Abstract
Gut functions, such as gastrointestinal motility, gastric secretion and pancreatic secretion, were reduced with age. Glucose tolerance is impaired, and the release of insulin and β-cell's sensitivity on glucose are reduced with age. However, a lot of controversial data have been reported as insulin concentrations after glucose ingestion are either higher or no different in elderly and young subjects. Thus, this study was aimed to investigate whether aging could affect pancreatic exocrine secretion and its action mechanisms. An isolated perfused rat pancreatic model was used to exclude the effects of external nerves or hormones. Pancreatic secretion was increased by CCK under 5.6 mM glucose background in the isolated perfused pancreas of young (3 months), 12 months and 18 months aged rats. There was no significant difference between young and aged rats. In 3 months old rats, CCK-stimulated pancreatic secretion was potentiated under 18 mM glucose background. However, the potentiation effects of endogenous insulin and CCK were not observed in 12 and 18 months old rats. Exogenous insulin also potentiated CCK-stimulated pancreatic secretion in 3 months old rats. Similarly, exogenous insulin failed to potentiate CCK-stimulated pancreatic secretion as that of 3 months old rats. Wet weight of pancreas and amylase content in pancreatic tissue were not changed with age. These results indicate that pancreatic exocrine secretion is reduced with age and endogenous insulin secretion and/or action is involved in this phenomenon.
Keywords: Aging, glucose, insulin, cholecystokinin, exocrine secretion
Aging changes functions variously in part of the body, including the gastrointestinal tract [1,2]. Also, aging causes obesity, diabetes and abnormality of glucose tolerance. The occurrence of these diseases, malabsorption and indigestion are very usual in old age, and these suggest that pancreatic function may be changed with age. Although the pancreas is a very important organ for digestion, effects of aging on pancreatic exocrine secretion are still unclear.
Insulin potentiates cholecystokinin (CCK)-stimulated pancreatic exocrine secretion and pancreatic polypeptide suppresses the potentiation action of insulin [3]. It has been reported that insulin receptor exists in the membrane of the pancreatic acinar cells [4-6], and insulin directly enters to the acinar cells [7]. These findings suggest that insulin directly acts acinar cells and has important role to maintain exocrine secretion in the acinar cells. On the other side, it has been reported that the abnormality of glucose tolerance increases with age and the insulin secretion on maximal glucose decreases in 2 months old rats than in 12 months old rats [8]. Glucose sensitivity and responsibility of the β-cell of aged rat are low in the isolated perfused pancreas of the young rat [9,10]. Thus, age-related changes in pancreatic exocrine secretion may be due to insulin release or insulin action. However, it is still not clear how aging alters pancreatic exocrine secretion and islet hormones affect pancreatic exocrine secretion.
The size of the acinar cell, which takes the charge of enzyme secretion, is reduced in old men and aged experimental animals [11]. Pancreatitis occurs frequently in old men; the patient, who has geriatric chronic pancreatitis accompanied pancreatic calcification, shows the atrophy of the acinar cell and fibrosis [12]. Also, it has been reported that pancreatic juice, bicarbonate and protein secretion, which are controlled by secretin and cholecystokinin, are reduced in aged rats [13]. These results suggest that aging can reduce pancreatic exocrine secretion and islet hormones can affect the reduction of pancreatic exocrine function. The present study was performed; therefore, to investigate the effects of age-related changes on pancreatic exocrine functions in 3, 12 and 18 months old rats.
Materials and Methods
Experimental animals
Male Sprague-Dawley rats (3, 12 and 18 months old) were used in the experiment, which succeeded in experimental animal center of Hallym University. The environment of breeding room was maintained at condition that temperature was 23±2℃ and relative humidity was 55±10%. Artificial lighting maintained 12 hours per day. The rats were anesthetized with a single intraperitoneal injection of 20% urethane (Sigma, USA) at a dose of 0.7 mL/100 g of body weight. The rats were sacrificed by an intravenous overdose injection of urethane after isolation of the pancreas. Food was forbidden from rats for 24 h before the experiment, but they were allowed to drink water freely. This animal study was conducted in accordance with the guidelines and the approval of the Institutional Animal Care and Use Committee of Hallym University (Hallym 2012-13).
Preparation of the isolated perfused pancreas
The isolated pancreases were prepared, according to the methods described previously [14,15]. In brief, the median line and the abdominal aorta was carefully dissected and cannulated with PE-50 tubing (I.D. 0.58 mm O.D. 0.97 mm: Clay Adams, USA) just above the celiac artery, and then tightly ligated just below the superior mesenteric artery. The pancreatic duct was also cannulated with Tygon microbore tubing (I.D. 1.27 mm, O.D. 2.28 mm; Fisher Scientific Co, USA) to drain the perfusate. To prevent a duodenal secretion inflow, each beginning and ending of duodenum was cannulated with plastic tubing.
