water extract of DS and examined whether the DS extract suppresses acute lung inflammation in an animal model. First, we set up two groups of seven week old C57BL/6 mice, one fed with PBS (average weight 22.58±0.45 g, n=12) and the other did with 1g DS in saline/kg body weight (average weight 22.47±0.43 g, n=12) for 15 days. At day 15 when the mice became 9 weeks old, the weight of the mice was measured again to examine any adverse effect of the formula, such as abnormal changes of body weight and anorexia. As shown in Fig. 1, DS feeding did not significantly affect the weight gaining of the mice (2nd and 4th columns; PBS-fed: DS-fed = 24.66±0.44 g: 24.49±0.48 g, p=0.7839). In addition, there was no death during the feeding period. These results suggest that the amount of DS administered in this experiment does not have an adverse effect to the mice
Fig. 1. Effect of DS on body weight of C57BL/6 mice.
The average weights of 7 week old mice, before and after feeding with either saline (n=12, 1st and 2nd columns) or with DS (n=12, 3rd and 4th columns) for 15 days, were measured. Data are presented as the mean ± SEM.
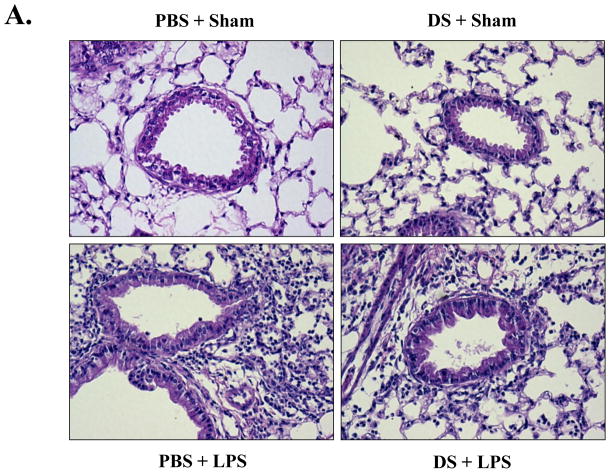
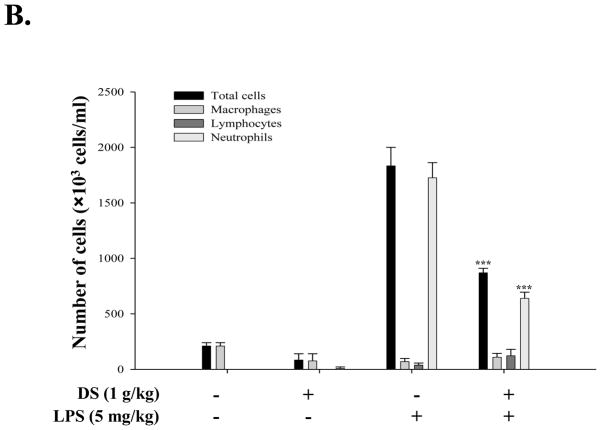
Next, each group of the mice, fed with either PBS or DS, were divided half, and one half of each group further received a single dose of intranasal LPS (5mg LPS/kg body weight, n=6). At 24 h after LPS administration, the mice were euthanized for the analysis of lung inflammation. As shown in Fig. 2A, histological analyses of lung sections reveal that the sham-treated mice, fed with either PBS or DS, maintained a normal lung histologic architecture, although the mouse fed with DS developed a mild interstitial inflammation (top two panels). However, the mice treated intranasally with LPS developed severe lung inflammation, as evidenced by the increase of cellular infiltrates and hyaline change in lungs, which was, however, substantially reduced by the treatment of DS (bottom two panels). Consistent with the results, differential counting of cells in BAL fluid showed that LPS treatment induced significant neutrophilic infiltration in the lungs, which was reduced to 52.54±0.75 % by DS treatment (Fig. 2B). These results clearly show that DS suppressed acute lung inflammation, suggesting that DS has an anti-inflammatory function.
Fig. 2. DS ameliorates acute lung inflammation.
(A) H&E stained lung sections of C57BL/6 mice. Mice, fed with PBS or DS for 15 days, received LPS intranasally (n=6 per group, bottom two panels). Similarly, mice, either fed with PBS or DS, were sham-treated with PBS (n=6 per group, top two panels). Data are representatives of at least five different areas of a lung (200X magnifications). (B) Infiltrates in bronchoalveolar lavage (BAL) fluid were counted. DS treatment significantly reduced infiltrates, compared to LPS-treated (n=6, *** P< 0.005). Data represent the mean±SEM of three independent counting.
