Abstract
The aim of this study was to investigate food intake, serum leptin levels, and leptin mRNA expression during the sexual cycle in rats. Female Wistar-Imamichi rats aged 8-10 weeks were used in this experiment. Food intake was measured during the light and dark phases (light on at 07:00 and off at 19:00) of the 4-day estrous cycle in female rats. Serum leptin levels were measured by ELISA, and leptin mRNA expression levels were analyzed using real-time PCR on diestrous- and proestrous-stage rats. Our results revealed that during the sexual cycle, food intake was significantly higher in the dark phase compared with the light phase. Food intake in proestrous females was significantly lower in the light and dark phases compared with the other groups. Serum leptin concentrations were significantly higher in both phases in proestrous rats compared with diestrous rats. There was a significant increase in leptin mRNA expression in adipose tissue during the proestrous period compared with the diestrous period. These findings suggest that increased leptin mRNA expression and serum leptin levels, which are induced by estrogen during the proestrous stage, may play a role in regulating appetitive behavior.
Keywords: Estrous cycle, food intake, leptin
The physiological control of feeding is one of many behavioral functions that are influenced by the hypothalamic-pituitary gonadal (HPG) axis [1]. For survival and reproductive success, reproductive hormones have co-adapted to modulate ingestive behavior, energy storage, and energy expenditure [2]. Hormones have multiple effects on food intake, body weight, carcass composition, and voluntary exercise in rats, suggesting that hormone-induced behavior changes may alter body weight and levels of adipose tissue [3,4]. Another study suggested that variations in the estrous cycle may affect meal size and body weight in female rats [5].
Leptin, a protein secreted from adipose tissue, has been proposed to be a satiety factor that reduces food intake and enhances energy expenditure, leading to reduced fat storage [6,7]. Plasma leptin levels are directly proportional to existing fat reserves. Leptin not only controls food intake, but it is also involved in reproductive neuroendocrine functions, which are controlled by the hypothalamus [8]. Leptin communicates the sufficiency of energy stores for reproduction by promoting the activity of GnRH neurons [9]. The response to leptin changes during hormonally induced estrous cycles in rats may cause cyclic feeding behavior in rats [1]. While food intake and body weight of rats fluctuates during the estrous cycle, it remains to be determined whether changes in leptin responses occur during the rat estrous cycle. The present study was conducted to determine the concentration of serum leptin and the expression of leptin mRNA in adipose tissue as well as food intake during the estrous cycle of intact female rats.
Materials and Methods
Animals
Twenty-four specific-pathogen-free Iar: Wistar-Imamichi female rats were obtained from the Imamichi Institute of Animal Reproduction (Tsuchiura, Ibaraki, Japan) at 7 weeks of age. The animals were seronegative for Mycoplasma pulmonis, Bacillus piliformis, Boldetella bronchiseotia, Streptococcus pneumoniae, and Sendai virus. They were housed in individual cages at a temperature of 24±2℃ in a light-controlled room (12:12 h light:dark cycle). Food (Oriental MF: Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water were provided ad libitum. The animals were allowed to acclimatize for seven days after arrival to the animal facilities. The animals were around 197±4.2 g and 9 weeks of age, when entering the study. The animal studies were approved by the Institutional Animal Care and Use Committee of the Nippon Veterinary and Life Science University and carried out in accordance with the National Institutes of Health, USA Guide for the Care and Use of Laboratory Animals. All efforts were undertaken according to the "3R principles" of the directive to reduce the number of animals used in this study, and optimize experimental protocols for obtaining maximum data from each tested animals.
Vaginal smear
On reaching the age of 8 weeks, estrous cycles were monitored daily using vaginal smears taken between 14:00 and 15:00 h. Smears were examined under a light microscope to estimate the relative preponderance of cornified epithelial cells, nucleated epithelial cells, and leukocytes [10]. Female rats that had experienced at least two consecutive 4-day estrous cycles were used for these studies.
Measurement of diet and leptin
Food consumption was measured by weighing the food hoppers at 07:00 and 19:00 h; a visual check was made for any diet dropped on the floor of the cage. Measurements were taken over the period of the 4-day estrous cycle.
