Abstract
Bone changes are common sequela of radiation therapy for cancer. The purpose of this study was to establish an experimental model of radiation-induced bone loss in adult mice using micro-computed tomography (µCT). The extent of changes following 2 Gy gamma irradiation (2 Gy/min) was studied at 4, 8, 12 or 16 weeks after exposure. Adult mice that received 1, 2, 4 or 6 Gy of gamma-rays were examined 12 weeks after irradiation. Tibiae were analyzed using µCT. Serum markers and biomechanical properties were measured and the osteoclast surface was examined. A significant loss of trabecular bone in tibiae was evident 12 weeks after exposure. Measurements performed after irradiation showed a dose-related decrease in trabecular bone volume fraction (BV/TV) and bone mineral density (BMD), respectively. The best-fitting dose-response curves were linear-quadratic. Taking the controls into accounts, the lines of best fit were as follows: BV/TV (%)= -0.071D2-1.799D+18.835 (r2=0.968, D=dose in Gy) and BMD (mg/cm3) = -3.547D2-14.8D+359.07 (r2=0.986, D=dose in Gy). Grip strength and body weight did not differ among the groups. No dose-dependent differences were apparent among the groups with regard to mechanical and anatomical properties of tibia, serum biochemical markers and osteoclast activity. The findings provide the basis required for better understanding of the results that will be obtained in any further studies of radiation-induced bone responses.
Keywords: Radiation, bone loss, murine model, microcomputed tomography
The long-term survival rate of cancer patients is increasing due to the improvement of cancer treatments and diagnostic techniques [1,2]. Specific and chronic complications may occur in these survivors. Cancer treatments that include radiation therapy can damage normal tissues [3,4]; further study is needed to clarify the prognosis and the cause of this situation. Bone loss due to high doses of irradiation therapy in adults has been identified in diagnostic radiographic images [5]. The primary effect of irradiation on bone is atrophy and the decrease of the functional components of the structure appears in these solid organs without reduction of the size. Several main factors have been considered as the etiology of the irradiation-mediated changes in bone, blood vessels, bone matrix, and cells [6], and these changes precede the spontaneous fractures that can occur following irradiation [7,8]. Irradiation of normal bone during cancer therapy can lead to atrophy and increased risk of fracture at several skeletal sites.
Animal models are commonly used in the study of skeletal biology and serve as good tools to define mechanisms on bone loss and skeletal fragility [9]. It is not clear if radiation results in bone changes [10-12]. Additional data are needed to characterize the radiation-induced changes in trabecular bone morphology in mice.
The objectives of the current study were to assess time- and dose-related changes in bone mineral density (BMD) and three-dimensional indices of trabecular microarchitecture in the tibiae.
In recent years, the use of high-resolution micro-computed tomography (µCT) imaging to evaluate trabecular and cortical bone structure in animal and human specimens has grown vastly. Bone histomorphometry is used to research the assorted bone diseases. Until recently, quantitative histologic techniques were the criterion for assessing trabecular and cortical bone architecture. The assessments are done on microscopic two-dimensional sections, and several methods have been suggested to extrapolate two-dimensional measurements to three-dimensions. Although histologic analyses supply unique data on cellularity and dynamic indicators of bone remodeling, they have restrictions with respect to assessment of bone microarchitecture because structural parameters are formed from stereologic analysis of a few two-dimensional sections, usually assuming that the inherent structure is plate-like [13]. In comparison, high-resolution three-dimensional imaging techniques, such as µCT, directly measure bone microarchitecture without relying on stereologic models. X-ray µCT is a recently developed imaging tool used to deduce three-dimensional architecture. Recently, discrepant results of two-dimensional histomorphometric measurements compared with three-dimensional outcomes obtained directly have been reported. µCT seems to be an interesting tool providing authentic morphometric results in less time than formal histomorphometry [14,15].
The effect of ionizing radiation on bone changes has not been studied in detail. Identifying the effects of radiation on the skeletal system requires an animal model. In an attempt to establish the murine model for radiation-induced bone loss, we investigated the skeletal response after gamma-irradiation.
Materials and Methods
Eight-week-old female C3H/HeN mice were obtained from a specific pathogen-free colony at Oriental Inc. (Seoul, Korea) and allowed 1 week for quarantine and acclimatization. The Institutional Animal Care and Use Committee at Chonnam National University approved the protocols used in this study (CNU IACUC-YB-2010-11), and the animals were cared for in accordance with the Guidelines for Animal Experiments. The animals were housed in a room that was maintained at 22±2℃ and relative humidity of 50±5%, with artificial lighting from 08:00-20:00 h and 13-18 air changes per hour. The animals were housed in groups of three per polycarbonate cage, and were given tap water and commercial rodent chow (Samyang Feed, Seoul, Korea) ad libitum.