The pancreas was perfused with modified Krebs-Henseleit solution (pH 7.4, 305 mosmol/kg water) through the celiac and superior mesenteric arteries at a flow rate of 1.2 mL/min, using a minipuls pump (Gilson, France). The perfusate contained 5.6 mM glucose (Sigma, USA), 0.1% bovine serum albumin (Sigma, USA) and 3% Dextran T-70 (Sigma, USA), and was continuously oxygenated with 95% O2 and 5% CO2. Krebs-Henseleit solution basically consists of NaCl 191.5 mM, NaHCO3 25 mM, KCl 5.6 mM, MgCl2 1 mM, NaH2PO4 1.15 mM and CaCl2 2.5 mM.
The pancreas was isolated with the duodenum, but separated from neighboring organs and tissues. The pancreas was placed in temperature-controlled experimental chamber at 37℃, which was also continuously supplied with Krebs-Henseleit solution at a flow rate of 0.35 mL/min and oxygenated. After equilibration period of 30 min, pancreatic juice was collected through the experiment every 15-min.
Effects of endogenous and exogenous insulin on action of CCK
To investigate the effects of CCK-8 under the hypoglycemic condition as a control experiment, the isolated pancreas was perfused with Krebs-Henseleit solution containing glucose at a concentration of 5.6 mM for 45 min, and then sulfated CCK-8 (Squibb, USA) was added to the perfusate during another 60 min at a concentration of 5 pM. For the purpose of observing effects of interaction of endogenous insulin and CCK, the isolated pancreas was perfused with a solution containing 18 mM glucose instead of 5.6 mM. To see the interaction of exogenous insulin and CCK, porcine insulin (Novo Nordisk) was added in the perfusate containing 5.6 mM glucose at a concentration of 100 nM instead of 18 mM glucose.
Measurement of amylase in pancreatic tissues
The rat pancreas was divided from other tissues and washed with 4℃ saline. Lymphnodes, fat tissues and vessels are removed from that pancreas. The pancreas was minced and put in polyethylene tube with phosphate buffer (20 mM, pH 8.0) 4 mL. Then the pancreas tissues were homogenized by tissue grinder (Plytron, USA) for 30 sec and centrifuged at a speed of 17,000 rpm for 30 min. After centrifuge, the suspension was collected.
Amylase assay in pancreatic juice
To investigate pancreatic digestive enzyme secretion, activity of representative enzyme α-amylase was measured. α-Amylase activity in the pancreatic juice was determined by the method of Rick and Stegbauer (1974) [16]. In brief, 1 mL starch solution (1%, w/v) is put in 1 mL phosphate buffer (10 mM KH2PO4, 10 mM Na2HPO4, 10 mM NaCl, pH 6.9) and mixed with 1000-3000 times diluted pancreatic juice (20 µL). Then it reacted in 37℃ incubator for 10 minutes. One % starch solution was heated in 100℃ for 20 minutes to melt and centrifuged in 4℃ at a speed of 3000 rpm for 20 minutes then the supernatant was used. After reaction, 2 mL dinitrosalicylic acid solution (1% 35, 55-dinitrosalycylic acid, 30% potassium sodium tartrate, 400 mM NaOH) was put in it and heated in 100℃ for 5 minutes to appear color of maltose, the reactive product. The reactive tube was cooling in room temperature for 30 minutes then measured a degree of extinction at 546 nm by spectrophotometer (Kontron, Swiss). Amylase activity 1 unit decided amount of amylase, which produce 1 µm maltose for 1 minute with above condition and converted each 15-min (amylase output; U/15 min). Pancreatic juice was collected in polyethylene tube, which has volume of 2.4 µL/cm then measured length to determine the volume flow. All reagents were bought from Sigma (USA).
Analysis of data
All data were expressed as the mean±SEM. The statistical significance of difference between groups was assessed with one-way ANOVA with Bonferroni's post-hoc test using GraphPad Prism version 4.0 for Windows XP (GraphPad Software, San Diego, CA, USA). The difference was considered significant when the P value was <0.05.