Rats in proestrous and diestrous were decapitated between 14:00-15:00 and 19:00-20:00 h, and blood samples were collected from the vena cava cuadalis and centrifuged at 2,200 g for 15 min at 4℃. The serum was stored at -35℃ until it was analyzed for leptin concentration using a commercially available ELISA Kit (Rat Leptin ELISA KIT(U-type) AKRLP-D10; Shibayagi Co., Ltd., Gunma, Japan)
Leptin mRNA expression
For mRNA studies, visceral abdominal fat was dissected and immediately frozen in liquid nitrogen and stored at -80℃ until RNA extraction. Total RNA was isolated using an RNeasy Lipid Tissue Midi kit (Qiagen, Maryland, USA) according to the manufacturer's instructions. RNA samples were quantified spectro-photometrically (Nano drop, ND-1000, V3.12, Houston, TX, USA), and the integrity was confirmed using a 1.5% agarose gel containing 2.2 M formaldehyde. Total RNA was transcribed into first strand cDNA for each sample using a SuperScript™ III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) in a final volume of 20 µL; 1 µg RNA sample was added with 1 µL of 20 µM oligo dT Primer, and DNase- and RNase-free water. Samples were incubated at 65℃ for 10 min and then placed on ice. Then, 4 µL of 5x first-stand buffer, 2 µL of 0.1M dithiothreitol (DTT), and 4 µL of 10 mM 2'-deoxy-nucleoside 5'-triphosphate mixed solution (dNTP) was added. Samples were pre-incubated at 42℃ for 2 min, and then 0.5 µL of SuperScript™ III RT was added. Samples were incubated at 42℃ for 1 h, 30 µL of TE buffer (Tris-hydrochloride buffer, pH 8.0, containing 1.0 mM EDTA) was added, and the samples were heated at 65℃ for 10 min to inactivate the RTase.
Real time RT-PCR was performed using a specific forward primer (5'-GACCAGACCCTGGCAGTCTA-3') and reverse primer (5'-CTCAGCATTCAGGGCTAAGG-3') to amplify the product. Samples were normalized using the housekeeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH) using a forward primer (5'-TGTCAGCAATGCATCCTGCA-3') and reverse primer (5'-CCGTTCAGCTCTGGGATGAC-3'). Real time RT-PCR was performed in a 25-µL final reaction volume using Platinum SYBR Green qPCR Supermix-UDG (Invitrogen, Carlsbad, CA, USA). Each real time RT-PCR contained 12.5 µL of Platinum SYBR Green qPCR Supermix-UDG, 10 µM of each primer, and an RNA template. Real-time RT-PCR was carried out in duplicate for each sample using an ABI 7500 Real-time RT-PCR System (Applied Biosystems, Foster City, CA, USA) with the following parameters: one cycle of 50℃ for 2 min (UDG incubation); 95℃ for 2 min; 40 cycles of 95℃ for 15 sec and 60℃ for 30 sec. Analysis of melting curves were performed with each series to confirm the specificity of the amplified products. To confirm the absence of genomic DNA signal, amplifications without reverse transcriptase were performed. Standard curves were prepared for tested and housekeeping genes. All expression data were normalized by dividing the amount of GAPDH used for the target gene by the amount used for the control.
Statistical analysis
Statistical analysis was carried out using commercially available software (Stat-View, SAS Institute, Cary, NC, USA). Student's t-test and generalized Wilcoxon rank sum test were used where appropriate.