Exposure to 137Cs-generated gamma-rays was conducted with Gammacell (Nordion, Ottawa, Canada). The mean dose rate of γ-rays was 2 Gy/min. The mice were irradiated to the whole body with 2 Gy of γ-rays. They were sacrificed at 4, 8, 12 or 16 weeks after exposure. The extent of changes following gamma irradiation (2 Gy/min) was studied at 4, 8, 12 or 16 weeks after exposure (6 mice for each group). Thirty mice that received 0, 1, 2, 4 or 6 Gy of gamma-rays were examined 12 weeks after irradiation.
Grip strength was assessed using a grip strength meter (GSM) designed by IWOO-Systems (Seoul, Korea). For testing, mice were gently held so their back legs were supported with one forelimb lightly restrained. The paw being tested was brought to the bar, the mouse was allowed about 1 s to establish a grip, and the mouse was then gently pulled back in one smooth motion until grip was released. The time between trials averaged 2 s. Positive grips were scored when the mouse grasped the bar immediately with all fingers and, after release, the paw was relaxed and not clenched. Gripping force was defined as the maximum force recorded on the GSM before the mouse released the bar. Mice were given four trials per session.
The blood samples from all the groups were withdrawn by the abdominal vein method to assess biochemical parameters. The animals were then sacrificed using ether anesthesia and the left tibiae was collected, cleaned of all non-osseous tissue, measured for length and weight, fixed in 10% neutral formalin for 48 h and stored in 70% ethanol. Tibia length was considered as the maximal distance between the proximal condyles and malleolus.
The freshly isolated right tibiae were assessed for their biomechanical strength using the tensile strength testing apparatus. Three-point bending tests were performed using a model 3344 apparatus (Instron, Norwood, MA, USA). The lateral surface of the tibia at the tibio-fibular junction was placed on the first point and proximal tibia on the other. A rounded press head compressed the middle of the tibial shaft until fracture occurred.
Serum calcium (Ca) and inorganic phosphorus (P) concentrations and serum alkaline phosphatase (ALP) activity were measured on an automatic analyzer (Fuji Dri-chem; Fujifilm, Tokyo, Japan) using a diagnostic slide. The levels of estradiol (E2) were determined using a specific and sensitive double-antibody radioimmunoassay kit (Estradiol Coat-A-count, Diagnostic Products, Los Angeles, CA, USA) on a gamma-ray counter (EG & G, Wallace, Finland).
Morphological measurements, including bone volume fraction (bone volume/tissue volume, BV/TV), trabecular thickness/separation/number (Tb.Th, Tb.Sp, Tb.N), structure model index (SMI), cortical BV and mean polar moment of inertia (pMOI) were calculated from the resulting µCT data for each mouse using a model 1172 apparatus (Skyscan, Kontich, Belgium). The regions of interest for analysis were the proximal tibia metaphysis. User-defined contours were outlined on every fifth slice of a 150 slice region extending 2.5 mm distally from the growth plate, starting at the point where the growth plate tissue was no longer visible in the grayscale CT slice. The proximal 90 slice region was used when analyzing the trabecular bone, and the most distal 60 slices were used when analyzing the cortical bone. For quantification of the trabecular BMD, the µCT was calibrated using two standard phantoms with a density of 0.25 and 0.75 g/cm3. The image slices were reconstructed and analyzed using CTan analyzer software (Skyscan, Kontich, Belgium).
Following tomographic analysis, the tibiae were decalcified using a formic acid solution and embedded and cut into sagittal sections with a thickness of 3 µm. Each slide was stained with tartrate-resistant acid phosphatase (TRAP) using a commercial kit (Sigma-Aldrich, St. Louis, MO, USA) to identify the osteoclast surface and counterstained with methyl green. Histomorphometric evaluation was performed from captured micrographs (400X) throughout the metaphysis, starting approximately 0.25 mm distal from the growth plate (to exclude the primary spongiosa) and extending a further 0.5 mm. Osteoclast surface measurements were quantified relative to total trabecular bone surface (Oc.S/BS).
The statistical significance of differences between the results in irradiated and control groups was determined by the two-tailed Student's t-test using a Graph PAD In Stat (GPIS) computer program (GraphPad Software, San Diego, CA, USA). A value of P<0.05 was considered statistically significant.