3. 2. DS activates Nrf2 and induces expression of Nrf2-regulated genes
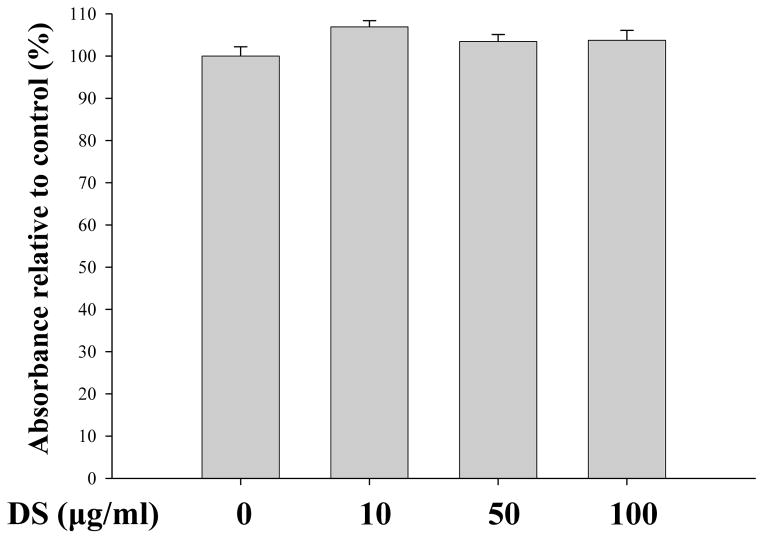
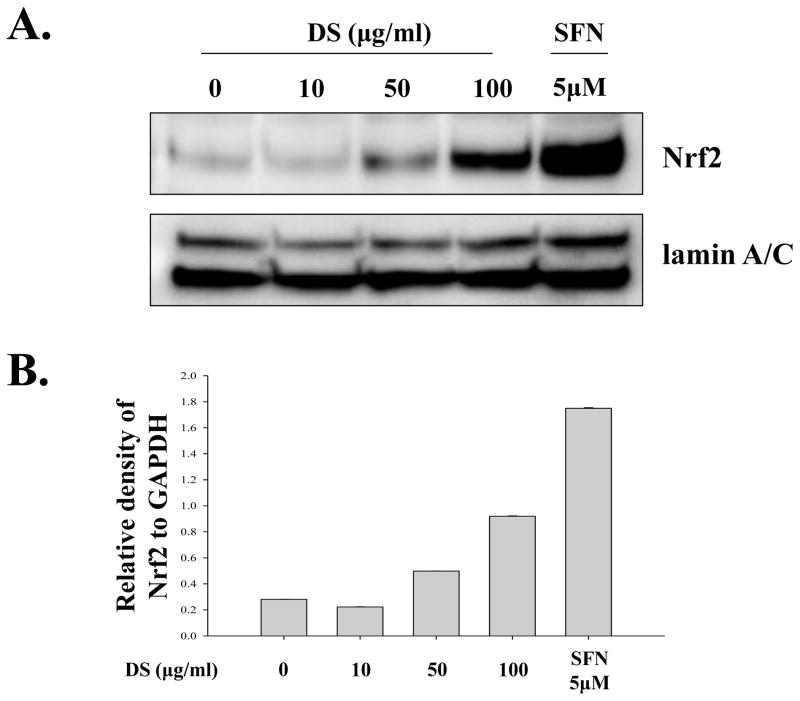
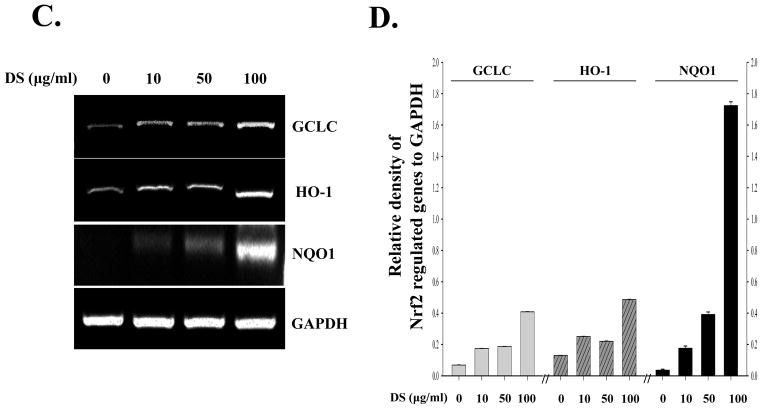
Since DS suppressed acute lung inflammation, we explored the possibility that DS activates Nrf2, a master transcription factor that is known to prevent from acute lung inflammation (Thimmulappa, et al. 2006;Chan and Kan,1999;Rangasamy, et al. 2005). For the study, we used RAW 264.7 cells, a murine macrophage cell line. First, we determined by performing MTT assay an optimal dose of DS, in which no significant cellular toxicity is apparent. RAW 264.7 cells were treated with various amounts of DS for 16 h, and the cytotoxicity was determined by MTT assay. As shown in Fig. 3, the amounts of DS ranging 10 to 100 μg/ml did not affect the viability of RAW 264.7. Next, we tested the possibility that the anti-inflammatory effect of DS is mediated by activating Nrf2. The cells were treated with the same amounts of DS determined above, along with Sulforaphane (5 μM), a well-documented Nrf2 activator(Johnson, et al. 2008).At 16h after treatment, the nuclear fraction of the treated cells was fractionated and analyzed by western blotting for the nuclear Nrf2, indicative of Nrf2 activation. The results in Fig. 4A and 4B show that the nuclear Nrf2 was detected in the treatment of 50 μg/ml of DS and became evident in the treatment of 100 μg/ml of DS, suggesting that DS indeed activates Nrf2. We examined whether Nrf2 activated by DS results in Nrf2-dependent gene expression. The cells were treated as described above, and total RNA of the treated cells was extracted for semi-quantitative RT-PCR analyses of GCLC, HO-1 and NQO-1, well-documented Nrf2 dependent genes. As shown Fig. 4C and 4D, DS induced the expression of Nrf2-dependent genes.