Results
Effects of CCK-8 on pancreatic exocrine secretion
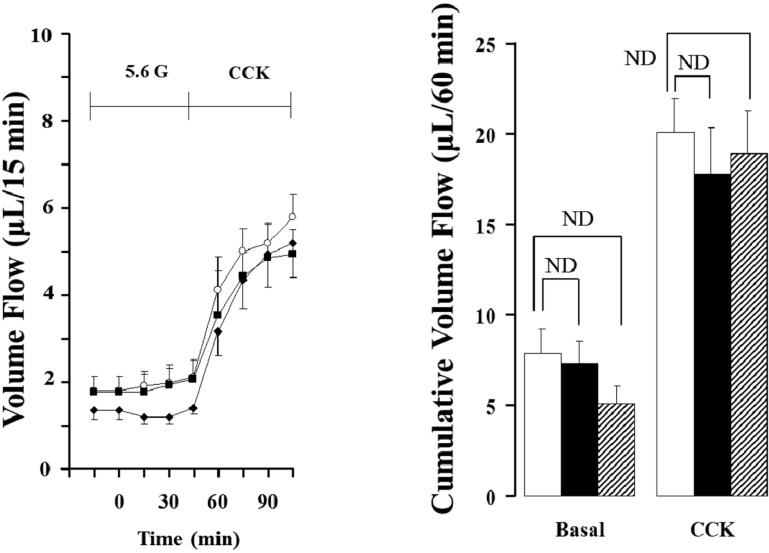
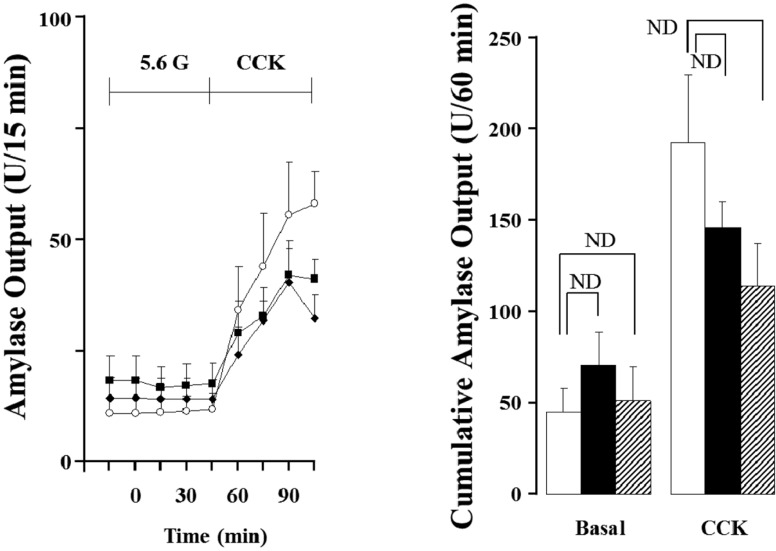
As shown in Figure 1 and 2, when the isolated perfused rat pancreas was perfused with CCK-8, pancreatic flow rate and amylase output were increased in all the groups after 15 min. After 45 min, each pancreatic flow rate in 3, 12 and 18 months old rats was 5.47±0.48 µL/15 min, 4.37±0.79 µL/15min and 4.94±0.76 µL/15min, respectively; all the rates were significantly elevated compared with basal secretion (P<0.05). Each amylase output was also significantly elevated to 55.80±11.07 U/15 min, 39.29±8.17 U/15 min and 37.17±7.41 U/15 min (P<0.05). The cumulative volume flow and amylase output secreted 60 min of CCK perfusion had no significant difference.
Figure 1.
Effect of cholecystokinin (CCK; 5 pmol) in pancreatic volume flow of the isolated rat pancreas under 5.6 mM glucose (G) background. Each point (○; 3 months old rats (M), ■; 12 months old rats (M), ♦; 18 months old rats (M) and bar (□; 3M, ■; 12M, ▨; 18M) represent the mean±S.E of 6 experiments. Cumulative volume flow was calculated from the sum of CCK effect minus basal secretion for 60 min. ND; no significant difference.
Figure 2.
Effect of cholecystokinin (CCK; 5 pmol) in pancreatic amylase output of the isolated rat pancreas under 5.6 mM glucose (G) background. Each point (○; 3 months old rats (M), ■; 12 months old rats (M), ♦; 18 months old rats (M) and bar (□; 3M, ■; 12M, ▨; 18M) represent the mean±S.E of 6 experiments. Cumulative amlylase output was calculated from the sum of CCK effect minus basal secretion for 60 min. ND; no significant difference.
Effects of endogenous insulin on pancreatic secretion stimulated by CCK-8
In order to induce endogenous insulin, the isolated rat pancreas was perfused with 18 mM glucose after perfused with 5.6 mM glucose background for 30 min. Then, CCK was added after 45 min.