Results
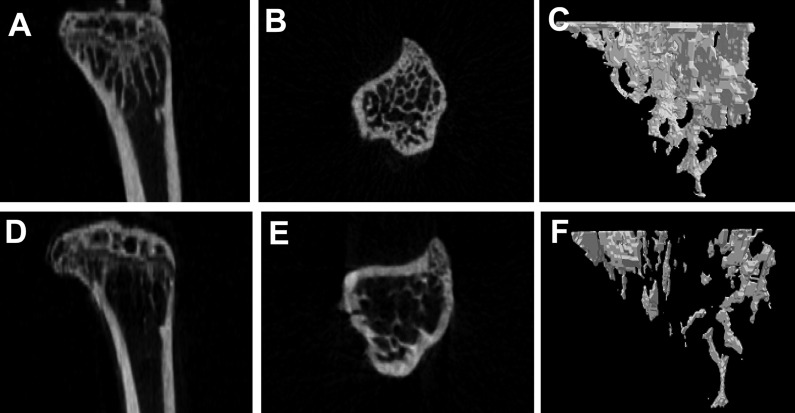
In the test of change at each observation time after 2 Gy irradiation, body weight and uterus weight did not differ among the groups. No differences were apparent among the experimental groups with regard to mechanical property, tibia length and tibia weight (Tables 1 and 2). The effects of gamma irradiation on serum biochemical markers are summarized in Table 3. As compared with the non-irradiated group, there was no difference in any of the markers. µCT revealed that proximal tibial metaphysis from irradiation group had less trabecular bone compared to the sham group (Figure 1). A slight change of bone loss was observed from 8 weeks after gamma-irradiation of trabecular bone, with the peak bone loss observed at 12 weeks based on the average of BV/TV and BMD. The decrease of value was observed in BV and pMOI at 16 weeks in case of cortical bone (Table 4).
Table 1.