Fig. 3. Measurement of the Cytotoxic Effect of DS.
MTT assay was performed to determine cytotoxicity of DS. RAW 264.7 cells were treated with indicated amounts of DS for 16 h prior to MTT assay. No significant cytotoxicity of DS was observed. Data represent the mean±SEM of three independent measurements.
Fig. 4. DS activates Nrf2.
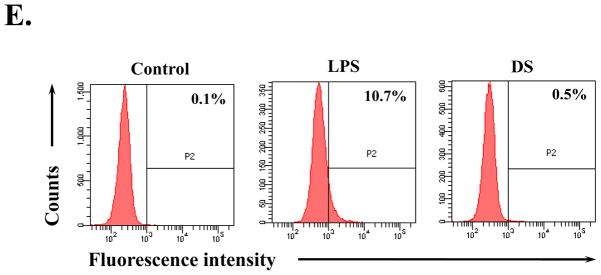
RAW 264.7 cells were treated with indicated amounts of DS for 16 h. The nuclear fraction of the treated cells was isolated, and the nuclear Nrf2, indicative of activation, was measured by western blot analysis(A), which was quantified by densitometric analysis with ImageJ (B). DS induced nuclear localization of Nrf2, like sulforaphane (SFN), a well-documented activator of Nrf2.Total RNA was extracted, and the expression of Nrf2-dependent genes, including GCLC, HO-1 and NQO-1, was analyzed by semi-quantitative RT-PCR (C), and relative expression of each gene against GAPDH was determined by densitometric analysis of each band (D). (E) FACS analysis was performed to measure ROS from the cells that were treated with LPS, an inducer of ROS, or DS. All experiment was repeated at least three times.
Since reactive oxygen species (ROS) exacerbates inflammation and activates Nrf2(Johnson, et al. 2008), it is possible that DS or other impurity, if any, in DS can induce ROS production, which, as a result, causes Nrf2 activation. To exclude this possibility, we measured ROS produced from DS treated cells. RAW 264.7 cells were treated with the highest amount of DS (100 μg/ml) used in this study for 16 h, and were stained with carboxy-H2DCFDA prior to flow cytometric analysis. As shown in Fig. 4E, unlike LPS, an inducer of ROS, DS did not elicit ROS production. In similar experiments in which the cells were treated for 4 h, DS yielded no ROS either (data not shown). Together, these results suggest that the anti-inflammatory effect of DS can be mediated by Nrf2 activation without involvement of ROS.
3. 3. DS suppresses NF-κB activation
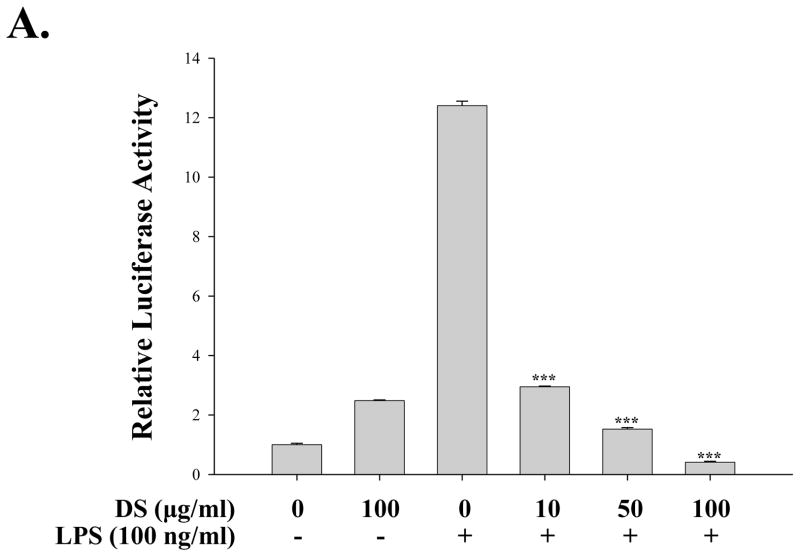
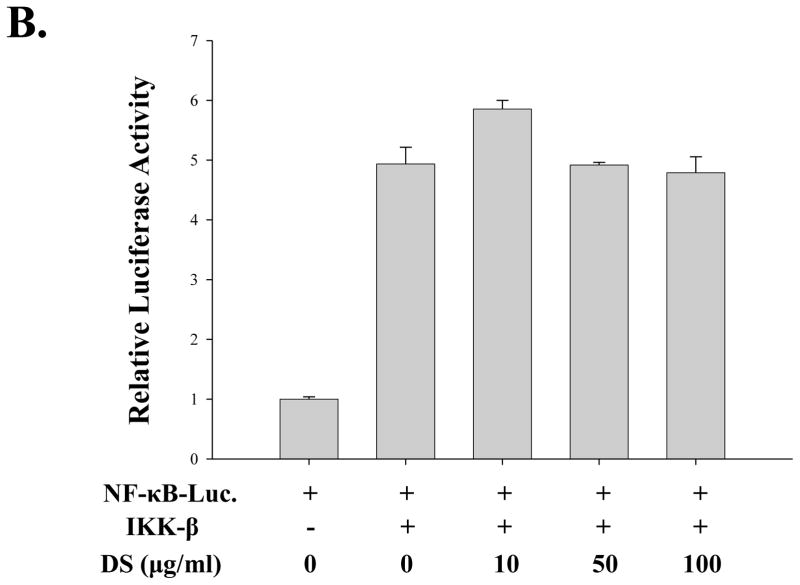
Since NF-κB is a well-documented transcription factor which is a master regulator of many of the pro-inflammatory cytokines, we tested the possibility that DS suppresses pro-inflammatory NF-κB activity. For these studies, we used a RAW 264.7 cell line that stably harbors an NF-κB-luciferase reporter construct (see materials and methods). As shown in Fig. 5A, treatment of the cell line with highly purified, TLR4 specific LPS (100 ng/ml) for 18 h yielded a strong luciferase activity, indicating that LPS treatment activates