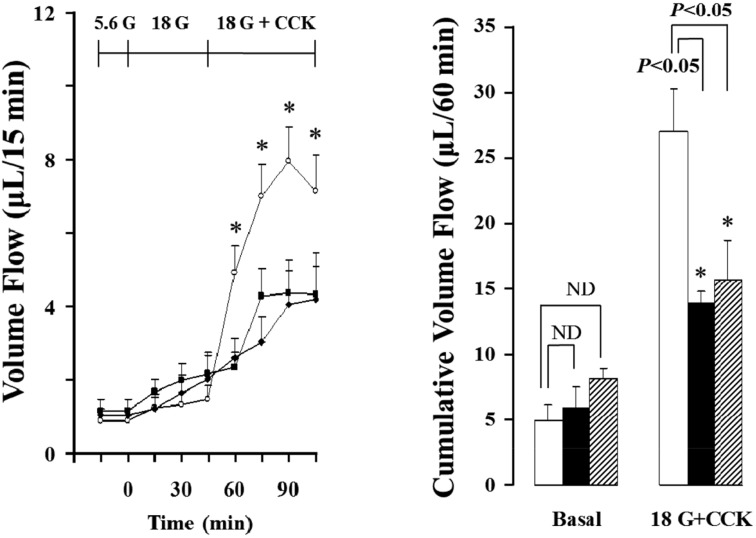
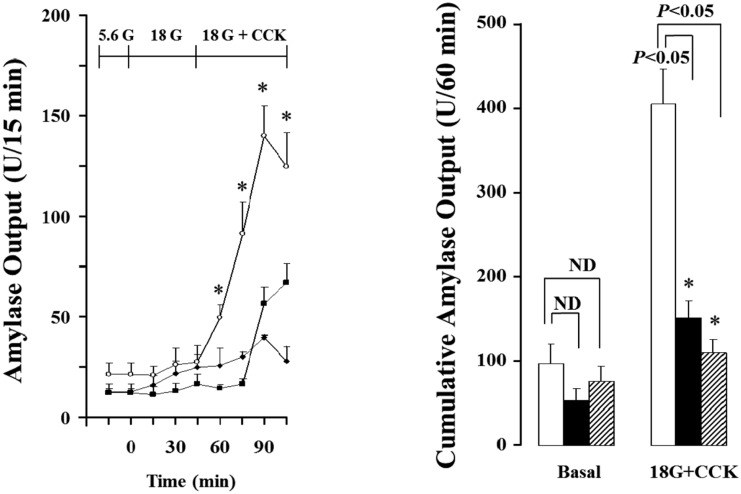
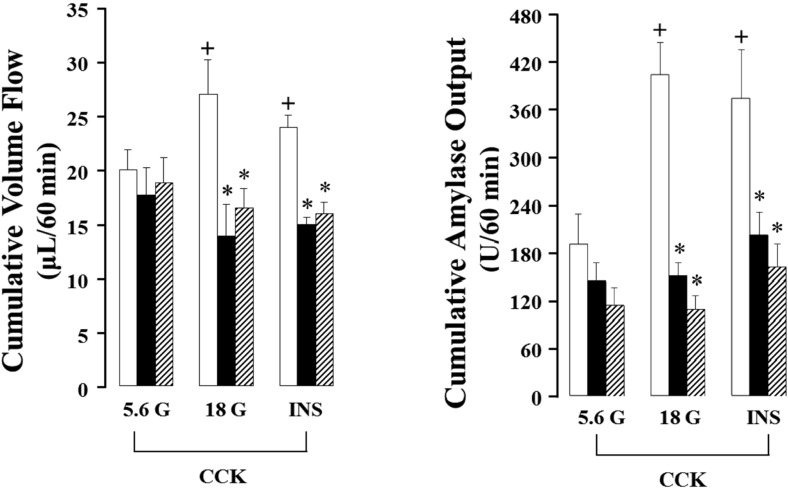
As shown in Figure 3 and 4, the isolated rat pancreas was perfused with CCK under 18mM glucose background, and pancreatic flow rate and amylase output were significantly elevated from basal secretion, each 0.90±0.16 µL/15 min, 21.59±5.41 U/15 min to maximum 7.98±0.92 µL/15 min, 140.06±14.82 U/15 min after 45 min in 3 months old rats. These results were increased 153% in pancreatic flow rate and 251% in amylase output after 45 min under 5.6 mM glucose. Also, total cumulative pancreatic flow rate and amylase output during 60 min were increased 36% and 111% than 5.6 mM glucose background. However, 12 and 18 months old rats did not represent similar results. The pancreatic flow rate in basal secretion was 1.05±0.16 µL/15 min and 1.17±0.31 µL/15 min, respectively. After increased glucose level to 18 mM and perfused with CCK for 45 min, each of them was elevated to 4.05±0.94 µL/15 min and 4.37±0.91 µL/15 min; however, they were not significant compared with 5.6mM background. Similarly, amylase outputs were each 10.55±2.49 U/15 min and 12.77±3.79 U/15 min in basal and they were increased to 48.66±10.45 U/15min and 40.12±3.11 U/15min after the perfusion of CCK for 45 min, but no significance. Likewise, cumulative total pancreatic flow rate and amylase output were not affected (Figure 5).