Time-related changes in body weight, uterus weight, tibia weight and length after gamma-irradiation with 2 Gy in 9-week-old mice
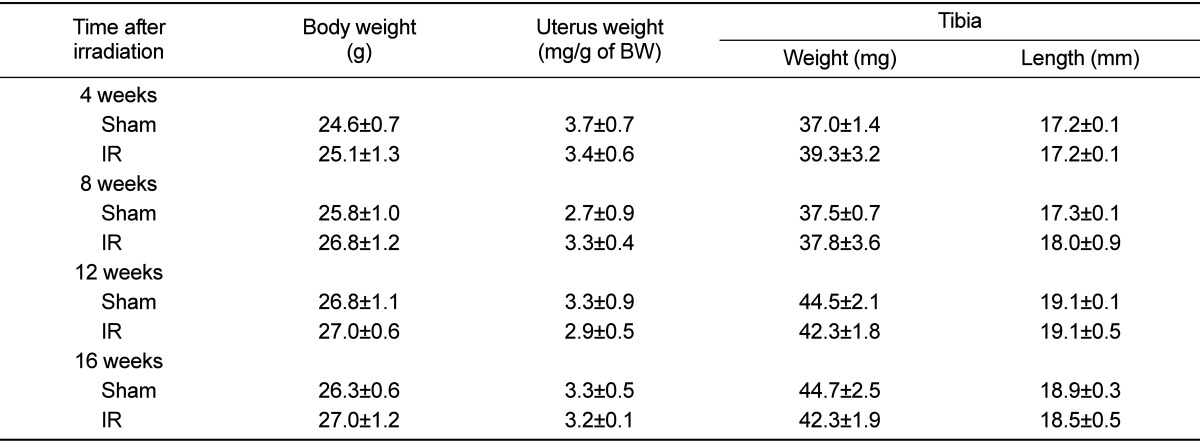
Data are expressed as means±SD (n=6).
Table 2.
Time-related changes in mechanical property after gamma-irradiation with 2 Gy in 9-week-old mice tibia

Data are expressed as means±SD (n=6) of maximum load (N).
Table 3.
Time-related changes in serum biochemical markers after gamma-irradiation with 2 Gy in 9-week-old mice
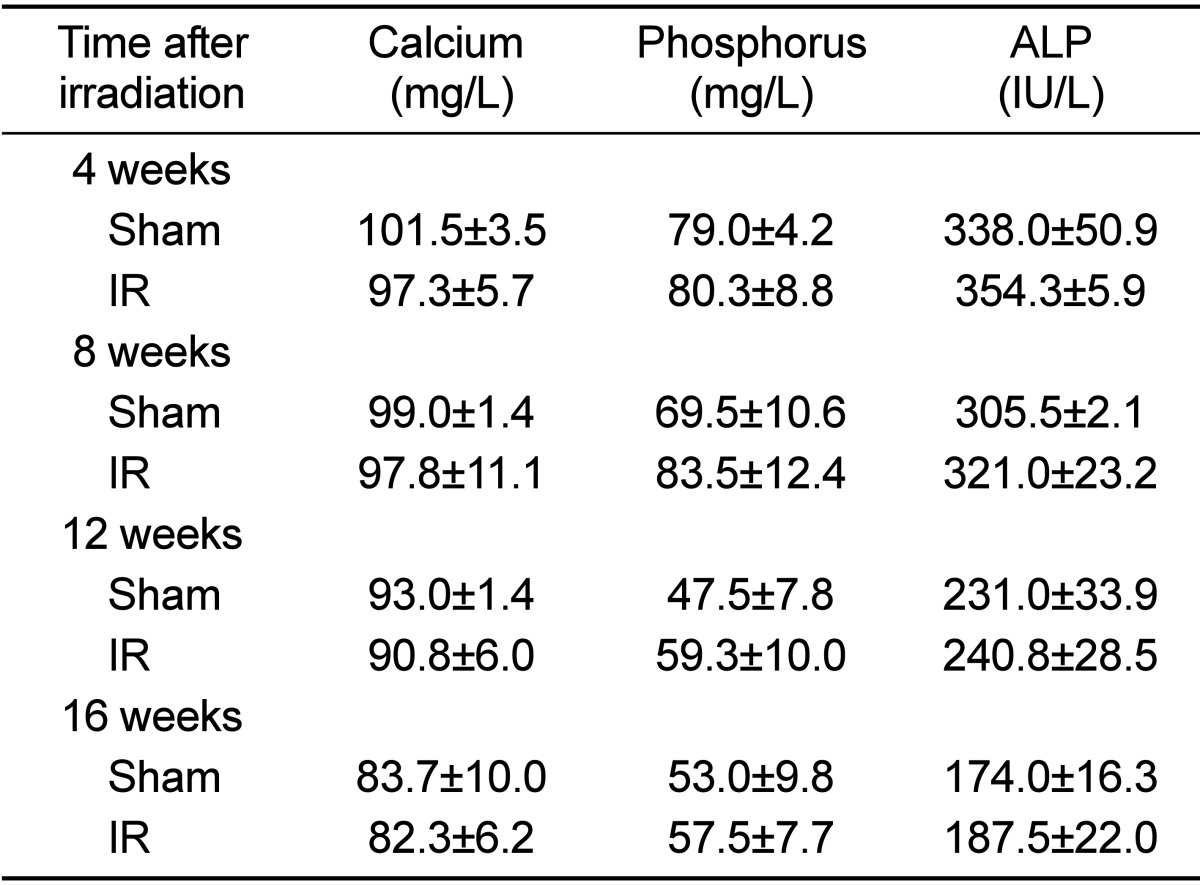
Data are expressed as means±SD (n=6).
ALP: alkaline phosphatase.
Figure 1.
Micro-CT images of the tibia for control (A, B, C) and irradiated (D, E, F) C3H/HeN mice. Cross sectional view (A, D), vertical view (B, E) and reconstructed three-dimensional image (C, F).
Table 4.
Time-related changes in trabecular and cortical bone properties after gamma-irradiation with 2 Gy in 9-week-old mice tibia
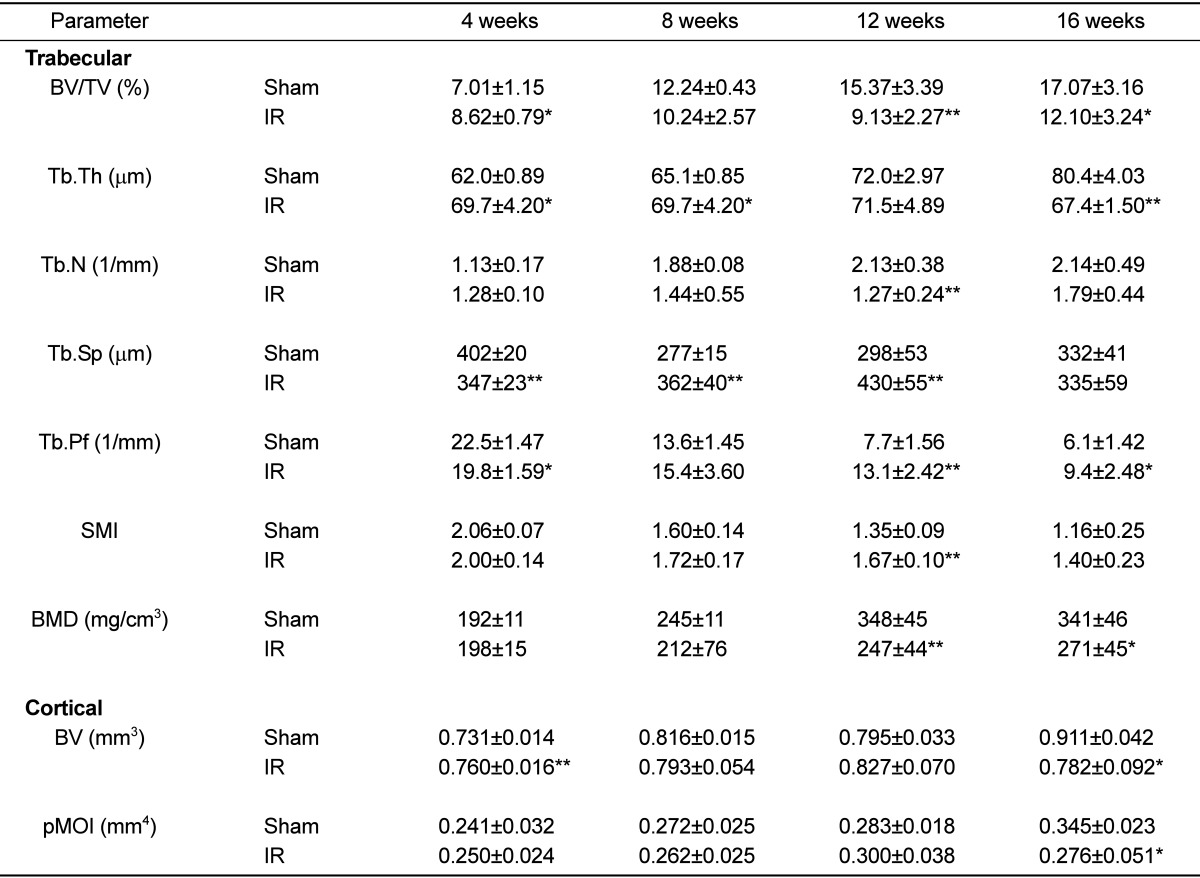
Data are expressed as means±SD (n=6).