NF-κB mediated transcription in the cell line (the 3rd column). Pretreatment of the cell line with DS significantly suppressed the NF-κB activity (from 4th to 6th columns), suggesting that DS impacts on TLR4 signaling. To obtain an insight into how DS regulates NF-κB activity in TLR4 signaling, we performed a transfection experiment with HEK293 cells that do not express TLR4.The cells were transfected with a plasmid encoding IKK-β, the major kinase for NF-κB activation which is regulated by inflammatory stimuli (Ghosh and Karin,2002), along with the NF-κB reporter construct. At 24h after transfection, the transfected cells were treated with various amounts of DS for 16 h prior to luciferase assay. As shown in Fig. 5B, DS had no significant impact on IKK-β driven NF-κB activation. These results suggest that DS suppresses NF-κB transcriptional activity not by directly affecting IKK, rather by affecting upstream of IKK in TLR4 signaling.
Fig. 5. DS suppresses NF-κB activity.
(A) An NF-κB reporter cell line derived from RAW 264.7 cells was treated with the indicated amounts of DS for 16 h prior to LPS treatment for 16 h. Luciferase activity was normalized by the amount of total proteins in cell lysate (*** P< 0.005, compared to the LPS-treated (the 3rd column)). (B) HEK 293 cells were transfected with either 0.1 μg of an empty plasmid (pcDNA 3.1) or a plasmid encoding IKK-β, along with 0.05 μg of NF-κB reporter construct. Total amount of transfected DNA was 0.2 μg. At 24 h after transfection, the transfected cells were treated with the indicated amounts of DS for 16 h. Luciferase activity was normalized to tk-Renilla luciferase activity. No significant effect was detected. Data represent the mean±SEM of three independent experiments.
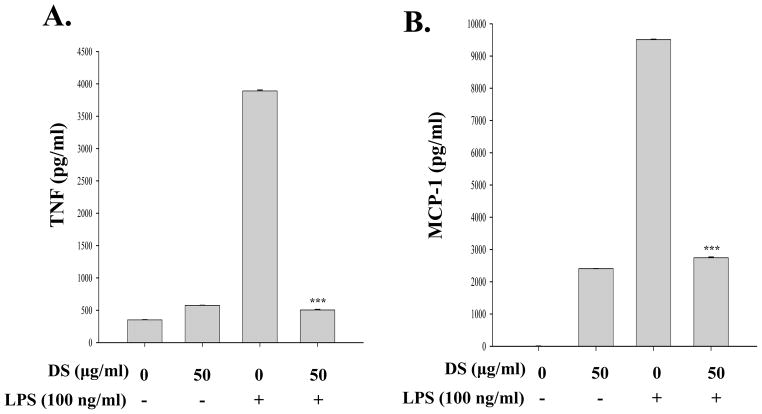
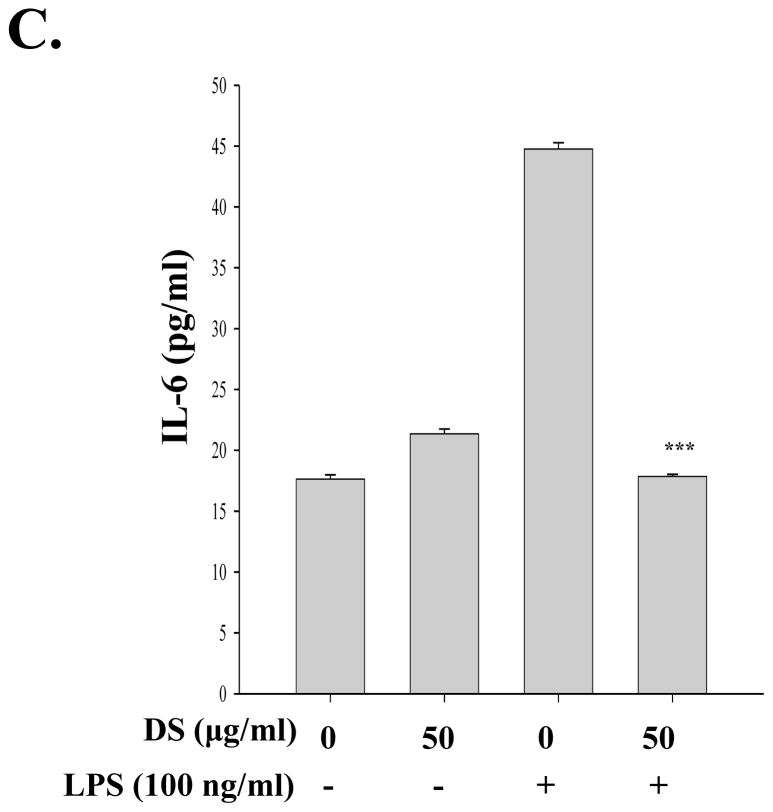
Since activated macrophages produce various inflammatory cytokines and chemokines including TNF-α, IL-1β, MCP-1, and IL-6 and NF-κB regulates the expression of these genes(Beutler and Rietschel,2003), we tested whether DS suppresses the production of NF-κB dependent pro-inflammatory molecules in macrophages. RAW 264.7 cells were treated with DS for 16 h and then with LPS (100 ng/ml). At 18 h after LPS treatment, the inflammatory molecules in the cell culture supernatant were measured by flow cytometric analysis. As shown in Fig. 6A, B and C, LPS treatment induced production of pro-inflammatory cytokines TNF-α, MCP-1, and IL-6 in RAW 264.7 cells, which was significantly suppressed by DS treatment. Taken together, these results demonstrate that DS suppresses NF-κB activity.