Figure 3.
Effect of cholecystokinin (CCK; 5 pmol) in pancreatic volume flow of the isolated rat pancreas under 18 mM glucose (G) background. Each point (○; 3 months old rats (M), ■; 12 months old rats (M), ♦; 18 months old rats (M) and bar (□; 3M, ■; 12M, ▨; 18M) represent the mean±S.E of 6 experiments. Cumulative volume flow was calculated from the sum of CCK effect minus basal secretion for 60 min *; The value is significantly (P<0.05) different from that of 3 months old rats. ND ; no significant difference.
Figure 4.
Effect of cholecystokinin (CCK; 5 pmol) in pancreatic amylase output of the isolated rat pancreas under 18 mM glucose (G) background. Each point (○; 3 months old rats (M), ■; 12 months old rats (M), ♦; 18 months old rats (M) and bar (□; 3M, ■; 12M, ▨; 18M) represent the mean±S.E of 6 experiments. Cumulative volume flow was calculated from the sum of CCK effect minus basal secretion for 60. min. *; The value is significantly (P<0.05) different from that of 3 months old rats. ND ; no significant difference.
Figure 5.
Effect of endogenous and exogenous insulin on cholecystokinin (CCK)-stimulated pancreatic volume flow and amylase output in the isolated rat pancreas. Each bar (□; 3 months old rats (M), ■; 12 months old rats (M), ▨; 18 months old rats (M)) represents the mean±S.E. of 5 experiments. *; The value is significantly (P<0.05) different from CCK-stimulated pancreatic volume flow and amylase output under 18 mM glucose (G) and exogenous insulin (INS) background of 3 months old rats. +; The value is significantly (P<0.05) different from CCK-stimulated pancreatic volume flow and amylase output under 18 mM glucose (G) and exogenous insulin (INS) background in each 3 months old pancreata.
Effects of exogenous insulin on pancreatic secretion simulated by CCK-8
Since endogenous insulin released by high concentration of glucose did not show the potentiation effect of insulin and CCK in 12 and 18 months rats as shown in Figure 3 and 4. Porcine insulin at a concentration of 100 nM was added to the perfusate together with CCK after 45 min to confirm the potentiation effect of exogenous insulin. At a concentration of 100 nM porcine insulin was added to the 5.6 mM glucose background, and then CCK was administered to the perfusate at 45 min later.
In 3 months old rats, exogenous insulin significantly (P<0.05) elevated the pancreatic flow rate and amylase output compared with that of the control (5.6 mM glucose background). However, exogenous insulin did not affect the pancreatic flow rate or increased amylase output; each 202.34±63.04 U/60 min and 162.63±30.26 U/60 min, 39 and 43% increase than control data, in 12 and 18 months old rats. However, the values are not significant between the young rats and aged rats.
Amylase activity in pancreatic tissues
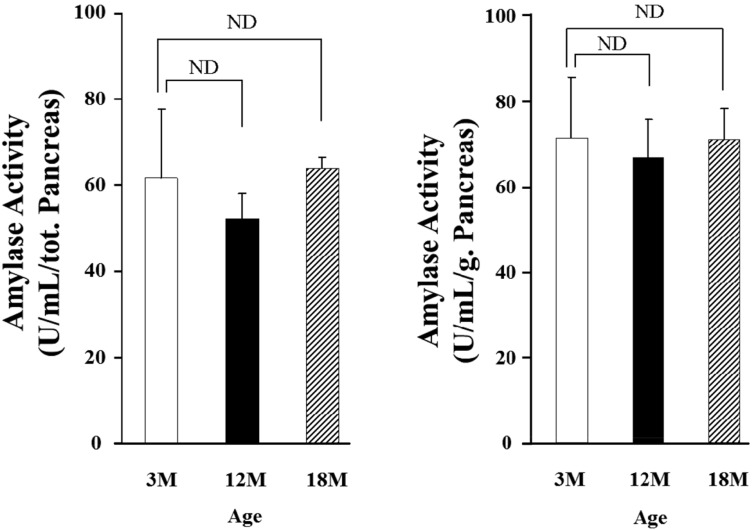
To investigate effects of aging on amylase activity in pancreatic tissue, pancreatic amylase content was measured. Age-related changes in wet weight of pancreas were shown in Table 1. As shown in the Table, there was no significance between pancreatic wet weight of the young and old rats. Also, amylase activity in the pancreatic tissue was measured (Fig. 6). The values were 72.01±14.22 U/mL, 67.43±8.76 U/mL and 71.70±7.13 U/mL in 3, 12 and 18 months old rats, respectively. Although the amylase activity in the pancreatic tissue was recalculated as a U/mL/g, the activity was also not significant among all the groups.