Results
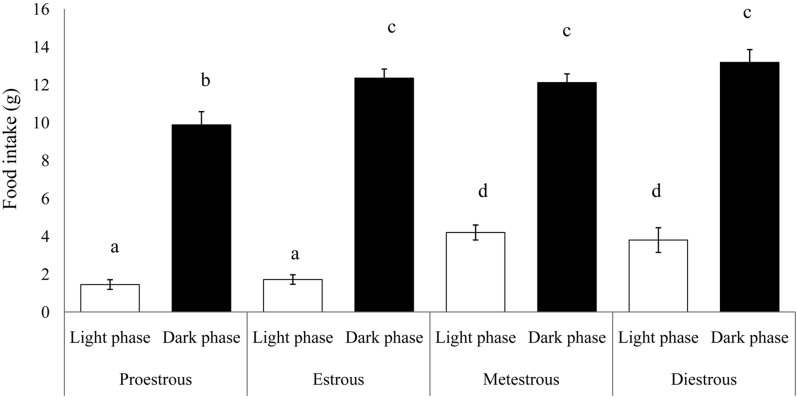
Food intake in intact female rats was measured over 4 days of the estrous cycle (Figure 1). Food intake was always greater (P<0.01) in the dark phase compared with the light phase, in all phases of the estrous cycle. Food intake in the dark phase in proestrous rats, however, was less (9.88±0.69 g) than that in the dark phase during the remaining of the estrous cycle (P<0.05). During the light phase, food consumption while in the proestrous or estrous phase, was lower than during the metestrous and diestrous periods (1.45±0.25 and 1.71±0.25 g, respectively) (P<0.01).
Figure 1.
Food intake in intact female rats through the 4-day estrous cycle. Data are presented as means±SEM (n=6). The different characters indicate significant differences between the periods of estrous cycle (P<0.01).
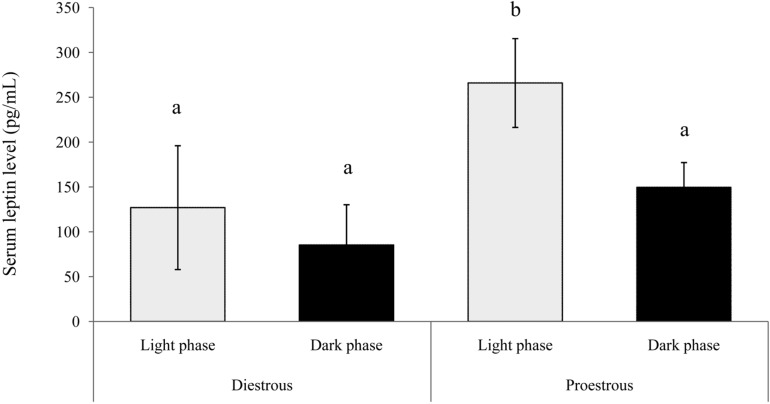
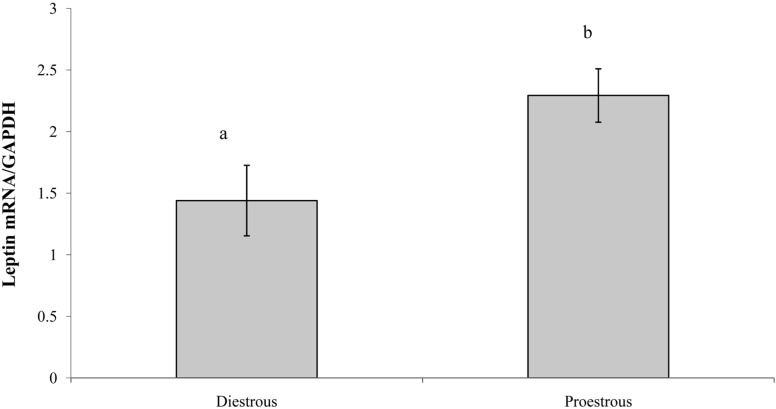
The concentration of serum leptin in proestrous and diestrous rats is presented in Figure 2. Serum leptin concentration was significantly higher (P<0.01) in the light phase of proestrous rats. The concentrations of serum leptin during the light and dark phases in diestrous rats were similar to that in the dark phase in proestrous rats. Similarly, leptin mRNA expression was higher in adipose tissue during the proestrous period (P<0.01) compared with the diestrous period (2.29±0.22 and 1.44±0.28 Leptin mRNA/GAPDH, respectively) (Figure 3).
Figure 2.
Serum leptin concentration in intact female rats during the proestrous and diestrous periods. Data are presented as means±SEM (n=6). The different characters indicate significant differences between the periods of estrous cycle (P<0.01).
Figure 3.
Leptin mRNA expression in adipose tissue during diestrous and proestrous. Data are presented means±SEM (n=6). The different characters indicate significant differences between the periods of estrous cycle (P<0.01).