Fig. 6. DS suppresses the expression of pro-inflammatory cytokines.
RAW 264.7 cells, pretreated with DS or PBS for 16h, were further treated with LPS for 16 h. The cell culture supernatant was collected, and pro-inflammatory cytokines were measured by ELISA. LPS treatment (3rd column in each panel) induced TNF-α (A), MCP-1 (B), and IL-6 (C), which were marked suppressed by DS (4th column in each panel). Data represent the mean±SEM of three independent experiments (*** P< 0.005, compared to the LPS-treated).
3. 4. DS enhances the expression of HO-1 and suppresses that of pro-inflammatory cytokines in inflamed mouse lungs
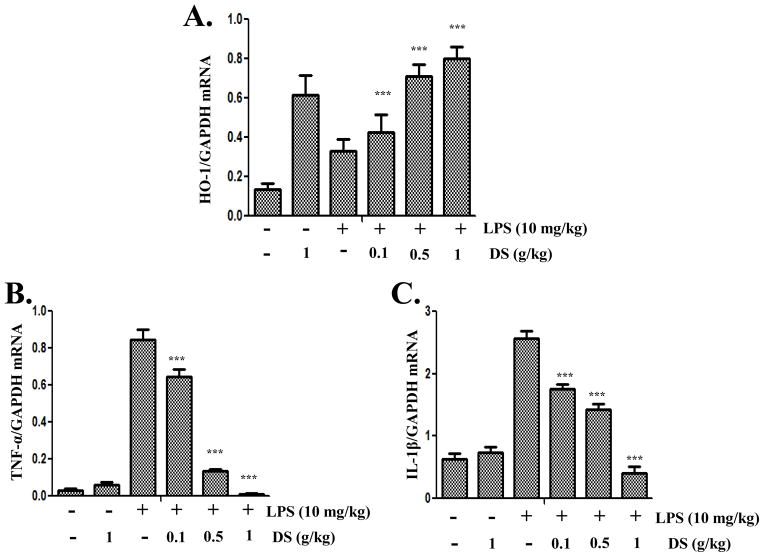
Lastly, we examined the effects of DS on the expression of Nrf2- and NF-κB-dependent genes in inflamed lungs. For the study, we performed the similar experiment described in Fig. 2. Mice (n=5 in each experimental group) were fed with either PBS or three different doses of DS (0.1, 0.5, and 1g/kg of a mouse) for 15 days. Differentially treated mice received an intranasal LPS (10mg/kg of a mouse) or sham. At 24 h after the treatments, lungs were harvested, from which total RNA was extracted for semi-quantitative RT-PCR analyses of Nrf2- and NF-κB-dependent gene expression. As shown in Fig. 7A, DS alone induced the expression of HO-1 in the lungs (the 2ndcolumn). LPS alone also did HO-1 expression (the 3rd column), which is likely due to ROS that were generated during inflammatory reaction in the lung. In mice treated with DS and LPS, DS enhanced the expression of HO-1 in a dose dependent manner(columns 2, 4, 5, and 6). On the other hand, similar analyses show that LPS instillation induced the expression of TNF-α (the 3rd column in Fig. 7B) and IL-1β(the 3rd column in Fig. 7C), which were however suppressed by DS in a dose dependent manner (columns4 to 6 in B and C). Together, these results suggest that DS induces the expression of an Nrf2-dependent gene and suppresses that of NF-κB dependent genes in the inflamed lungs.
Fig. 7. DS enhances the expression of HO-1 and suppresses that of pro-inflammatory cytokines in mouse lungs.