*P<0.05, **P<0.01, vs sham group at corresponding times and parameters.
BV/TV: bone volume density; Tb.Th: trabecular thickness; Tb.N: trabecular number; Tb.Sp: trabecular separation; Tb.Pf: trabecular bone pattern factor; SMI: structure model index; BMD: trabecular volumetric mineral density; BV: cortical bone volume; pMOI: polar moment of inertia.
There was no dose-dependent change of irradiation in grip strength, body weight, weight and length of tibia in the test of bone change observation at 12 weeks after irradiation of each dose (Tables 5 and 6). Decreased Ca and increased of P and ALP in serum chemistry were observed in mice irradiated with a high dose (6 Gy). However, no dose-dependent change of irradiation and no change of estrogen were evident in any test group (Table 7). Trabecular bone loss was markedly enhanced by irradiation in a dose-dependent manner. The µCT values indicated a changed trabecular bone loss in 2 Gy irradiated mice, with a rapid change evident upon increasing radiation dose. There was no change in BV of compact bone and pMOI was decreased in mice irradiated with more than 2 Gy compared to the non-irradiated control group, but with no dose-dependent decrease (Table 8). The dose-response curves were analyzed using the best-fitting curve model. The curves were linear-quadratic and a significant relationship was found between the bone change and dose. Taking the controls into accounts, the lines of best fit were:
Table 5.
Dose-related changes in grip strength at 12 weeks after gamma-irradiation in 9-week-old mice
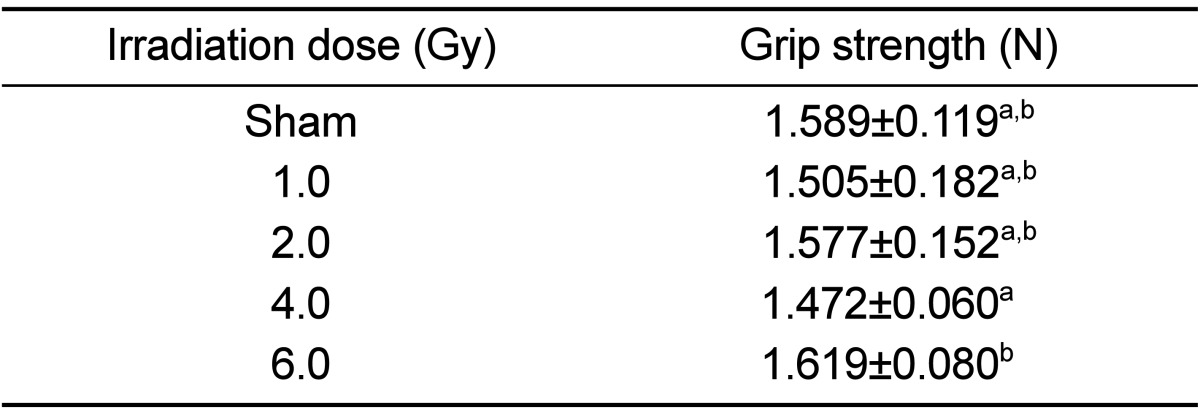
Data are expressed as means±SD (n=6).
a,bValues with different superscripts in the same parameter are significantly different (P<0.05).
Table 6.