Discussion
The present study demonstrates that food intake in intact female rats is higher in the dark phase compared with the light phase during the normal estrous cycle. Our results are in agreement with previous findings, which demonstrated that food intake in rats occurs mainly during the dark phase of the diurnal cycle [11-13]. Our observation that decreased food intake occurs during the light and dark phases of proestrous is similar to previous results showing that food intake is greatly reduced during proestrous [12]. However, Brobeck et al. [14] reported that decreased food intake occurred during the estrous phase. This pattern is a result of the estrogen surge that occurs around the time of increased physical activity and decreased eating activity, whereas estrogen levels, which alter thresholds of hypothalamic sensitivity, peak in the early morning of proestrous [12,14]. Decrease in food intake was facilitated by decreasing meal size due to a direct influence of estradiol on the CNS appetite centers, affecting the mechanism that terminates short-term food intake. These are not secondary to changes in the level of total daily food intake [15]. In the present study, serum leptin levels in proestrous and diestrous rats were high in the light phase compared with the dark phase. The levels were found in the light phase more highly in proestrous than diestrous rats. Leptin mRNA expression in adipose tissue was also higher in proestrous compared with diestrous rats. These results suggest that serum leptin levels are positively regulated by estrogen, as serum leptin concentrations peaked during periods of elevated estrogen activity in normal cycles. In contrast, serum leptin levels increased during the dark phase [13]. Bennet et al. [16] and Tanaka et al. [17] demonstrated that serum leptin levels increase during proestrous in rats. Evaluation of the body kinetics of leptin show that its half-life is only 9.4 min, indicating that the production rate varies significantly depending on hormonal factors such as the serum estrogen levels [18]. Other reports showed that serum leptin concentrations do not change significantly during the estrous cycle in rats [19-21]. Although some studies reported that estrogen stimulates the concentration of serum leptin and leptin mRNA expression in rat adipose tissue, which is mediated by the adipocyte estrogen receptor [1,17,22,23]. Sjoholm et al. [1] reported that hormonally induced changes in the responsiveness of leptin during the estrous cycle, particularly during the proestrous and estrous periods, and the mechanism of estrogen regulation of food intake involve an upregulation of leptin-receptor abundance and leptin sensitivity.
Leptin, which has been implicated in the regulation of reproduction, could act at multiple levels within the reproductive axis [9]. It prevents disruption of the estrous cycle in females, but only minimally restores LH level in males [24]. Leptin regulation of the reproductive system is mediated by its hypothalamic receptor eliciting the release of GnRH, which subsequently induces the synthesis and release of FSH and LH [9]. Tanaka et al. [17] reported that the elevation of serum leptin in the proestrous period might mediate the estrogen action that triggers preovulatory LH secretion. The LH surge is necessary for ovulation, which can induce estrogen levels during proestrous period.
In conclusion, our study show that food intake changes during the estrous cycle, and serum leptin concentration and leptin mRNA expression levels peak during proestrous in virgin female rats. Our data suggest that changes in serum leptin concentration during the estrous cycle may regulate cyclic feeding behavior in rats.
Acknowledgments
The authors thank RS Nakamura, Department of Comparative Medicine, School of Veterinary Medicine, Nippon Veterinary and Life Science University, for technical assistance. This study was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, of Japan (No. 05680742) to TRS.