Table 1.
Wet weight of pancreas in 3, 12 and 18 months old rats
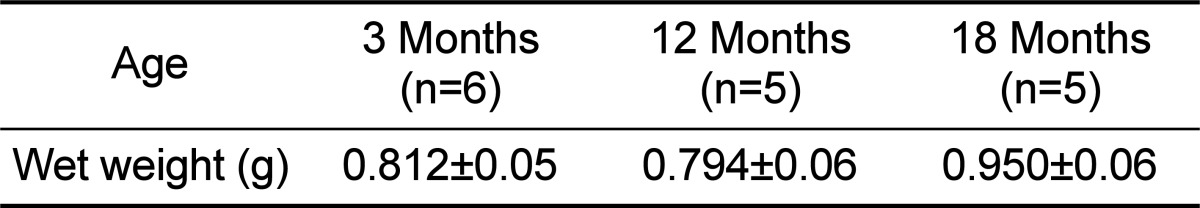
There was no significant difference in the wet weigh of the pancreas.
Figure 6.
Amylase activity in pancreatic tissue. Each bar (□; 3 months old rats (M), ■; 12 months old rats (M), ▨; 18 months old rats (M)) represents the mean±S.E. of 5-6 experiments. ND : no significant difference.
Discussion
In the present study, we used the isolated perfused rat pancreas to exclude the effects of the external nerves or gastrointestinal hormones. When the pancreas was perfused with 5.6 mM glucose, the basal secretion of pancreatic flow rate and amylase output was not significant in all the groups. The pancreatic flow rate and amylase output were elevated by the administration of CCK-8 in all the groups; however, there was no significant difference between the young and aged rats. These results indicated that pancreatic exocrine secretion does not depend on age in basal or CCK-induced secretion. The other side, since the pancreas has insuloacinar axis between the endocrine part and exocrine part, islet hormones could affect exocrine secretion. Especially insulin, one of islet hormones, potentiates the action of CCK and increases pancreatic exocrine secretion [14,17,18]. In the 3 months old rats, we observed that the pancreatic exocrine secretion, induced by CCK, was potentiated by endogenous insulin. These data well agreed with the reports that pancreatic exocrine secretion, induced by feeding or mixture of physiological concentration of CCK and secretin, was suppressed with the administration of insulin antibody in rats [19]. In our present experiment, interestingly, the potentiation effect of insulin on the action of CCK did not appear in the 12 and 18 months old rats. When the isolated pancreas was perfused with 18 mM glucose and CCK in the aged rats, the pancreatic exocrine secretion only increased to the similar level, which was perfused with 5.6 mM glucose and CCK. Our results suggest that there is a possible defect of insulin release or insulin synthesize or insulin action or other reasons with age. Therefore, to confirm whether insulin secretion induced by high concentration of glucose decreases or not with age, exogenous porcine insulin at a concentration of 100 nM was administered with CCK in the 12 or 18 months old rats. Amylase output induced by exogenous insulin and CCK was higher than that induced by CCK alone in the 12 and 18 months old rats, each 39 and 43%, respectively. However, it was significantly less than the 3 months old rats. Pancreatic flow rate was not affected in the 12 and 18 months old rats. A fact that amylase output in the aged rats was increased by exogenous insulin suggests that the insulin secretion was diminished by aging. These results are in good accord with the previous reports that there were not related with obesity, distribution of fat or physiological activity, insulin secretion, which are diminished by aging in humans [10,20,21]. One hand, it is not yet known what factor makes insulin secretion decrease by aging. However, it was reported that the decrease of insulin secretion resulted in the decrease of β-cell reactivity about glucose [22]. In addition, although there was high concentration of insulin in the rat pancreatic islet, β-cells, which secrets insulin, had problem on the mechanism of secretion even if it stimulated [23]. Furthermore, it was reported that insulin secretion about glucose was diminished because the adenylate cyclase activity of islet and oxidation of islet glucose were reduced by aging [24]. However, since the present study did not measure the insulin release in the perfusate eliminated through the portal vein, we could not conclude that it resulted in the decrease of insulin secretion. Therefore, further study must be carried out to measure insulin output in the perfusate.