Dose-related changes in body weight, tibia weight and length at 12 weeks after gamma-irradiation in 9-week-old mice
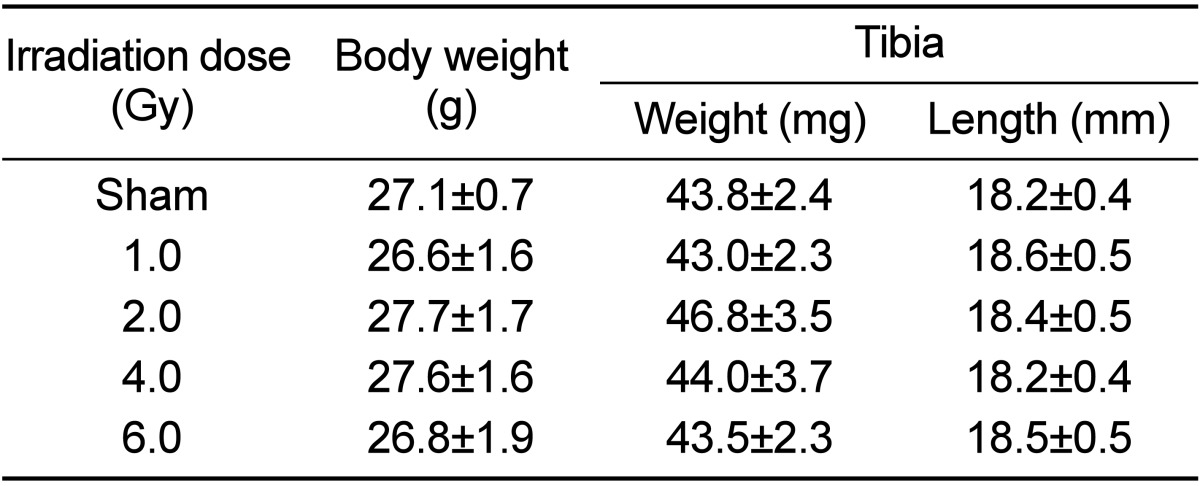
Data are expressed as means±SD (n=6).
Table 7.
Dose-related changes in serum biochemical markers at 12 weeks after gamma-irradiation in 9-week-old mice
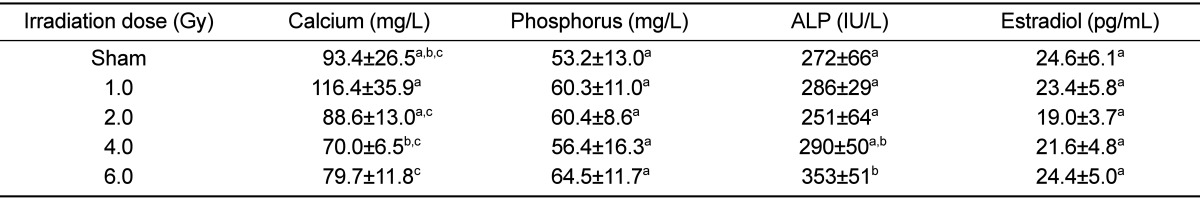
Data are expressed as means±SD (n=6).
a,b,cValues with different superscripts in the same parameter are significantly different (P<0.05).
ALP: alkaline phosphatase.
Table 8.
Dose-related changes in trabecular and cortical bone properties at 12 weeks after gamma-irradiation in 9-week-old mice tibia
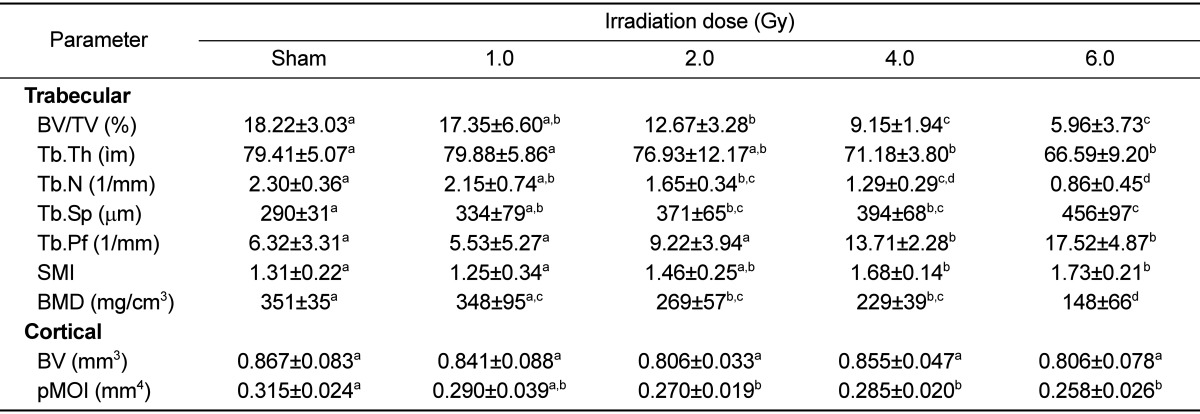
Data are expressed as means±SD (n=6).
a,b,c,dValues with different superscripts in the same parameter are significantly different (P<0.05).
BV/TV: bone volume density; Tb.Th: trabecular thickness; Tb.N: trabecular number; Tb.Sp: trabecular separation; Tb.Pf: trabecular bone pattern factor; SMI: structure model index; BMD: trabecular volumetric mineral density; BV: cortical bone volume; pMOI: polar moment of inertia.
| BV/TV (%)= -0.071D2-1.7986D+18.835 (r2=0.968) |
| BMD (mg/cm3)= -3.5466D2-14.8D+359.07 (r2=0.9862) |
where D is the irradiation dose in Gy.