Mice (n=5 in each group), fed with either PBS or the indicated amounts of DS, received an intranasal LPS or sham. The expressions of HO-1 (A), TNF-α (B), and IL-1β (C)in the lungs were measured by semi-quantitative RT-PCR and subsequently densitometric analyses of PCR bands. Relative expression of each gene was normalized against that of GAPDH. Data represent the mean±SEM (*** P< 0.05, compared to the LPS-treated).
4. Discussion
Inflammation is an important component of innate immune response that in many ways prevents tissue damage and helps tissue healing. However, when uncontrolled, it results in tissue damage(Serhan, et al., 2008). In accordance with this notion, accumulating evidence shows that dysregulated inflammation is closely associated with a variety of disorders including cancer, autoimmune diseases, diabetes, and neuronal diseases (Nathan and Ding,2010). Therefore, curtailing and regulating inflammation could be an important measure for effective treatment of these diseases. In many Asian countries, inflammation-associated diseases have long been treated with the herbal extracts as part of traditional medicine (Yu, et al., 2006).For instance, the roots of Aralia continentalis Kitagawa (Araliaceae)have long been used to treat rheumatism, lumbago, and lameness (Lim, et al.,2009). Given the extensive history of safety and effective usage of medicinal herbs, it is possible that these remedies could provide significant health benefits to patients who suffer from these crippling disorders. However, there is little understanding of mechanisms by which these herbal extract execute their effects. This has hampered the integration of these traditional remedies into mainstream medicine. In this study, we postulate that the therapeutic effect of DS is associated with regulation of inflammation, and sought to elucidate the mechanisms by which these anti-inflammatory effects are executed. Our results show that pretreatment of mice with DS suppressed acute lung inflammation induced by LPS. In vitro studies using macrophage cell lines show that DS activated anti-inflammatory factor Nrf2 but suppressed pro-inflammatory factor NF-κB. Similarly, in inflamed lungs, DS enhanced the expression of HO-1, an Nrf2-dependent gene, but suppressed the expression of TNF-α and IL-1β, NF-κB dependent genes. These results suggest that the therapeutic effects of DS stem from, at least in part, its anti-inflammatory activity, which is mediated by differential regulation of Nrf2 and NF-κB activities.
Nrf2 is a key transcription factor with anti-oxidant effects which has been shown to be protective in various inflammatory diseases, as evidenced by genetic ablation of nrf2in mice that show exacerbation of acute lung inflammation, smoke-induced emphysema and asthma (Chan and Kan,1999;Mochizuki, et al.,2005;Rangasamy, et al.,2004;Rangasamy, et al.,2005). However, Nrf2 is activated in pathologic conditions by oxidative stress that is culminated during inflammatory responses. For example, ROS produced by macrophages and neutrophils create an oxidative environment, which results in activation of Nrf2 and subsequent induction of Nrf2-dependent gene expression in inflicted cells(Thimmulappa, et al. 2006). Activated Nrf2 induces the expression of ROS-scavenging enzymes so as to protect from collateral tissue damage caused by ROS, contributing to suppression and possibly resolution of inflammation. Thus, activation of Nrf2 without generating ROS would be an attractive approach to curtail inflammation. Our results show that DS activated Nrf2 without generating ROS and that DS induced Nrf2 dependent protective genes in RAW 264.7 cells and in inflamed mouse lungs. Although it would be necessary to employ nrf2−/− mice to definitively determine whether DS exerts its anti-inflammatory effect through Nrf2, our results suggest that DS can be useful in treating various inflammatory diseases that have been shown to associate with deregulation of the expression of Nrf2.
Secondly, our results show that DS suppressed the transcriptional activity of NF-κB in vitro and that DS in vitro and in inflamed lungs inhibited the induction of several NF-κB dependent genes including TNF-α, IL-1β, and IL-6(Alcamo, et al., 2001;Libermann and Baltimore,1990).These results suggest that DS is a potent anti-inflammatory herbal formula that exertsits anti-inflammatory effects by suppressing the pro-inflammatory factor NF-κB and pro-inflammatory gene expression and by activating the anti-inflammatory factor Nrf2 and Nrf2-dependent gene expression. Potent anti-inflammatory drugs that control both Nrf2 and NF-κB are not unprecedented. For example, 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl] imidazole (CDDO-IM), a triterpenoids derivative that has been developed and tested as an anti-inflammatory drug for renal diseases and cancer, activates Nrf2 but suppresses NF-κB(Liby, et al., 2005).
It is interesting that, as shown in this study, DS, which has been traditionally prescribed for the treatment of inflammation by physical trauma, lowered the level of IL-6 because increased IL-6 is clinically correlated with exacerbation of trauma and the trauma-associated inflammation, which makes IL-6 an important biomarker for trauma (Jawa, et al., 2011b;Biffl, et al. 1996b). Since IL-6 is pleiotropic, affecting other diseases including atherosclerosis, myocardial infarction, obesity and asthma (Jawa, et al., 2011a;Biffl, et al., 1996a), DS is likely to be effective in ameliorating a variety of diseases that involve IL-6.