In the present study, in spite of perfusion with exogenous insulin in the aged rats, the pancreatic flow rate did not change, and the amylase output was elevated a little, so it is supposed that other reasons besides can decrease the insulin secretion induced by the high concentration of glucose. For example, it was reported that plasma insulin concentration was not affected by aging in the fasting state [25]. In addition, it was reported that pancreatic insulin content did not differ between young and old rats; however, some researchers reported that pancreatic total insulin content was elevated by aging [8,26]. So, it is not clear how pancreatic insulin content changes by aging. It is clearly known that the pancreas secretes insulin and somatostatin and that somatostatin content in the islet is increased by aging and it acts an inhibitory effect on insulin secretion [27]. Therefore, it has been suggested that the content and activity of islet hormones besides insulin, which affects pancreatic exocrine secretion, can be changed with age.
It was reported that, in old humans, pancreatic anteroposterior diameter was significantly reduced, and lobulation and parenchymal fatty change became more evident [28]. As our results, 3 months old rats were significantly lower than 12 and 18 months old rats in CCK-stimulated pancreatic exocrine function, but there was no significant difference in the wet weigh of the pancreas. Also, pancreatic amylase content had no significance. Therefore, it was supposed that pancreatic amylase content was not affected by aging. We concluded from the present investigations that pancreatic exocrine secretion is diminished by the alteration of pancreatic function related with aging in rats and it is supposed that insulin, one of islet hormone, affects it. These phenomena may be due to a decrease in both insulin secretion and insulin action in aged rats.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology(2010-0008488).
References
- 1.Werner I, Hambraeus L. The digestive capacity of elderly people. The effect of a high protein diet. Acta Soc Med Ups. 1971;76(5-6):239–242. [PubMed] [Google Scholar]
- 2.Sonnenberg A, Koch TR. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci. 1989;34(4):606–611. doi: 10.1007/BF01536339. [DOI] [PubMed] [Google Scholar]
- 3.Lee YL, Kwon HY, Park HS, Lee TH, Park HJ. The role of insulin in the interaction of secretin and cholecystokinin in exocrine secretion of the isolated perfused rat pancreas. Pancreas. 1996;12(1):58–63. doi: 10.1097/00006676-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron JJ, Rachubinski R, Searle N, Sikstrom R, Borts D, Bastian P, Posner BI. Radioautographic visualization of in vivo insulin binding to the exocrine pancreas. Endocrinology. 1980;107(4):1069–1080. doi: 10.1210/endo-107-4-1069. [DOI] [PubMed] [Google Scholar]
- 5.Sankaran H, Iwamoto Y, Korc M, Williams JA, Goldfine ID. Insulin action in pancreatic acini from streptozotocin-treated rats. II. Binding of 125I-insulin to receptors. Am J Physiol. 1981;240(1):G63–G68. doi: 10.1152/ajpgi.1981.240.1.G63. [DOI] [PubMed] [Google Scholar]
- 6.Sjodin L, Holmberg K, Lyden A. Insulin receptors on pancreatic acinar cells in guinea pigs. Endocrinology. 1984;115(3):1102–1109. doi: 10.1210/endo-115-3-1102. [DOI] [PubMed] [Google Scholar]
- 7.Goldfine ID, Kriz BM, Wong KY, Hradek G, Jones AL, Williams JA. Insulin action in pancreatic acini from streptozotocin-treated rats. III. Electron microscope autoradiography of 125I-insulin. Am J Physiol. 1981;240(1):G69–G75. doi: 10.1152/ajpgi.1981.240.1.G69. [DOI] [PubMed] [Google Scholar]
- 8.Curry DL, Reaven G, Reaven E. Glucose-induced insulin secretion by perfused pancreas of 2- and 12-mo-old Fischer 344 rats. Am J Physiol. 1984;247(3 Pt 1):E385–E388. doi: 10.1152/ajpendo.1984.247.3.E385. [DOI] [PubMed] [Google Scholar]
- 9.Aizawa T, Komatsu M, Sato Y, Ishihara F, Suzuki N, Nishii N, Hashizume K, Yamada T. Insulin secretion by the pancreatic beta cell of aged rats. Pancreas. 1994;9(4):454–459. doi: 10.1097/00006676-199407000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Elahi D, Muller DC, Andersen DK, Tobin JD, Andres R. The effect of age and glucose concentration on insulin secretion by the isolated perfused rat pancreas. Endocrinology. 1985;116(1):11–16. doi: 10.1210/endo-116-1-11. [DOI] [PubMed] [Google Scholar]