Measurement of positive osteoclast length after TRAP staining revealed a length of 19% in the non-irradiated control group with no change according to irradiation dose (Figure 2, Table 9).
Figure 2.
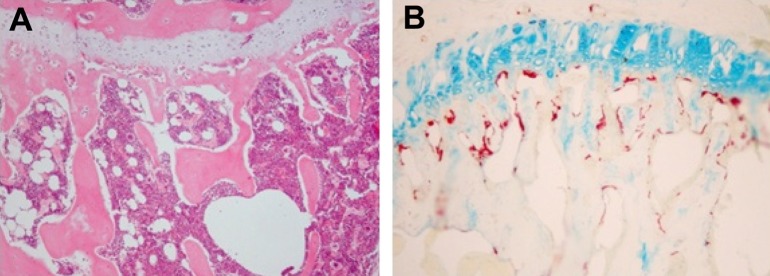
Hematoxylin & eosin (A) and tartrate-resistant acid phosphatase (TRAP) staining (B) of trabecular bone in tibia (× 40).
Table 9.
Dose-related changes in osteoclast surface as a percentage of total bone surface (Oc.S/BS) at 12 weeks after gamma-irradiation in 9-week-old mice
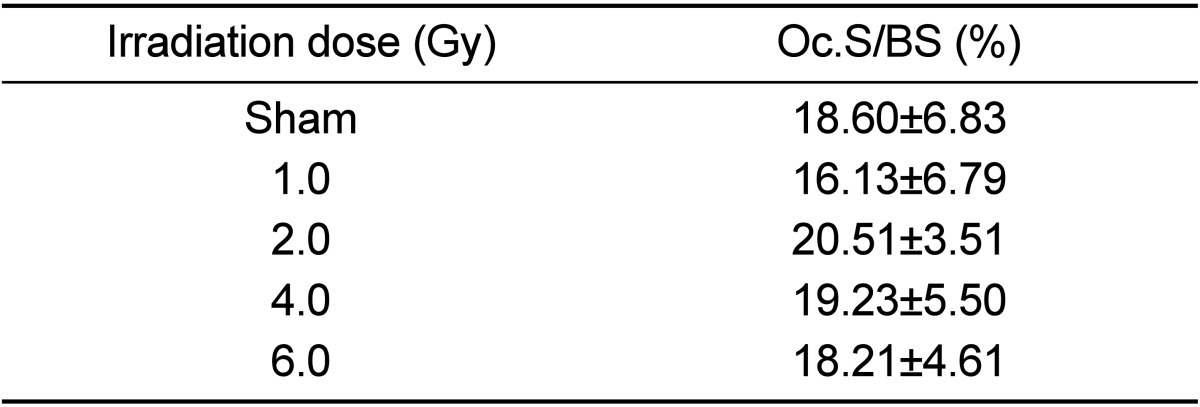
Data are expressed as means±SD (n=6).
Discussion
Despite widespread use of mice in the study of musculoskeletal disease, the radiation-induced changes in murine bone structure and BMD changes are not well characterized. Thus, we assessed radiation-induced changes in BMD, and trabecular and cortical microarchitecture in the tibiae of mice.
Advancements in bone imaging techniques through µCT have improved our ability to make quantitative measurements of trabecular bone architecture in experimental animals. Murine models have given experimenters the advantage of large populations of genetically defined individuals available for study [16]. In addition, the mouse has proven to be a good model for studying issues related to the genetic regulation of peak bone mass, sexual dimorphism of the bone, disuse and senile-related loss [17].
There was no change of mechanical property, lengths and weights of tibia, and serum biochemical markers in the test of time-related change after 2 Gy gamma irradiation. Therefore, it is not considered to be sensitive index. There was no change of the value in uterine weight and it is considered not to induce ovary damage by irradiation and subsequent secondary hormonal bone loss. The significant loss of trabecular bone in multiple indices was observed at 12 weeks after irradiation. The change of compact bone was observed from 16 weeks. However, as the change of trabecular bone was considered to be a standard of index in the study of observation in osteoporosis [11], the observation time in bone loss by irradiation of adult mice was considered to be appropriate at 12 weeks after irradiation when we comprehensively analyzed the results.
There were no changes of grip strength, body weight, weight and length of tibiae 12 weeks after irradiation at each dose. There was no dose-dependent change of serum chemistry, therefore, it was not considered to be sensitive index. There was no particular change of the estrogen level between irradiated and sham mice, consistent with the interpretation that radiation-induced bone loss has no relation with hormone change by radiation-induced ovary damages. The multiple indices showed the change of bone loss from 2 Gy in the observation of trabecular bone change and dose-dependent results. It is considered that the change of trabecular bone can be an indicator of irradiation-related bone loss because the change of compact bone was not dose-dependent. No change of osteoclast activity was observed during this study period. The loss of bone mass after irradiation has been hypothesized to occur as a result of damage to osteoblast or increase in osteoclast activity [12,18]. The decrease of ALP or increase of osteoclast activity was not recognized at the observation time of 12 week. Therefore, in verification of bone loss due to increase of osteoclast activity or bone loss due to decrease of osteoblast activity, it can be considered that a previous duration-set-up of the observation time in trabecular bone change is needed.