It is unclear how DS activates Nrf2 and suppresses NF-κB. Since DS is a formula composed of various constituents, it is highly likely that the strong anti-inflammatory effect of DS results from a collective effect of multiple natural compounds. Accumulating documents have reported key compounds in each herb comprising DS. In Angelica gigas, one of the nine herbs in DS, decursin (Kim, et al., 2010) and decursinolangelate(Li, et al., 2011) were reported to suppress inflammatory responses by regulating Nrf2 and NF-κB simultaneously. Similarly, Linderastrychnifolia contains alkaloids that down-regulate NF-κB (Luo, et al., 2009) and lindenenyl acetate that can activate Nrf2(Li, et al., 2009). In addition, Caesalpiniasappan contains brazilin that suppresses NF-κB activity (Bae, et al., 2005) and isoliquiritigenin 2′-methyl ether that can activate Nrf2 (Lee, et al., 2010). Thus, it is clear that most herbs comprising DS can both activate Nrf2 and suppress NF-κB. It is however interesting that, in our hands, only the water extract of Carthamustinctorius activates strongly Nrf2 (unpublished results). These results may reflect the different compositions of an extract depending on extraction methods.
Several layers of mechanisms regulate Nrf2 activation. One of the well-established mechanisms is through Keap1, an inhibitor that binds to and mediates a continual degradation of Nrf2, and any modification of the key cysteine residues of Keap1 results in activation of Nrf2 (Kaspar, et al., 2009). Through xenobiotics metabolism, organic molecules undergo modifications to be soluble, in which process oxidants are generated and can activate Keap1 (Estabrook,2003). In this study, we used an aqueous extract of DS, the constituents of which are hydrophilic and membrane-impermeable. Thus, it is conceivable that some of the constituents could be selectively transported into the cells and formed adducts of Keap1, which will dissociate Keap1 from Nrf2 and result in Nrf2-dependent gene expression. Alternative possibility is that since serine kinases, including PKC, MAPK and PERK, phosphorylate and activate Nrf2 (Cullinan, et al., 2003;Yu, et al., 2000;Zipper and Mulcahy,2000), the constituents of DS act on receptors that signals to activate the kinases to phosphorylate Nrf2. This possibility is upheld by our results showing that DS suppress NF-κB activity induced by LPS, but not by IKK-β, which suggests the possibility that the constituents of DS competes with LPS for TLR4 receptor.
Understanding the precise mechanisms of the anti-inflammatory effects of DS should be founded on the identification of the constituents exerting those effects. It is possible that various constituents in DS directly or cooperatively with other constituents for the anti-inflammatory effect. However, it is also likely that some of the constituents work differently, promoting inflammation. Some of our results are in line with this notion. As shown in Fig. 5A and Fig. 6B, DS alone can activate NF-κB and the expression of some of NF-κB dependent genes slightly. However, as shown in Fig. 2B, the net effect of DS in an acute lung inflammation animal model was suppression of inflammation.
5. Conclusion
We examined the anti-inflammatory effect of DS, a formula that has been prescribed as a traditional Asian medicine for the treatment of inflammation induced by physical trauma. We found that DS activated Nrf2, a key anti-inflammatory factor, and suppressed NF-κB, a well-established pro-inflammatory factor in vitro. In an acute lung injury animal model, DS enhanced the expression of an Nrf2 dependent gene and suppressed that of NF-κB dependent genes, ameliorating acute lung inflammation. Thus, we propose the possibility that the therapeutic effects of DS are executed by differentially regulating key transcription factors that control the inflammatory response. These results unveil important mechanism by which traditional oriental remedies exert anti-inflammatory effects and thus can be used as complementary and alternative medicines for devastating diseases.
Acknowledgments
This work was financially supported by grants from KRIBB Research Initiative Program, Korea Research Council of Fundamental Sciences& Technology (NTM1000913), and Ministry of Education Science and Technology (KGM2250911).
Abbreviations
- NF
nuclear factor
- E
erythroid
- Nrf2
2 related factor 2
- GCLC
glutamate-cysteine ligase catalytic subunit
- NQO1
NAD(P)H:quinine oxidoreductase-1
- HO-1
heme oxygenase-1
- IKK
inhibitory κB kinase
Footnotes
Disclosures
The authors do not have a commercial or other association that might have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alcamo E, Mizgerd JP, Horwitz BH, Bronson R, Beg AA, Scott M, Doerschuk CM, Hynes RO, Baltimore D. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-kappa B in leukocyte recruitment. Journal of Immunology. 2001;167:1592–1600. doi: 10.4049/jimmunol.167.3.1592. [DOI] [PubMed] [Google Scholar]
- Bae IK, Min HY, Han AR, Seo EK, Lee SK. Suppression of lipopolysaccharide-induced expression of inducible nitric oxide synthase by brazilin in RAW 264.7 macrophage cells. European Journal of Pharmacology. 2005;513:237– 242. doi: 10.1016/j.ejphar.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nature Reviews Immunology. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- Biffl WL, Moore EE. Splanchnic ischemia/reperfusion and multiple organ failure. British Journal of Anaesthesia. 1996a;77:59–70. doi: 10.1093/bja/77.1.59. [DOI] [PubMed] [Google Scholar]
- Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Annals of Surgery. 1996b;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart MK. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arteriosclerois, Thrombosis, and Vascular Biology. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proceedings of the National Academy of Sciences of the United State of America. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. American Journal of Respiratory Cell and Molecular Biology. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB Journal. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Molecular and Cellular Biololgy. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook RW. A passion for P450s (rememberances of the early history of research on cytochrome P450) Drug Metabolism and Disposition. 2003;31:1461–1473. doi: 10.1124/dmd.31.12.1461. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and Biophysical Research Communications. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radical Biology and Medicine. 2000;29:254–262. doi: 10.1016/s0891-5849(00)00306-3. [DOI] [PubMed] [Google Scholar]
- Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Analytic review: Interleukin-6 in surgery, trauma, and critical care: part I: basic science. Journal of Intensive Care Medicine. 2011a;26:3–12. doi: 10.1177/0885066610395678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implication. Journal of Intensive Care Medicine. 2011b;26:73–87. doi: 10.1177/0885066610384188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Annals of the New York Academy of Sciences. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo M, Kwon M, Sadikot RT, Kingsley PJ, Marnett LJ, Blackwell TS, Peebles RS, Jr, Urade Y, Christman JW. Induction and function of lipocalin prostaglandin D synthase in host immunity. Journal of Immunology. 2007;179:2565–2575. doi: 10.4049/jimmunol.179.4.2565. [DOI] [PubMed] [Google Scholar]
- Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Biology and Medicine. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park SH, Kim YW, Ha JM, Bae SS, Lee GS, Cho SI, Choi BT, Shin HK. The Traditional Herbal Medicine, Dangkwisoo-San, Prevents Cerebral Ischemic Injury through Nitric Oxide-Dependent Mechanisms. Evidence-based Complementary and Alternative Medicine. 2011;2011:718302. doi: 10.1155/2011/718302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Lee MY, Kim JH, Suk K, Lee WH. Decursinol angelate blocks transmigration and inflammatory activation of cancer cellsthrough inhibition of PI3K, ERK and NF-kappaB activation. Cancer Letter. 2010;296:35–42. doi: 10.1016/j.canlet.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Lee YM, Jeong GS, Lim HD, An RB, Kim YC, Kim EC. Isoliquiritigenin 20-methyl ether induces growth inhibition and apoptosis in oral cancer cells via heme oxygenase-1. Toxicology in Vitro. 2010;24:776–782. doi: 10.1016/j.tiv.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Li B, Jeong GS, Kang DG, Lee HS, Kim YC. Cytoprotective effects of lindenenyl acetate isolated from Lindera strychnifolia on mouse hippocampal HT22 cells. European Journal of Pharmacology. 2009;614:58–65. doi: 10.1016/j.ejphar.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Li L, Li W, Jung SW, Lee YW, Kim YH. Protective effects of decursin and decursinol angelate against amyloid β-protein-induced oxidative stress in the PC12 cell line: the role of Nrf2 and antioxidant enzymes. Bioscience, Biotechnology, and Biochemistry. 2011;75:434–442. doi: 10.1271/bbb.100606. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Molecular and Cellular Biololgy. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Research. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- Lim H, Jung HA, Choi JS, Kim YS, Kang SS, Kim HP. Anti-inflammatory activity of the constituents of the roots of Aralia continentalis. Archives of Pharmacological Research. 2009;32:1237–1243. doi: 10.1007/s12272-009-1909-3. [DOI] [PubMed] [Google Scholar]
- Luo Y, Liu M, Yao X, Xia Y, Dai Y, Chou G, Wang Z. Total alkaloids from Radix Linderae prevent the production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 cells by suppressing NF-kB and MAPKs activation. Cytokine. 2009;46:104–110. doi: 10.1016/j.cyto.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Minino AM, Anderson RN, Fingerhut LA, Boudreault MA, Warner M. Deaths: injuries, 2002. National Vital Statistics Report. 2006;54:1–124. [PubMed] [Google Scholar]
- Mochizuki M, Ishii Y, Itoh K, Iizuka T, Morishima Y, Kimura T, Kiwamoto T, Matsuno Y, Hegab AE, Nomura A, Sakamoto T, Uchida K, Yamamoto M, Sekizawa K. Role of 15-deoxy delta(12,14) prostaglandin J2 and Nrf2 pathways in protection against acute lung injury. American Journal of Respiratory and Critical Care Medicine. 2005;171:1260–1266. doi: 10.1164/rccm.200406-755OC. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. Journal of Clinical Investigation. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. Journal of Experimental Medicine. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. Journal of Clinical Investigation. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. New England Journal of Medicine. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Yu HQ, Rafi MM, Ho CT. Targeting inflammation using Asian herbs. In: Ho CT, editor. Herbs: Challenges in Chemistry and Biology. Oxford: Oxford University Press; 2006. pp. 266–280. [Google Scholar]
- Yu R, Chen C, Mo YY, Hebbar V, Owuor ED, Tan TH, Kong AN. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. Journal of Biological Chemistry. 2000;275:39907–39913. doi: 10.1074/jbc.M004037200. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Sun Z, Habib GM, Lieberman MW, Hannink M. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteosome-independent pathway. Journal of Biological Chemistry. 2005;280:30091–30099. doi: 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- Zipper LM, Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochemical and Biophysical Research Communications. 2000;278:484–492. doi: 10.1006/bbrc.2000.3830. [DOI] [PubMed] [Google Scholar]