- 11.Morgan ZR, Feldman M. The liver, biliary tract and pancreas in the aged: an anatomic and laboratory evaluation. J Am Geriatr Soc. 1957;5(1):59–65. doi: 10.1111/j.1532-5415.1957.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 12.Ammann R, Sulser H. ["Senile" chronic pancreatitis; a new nosologic entity? Studies in 38 cases. Indications of a vascular origin and relationship to the primarily painless chronic pancreatitis] Schweiz Med Wochenschr. 1976;106(13):429–437. [PubMed] [Google Scholar]
- 13.Khalil T, Fujimura M, Townsend CM, Jr, Greeley GH, Jr, Thompson JC. Effect of aging on pancreatic secretion in rats. Am J Surg. 1985;149(1):120–125. doi: 10.1016/s0002-9610(85)80020-9. [DOI] [PubMed] [Google Scholar]
- 14.Park HJ, Lee YL, Kwon HY. Effects of pancreatic polypeptide on insulin action in exocrine secretion of isolated rat pancreas. J Physiol. 1993;463:421–429. doi: 10.1113/jphysiol.1993.sp019602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanno T, Saito A. The potentiating influences of insulin on pancreozymin-induced hyperpolarization and amylase release in the pancreatic acinar cell. J Physiol. 1976;261(3):505–521. doi: 10.1113/jphysiol.1976.sp011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang ZE, Shin BN, Kim IH, Lee HJ, Yong JH, Lee MJ, Won MH, Lee YL. Roles of Non-cholinergic Intrapancreatic Nerves, Serotonergic Nerves, on Pancreatic Exocrine Secretion in the Isolated Perfused Rat Pancreas. Korean J Physiol Pharmacol. 2011;15(5):307–312. doi: 10.4196/kjpp.2011.15.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garry DJ, Garry MG, Williams JA, Mahoney WC, Sorenson RL. Effects of islet hormones on amylase secretion and localization of somatostatin binding sites. Am J Physiol. 1989;256(5 Pt 1):G897–G904. doi: 10.1152/ajpgi.1989.256.5.G897. [DOI] [PubMed] [Google Scholar]
- 18.Saito A, Williams JA, Kanno T. Potentiation of cholecystokinin-induced exocrine secretion by both exogenous and endogenous insulin in isolated and perfused rat pancreata. J Clin Invest. 1980;65(4):777–782. doi: 10.1172/JCI109727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KY, Zhou L, Ren XS, Chang TM, Chey WY. An important role of endogenous insulin on exocrine pancreatic secretion in rats. Am J Physiol. 1990;258(2 Pt 1):G268–G274. doi: 10.1152/ajpgi.1990.258.2.G268. [DOI] [PubMed] [Google Scholar]
- 20.Reaven E, Curry D, Moore J, Reaven G. Effect of age and environmental factors on insulin release from the perfused pancreas of the rat. J Clin Invest. 1983;71(2):345–350. doi: 10.1172/JCI110775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52(7):1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 22.Scheen AJ, Sturis J, Polonsky KS, Van Cauter E. Alterations in the ultradian oscillations of insulin secretion and plasma glucose in aging. Diabetologia. 1996;39(5):564–572. doi: 10.1007/BF00403303. [DOI] [PubMed] [Google Scholar]
- 23.Borg LA, Dahl N, Swenne I. Age-dependent differences in insulin secretion and intracellular handling of insulin in isolated pancreatic islets of the rat. Diabete Metab. 1995;21(6):408–414. [PubMed] [Google Scholar]
- 24.Lipson LG, Bush MJ, Tietjen GE, Yoon A. Role of the adenylate cyclase system in altered insulin release from islets of Langerhans of aging rats. Acta Endocrinol (Copenh) 1981;96(2):222–226. doi: 10.1530/acta.0.0960222. [DOI] [PubMed] [Google Scholar]
- 25.Draznin B, Steinberg JP, Leitner JW, Sussman KE. The nature of insulin secretory defect in aging rats. Diabetes. 1985;34(11):1168–1173. doi: 10.2337/diab.34.11.1168. [DOI] [PubMed] [Google Scholar]
- 26.Burch PT, Berner DK, Leontire A, Vogin A, Matschinsky BM, Matschinsky FM. Metabolic adaption of pancreatic islet tissue in aging rats. J Gerontol. 1984;39(1):2–6. doi: 10.1093/geronj/39.1.2. [DOI] [PubMed] [Google Scholar]
- 27.Casad RC, Jr, Adelman RC. Aging enhances inhibitory action of somatostatin in rat pancreas. Endocrinology. 1992;130(4):2420–2422. doi: 10.1210/endo.130.4.1347743. [DOI] [PubMed] [Google Scholar]
- 28.Sato T, Ito K, Tamada T, Sone T, Noda Y, Higaki A, Kanki A, Tanimoto D, Higashi H. Age-related changes in normal adult pancreas: MR imaging evaluation. Eur J Radiol. 2012;81(9):2093–2098. doi: 10.1016/j.ejrad.2011.07.014. [DOI] [PubMed] [Google Scholar]