Fairall and colleagues[1] provide additional evidence for task-shifting in HIV care. But, like previous reports[2,3], they describe sub-optimal retention and virologic suppression rates and a lack of evidence of long-term effectiveness and sustainability of task-shifting.
We prospectively evaluated an ART programme at a rural primary-care clinic in South Africa, with extensive task-shifting to nurses who assessed and managed HIV patients monthly according to contemporary treatment guidelines but referred to an on-site doctor for ART initiation or complications.
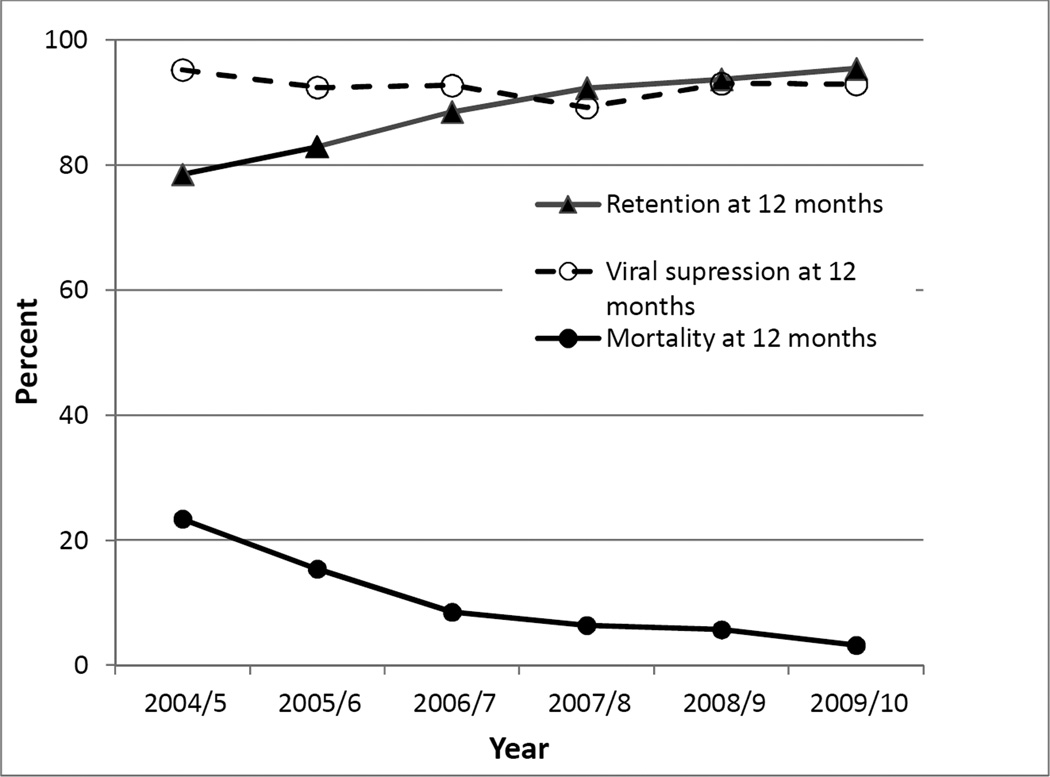
Between 2004 and 2010, 3733 ART-naïve adults were enrolled in care and 1585 commenced ART. 3130 of 3733 patienst (83·8%) remained in pre-ART care between registration and CD4 testing, and 2648 of 3130 (84.6%) remained between CD4 testing and ART initiation; these results are appreciably higher than in similar settings [4]. To evaluate how treatment outcomes changed between 2004 and 2010, initiation cohorts were defined by the year of ART commencement (Appendix). CD4 counts at baseline rose suggesting earlier commencement of ART but treatment regimens remained similar. Mortality rates at 6,12 or 24 months declined significantly within cohorts and temporally (p<0·0001). At each timepoint evaluated, 88·9–97·4% of patients achieved virologic suppression, and the proportions were sustained until 60 months. Retention at 12 or 24 months averaged 88.0% and 81·7% respectively and improved between 2004 and 2010 (p<0·0001) (Figure 1).
Figure 1.
Trends in retention, mortality and viral suppression rates at 12 months post ART initiation in patients enrolled in the CAPRISA AIDS Treatment Programme in rural South Africa each year from 2004 to 2010
While our data are limited to one clinic without a comparator, they demonstrate that task-sharing can realistically achieve better outcomes than reported[1,2,3,4,5]. The temporal changes point to a sustained improvement in treatment outcomes with time.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contributions of the CAPRISA AIDS Treatment team for providing care. We thank the CAPRISA Vulindlela community advisory board and community of Vulindlela for supporting this program and the Mahlase family for their leadership in inviting us to offer care in this community. We thank the KwaZulu-Natal Department of Health and the doctors and nurses at the Mafakathini Primary care clinic for their professional support in clinical care.
Role of the Funding Source
Patient care in the CAPRISA AIDS Treatment project is supported by the KwaZulu-Natal Department of Health, the Global Fund to fight AIDS, Tuberculosis and Malaria and the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). We thank the US National Institutes for Health’s Comprehensive International Program of Research on AIDS (CIPRA, grant # AI51794) for the research infrastructure. VN was supported by LIFELab and the Columbia University-South Africa Fogarty AIDS International Training and Research Program (AITRP, grant # D43 TW000231). None of the funding sources had any role in the writing of the manuscript of the decision to submit it for publication. The corresponding author had full access to the data and final responsibility to submit for publication.
Footnotes
Authors Contributions
VN and QAK conceived and designed the study. VN, KN and NY collected the data. NY performed data analyses steps and the data was interpreted by all of the authors listed.
Conflicts of Interest
SSAK is a member of the PEPFAR Scientific Advisory Board and the WHO Strategic Use of Antiretrovirals Committee. The other authors declare no conflicts of interest.
Ethics Committee Approval
This study was approved by the University of KwaZulu Natal Biomedical Research Ethics Committee.
References
- 1.Fairall L, Bachmann MO, Lombard C, Timmerman V, Uebel K, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012 doi: 10.1016/S0140-6736(12)60730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanne I, Orrell C, Fox MP, Conradie F, Ive P, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffar S, Amuron B, Foster S, Birungi J, Levin J, et al. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet. 2009;374:2080–2089. doi: 10.1016/S0140-6736(09)61674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS medicine. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. The Lancet infectious diseases. 2010;10:155–166. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.