Although some studies have shown significant correlation between grip strength, biomechanical property and BMD [19,20], there was no significant correlation among these markers in the current study.
The present data indicate that there is a quantitative bone loss in tibiae produced by various doses of radiation. The yield of BV/TV and BMD appeared to be linear-quadratically related to dose. The results indicate that the frequency of bone loss following the dose of 2 Gy was significantly higher than for sham-irradiation, and increased with increasing dose. With this approach it would be possible to detect the effects of doses between 2 Gy to 6 Gy at 12 weeks after irradiation. Therefore, the bone change assay using µCT is a relatively new and simple quantification of radiation injury in mice. Further mechanistic studies on the effect of radiation-induced bone changes in mice will be needed to extrapolate the experimental data for radiation protection to humans.
Acknowledgments
This work was supported by a National Research Foundation (NRF) of Korea grant funded by the Korean Government.
References
- 1.Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: challenge and opportunity. Semin Radiat Oncol. 2003;13(3):248–266. doi: 10.1016/S1053-4296(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80(Spec No 1):S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 4.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16(2):130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 5.Howland WJ, Loeffler RK, Starchman DE, Johnson RG. Postirradiation atrophic changes of bone and related complications. Radiology. 1975;117(3 Pt 1):677–685. doi: 10.1148/117.3.677. [DOI] [PubMed] [Google Scholar]
- 6.Ergün H, Howland WJ. Postradiation atrophy of mature bone. CRC Crit Rev Diagn Imaging. 1980;12(3):225–243. [PubMed] [Google Scholar]
- 7.Heuch F, Lauritzen C. Veränderungen von mineralgehalt und struktur des femur nach gyakologischer stralentherapie. Stralentherapie. 1967;66:87–92. [Google Scholar]
- 8.Chen HH, Lee BF, Guo HR, Su WR, Chiu NT. Changes in bone mineral density of lumbar spine after pelvic radiotherapy. Radiother Oncol. 2002;62(2):239–242. doi: 10.1016/s0167-8140(02)00002-6. [DOI] [PubMed] [Google Scholar]
- 9.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22(8):1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 10.Szymczyk KH, Shapiro IM, Adams CS. Ionizing radiation sensitizes bone cells to apoptosis. Bone. 2004;34(1):148–156. doi: 10.1016/j.bone.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Bandstra ER, Pecaut MJ, Anderson ER, Willey JS, De Carlo F, Stock SR, Gridley DS, Nelson GA, Levine HG, Bateman TA. Long-term dose response of trabecular bone in mice to proton radiation. Radiat Res. 2008;169(6):607–614. doi: 10.1667/RR1310.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willey JS, Lloyd SA, Robbins ME, Bourland JD, Smith-Sielicki H, Bowman LC, Norrdin RW, Bateman TA. Early increase in osteoclast number in mice after whole-body irradiation with 2 Gy X rays. Radiat Res. 2008;170(3):388–392. doi: 10.1667/RR1388.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 14.Chappard D, Retailleau-Gaborit N, Legrand E, Baslé MF, Audran M. Comparison insight bone measurements by histomorphometry and microCT. J Bone Miner Res. 2005;20(7):1177–1184. doi: 10.1359/JBMR.050205. [DOI] [PubMed] [Google Scholar]
- 15.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 16.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18(5):397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 17.Bower AL, Lang DH, Vogler GP, Vandenbergh DJ, Blizard DA, Stout JT, McClearn GE, Sharkey NA. QTL analysis of trabecular bone in BXD F2 and RI mice. J Bone Miner Res. 2006;21(8):1267–1275. doi: 10.1359/jbmr.060501. [DOI] [PubMed] [Google Scholar]
- 18.Willey JS, Lloyd SA, Nelson GA, Bateman TA. Ionizing Radiation and Bone Loss: Space Exploration and Clinical Therapy Applications. Clin Rev Bone Miner Metab. 2011;9(1):54–62. doi: 10.1007/s12018-011-9092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya A, Watts NB, Davis K, Kotowski S, Shukla R, Dwivedi AK, Coleman R. Dynamic bone quality: a noninvasive measure of bone's biomechanical property in osteoporosis. J Clin Densitom. 2010;13(2):228–236. doi: 10.1016/j.jocd.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Monaco M, Di Monaco R, Manca M, Cavanna A. Handgrip strength is an independent predictor of distal radius bone mineral density in postmenopausal women. Clin Rheumatol. 2000;19(6):473–476. doi: 10.1007/s100670070009. [DOI] [PubMed] [Google Scholar]