References
- 1.Sjoholm K, Carlssom B, Carlsson LMS. Hypothalamic response to leptin changes during a hormonally induced estrous cycle in rats. Cent Eur J Biol. 2006;1:221–234. [Google Scholar]
- 2.Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Gray JM, Wade GN. Food intake, body weight, and adiposity in female rats: actions and interactions of progestins and antiestrogens. Am J Physiol. 1981;240(5):E474–E481. doi: 10.1152/ajpendo.1981.240.5.E474. [DOI] [PubMed] [Google Scholar]
- 4.Wade GN, Heller HW. Tamoxifen mimics the effects of estradiol on food intake, body weight, and body composition in rats. Am J Physiol. 1993;264:R1219–R1223. doi: 10.1152/ajpregu.1993.264.6.R1219. [DOI] [PubMed] [Google Scholar]
- 5.Tritos NA, Segal-Lieberman G, Vezeridis PS, Maratos-Flier E. Estradiol-induced anorexia is independent of leptin and melanin-concentrating hormone. Obes Res. 2004;12(4):716–724. doi: 10.1038/oby.2004.84. [DOI] [PubMed] [Google Scholar]
- 6.Koochmeshgi J. Reproductive switch and aging: the case of leptin change in dietary restriction. Ann N Y Acad Sci. 2004;1019:436–438. doi: 10.1196/annals.1297.079. [DOI] [PubMed] [Google Scholar]
- 7.Saito TR, Suzuki M, Aoki-Komori S, Tanaka M. Food intake and leptin concentrations of lactating rats nursing various sized litters. Reprod Med Biol. 2005;4:203–206. doi: 10.1111/j.1447-0578.2005.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin B, Golden E, Carlson OD, Egan JM, Mattson MP, Maudsley S. Caloric restriction: impact upon pituitary function and reproduction. Ageing Res Rev. 2008;7(3):209–224. doi: 10.1016/j.arr.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67(6):370–376. doi: 10.1159/000054335. [DOI] [PubMed] [Google Scholar]
- 10.Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 11.Asakuma S, Hiraku O, Kurose Y, Kobayashi S, Terashima Y. Diurnal rhythm of cerebrospinal fluid and plasma leptin levels related to feeding in non-lactating and lactating rats. J Endocrinol. 2004;180(2):283–286. doi: 10.1677/joe.0.1800283. [DOI] [PubMed] [Google Scholar]
- 12.ter Haar MB. Circadian and estrual rhythms in food intake in the rat. Horm Behav. 1972;3(3):213–219. doi: 10.1016/0018-506x(72)90034-7. [DOI] [PubMed] [Google Scholar]
- 13.Nagatani S, Guthikonda P, Foster DL. Appearance of a nocturnal peak of leptin secretion in the pubertal rat. Horm Behav. 2000;37(4):345–352. doi: 10.1006/hbeh.2000.1582. [DOI] [PubMed] [Google Scholar]
- 14.Brobeck JR, Wheatland M, Strominger JL. Variations in regulation of energy exchange associated with estrus, diestrus and pseudopregnancy in rats. Endocrinology. 1947;40(2):65–72. doi: 10.1210/endo-40-2-65. [DOI] [PubMed] [Google Scholar]
- 15.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17(2):201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 16.Bennett PA, Lindell K, Wilson C, Carlsson LM, Carlsson B, Robinson IC. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology. 1999;69(6):417–423. doi: 10.1159/000054444. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, Nakaya S, Kumai T, Watanabe M, Tateishi T, Shimizu H, Kobayashi S. Effects of estrogen on serum leptin levels and leptin mRNA expression in adipose tissue in rats. Horm Res. 2001;56(3-4):98–104. doi: 10.1159/000048099. [DOI] [PubMed] [Google Scholar]
- 18.Zeng J, Patterson BW, Klein S, Martin DR, Dagogo-Jack S, Kohrt WM, Miller SB, Landt M. Whole body leptin kinetics and renal metabolism in vivo. Am J Physiol. 1997;273:E1102–E1106. doi: 10.1152/ajpendo.1997.273.6.E1102. [DOI] [PubMed] [Google Scholar]
- 19.Amico JA, Thomas A, Crowley RS, Burmeister LA. Concentrations of leptin in the serum of pregnant, lactating, and cycling rats and of leptin messenger ribonucleic acid in rat placental tissue. Life Sci. 1998;63(16):1387–1395. doi: 10.1016/s0024-3205(98)00405-6. [DOI] [PubMed] [Google Scholar]
- 20.Frederich RC, Löllmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96(3):1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha M, Grueso E, Puerta M. The anorectic effect of oestradiol does not involve changes in plasma and cerebrospinal fluid leptin concentrations in the rat. J Endocrinol. 2001;171(2):349–354. doi: 10.1677/joe.0.1710349. [DOI] [PubMed] [Google Scholar]
- 22.Kristensen K, Pedersen SB, Richelsen B. Regulation of leptin by steroid hormones in rat adipose tissue. Biochem Biophys Res Commun. 1999;259(3):624–630. doi: 10.1006/bbrc.1999.0842. [DOI] [PubMed] [Google Scholar]
- 23.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140(4):1567–1574. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- 24.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]