Abstract
During fasting and after exercise, skeletal muscle efficiently switches from carbohydrate to lipid as the main energy source to preserve glycogen stores and blood glucose levels for glucose-dependent tissues. Skeletal muscle cells sense this limitation in glucose availability and transform this information into transcriptional and metabolic adaptations. Here we demonstrate that AMPK acts as the prime initial sensor that translates this information into SIRT1-dependent deacetylation of the transcriptional regulators PGC-1α and FOXO1, culminating in the transcriptional modulation of mitochondrial and lipid utilization genes. Deficient AMPK activity compromises SIRT1-dependent responses to exercise and fasting, resulting in impaired PGC-1α deacetylation and blunted induction of mitochondrial gene expression. Thus, we conclude that AMPK acts as the primordial trigger for fasting- and exercise-induced adaptations in skeletal muscle and that activation of SIRT1 and its downstream signalling pathways are improperly triggered in AMPK deficient states.
Keywords: AMPK, SIRT1, PGC-1α, energy expenditure, FOXO1
Introduction
During fasting or energy deficits, the limiting glucose availability must be compensated by an increase in mitochondrial fatty acid oxidation in skeletal muscle and other peripheral tissues to preserve blood glucose levels and supply glucose-dependent tissues, such as the brain or red blood cells (Cahill et al., 1966). The lack of metabolic flexibility to correctly adapt to energy demands and nutrient availability constitutes a burden on energy homeostasis, ultimately leading to metabolic disease (Kelley and Mandarino, 2000). Metabolic adaptations in muscle are driven by coordinated transcriptional responses to promote the mitochondrial use of lipid substrates as a source of energy (de Lange et al., 2006). Such alterations in gene expression patterns are achieved through the modulation of transcriptional regulators, as the coactivator PGC-1α or the FOXO family of transcription factors, both of which are intimately linked to the regulation of mitochondrial and fatty acid metabolism (Gross et al., 2008; Handschin and Spiegelman, 2006). The activities of PGC-1α (Rodgers et al., 2005) and FOXOs (Brunet et al., 2004) are critically influenced by the control of their acetylation levels through the type III NAD+-dependent deacetylase SIRT1. As a result, SIRT1 acts as master regulator of muscle adaptations to nutrient availability (Fulco et al., 2008; Gerhart-Hines et al., 2007). Notable, SIRT1 knock-down prevents the induction of mitochondrial and lipid oxidation genes in glucose restricted myotubes (Gerhart-Hines et al., 2007). How SIRT1 activity is regulated is still largely unknown, even thought it has been hypothesized that NAD+ availability, for which SIRT1 acts as a sensor, plays a major role (Gerhart-Hines et al., 2007).
Another metabolic sensor with strong impact on transcriptional responses is the AMP-activated protein kinase (AMPK), a heterotrimeric Ser/Thr kinase composed of one catalytic (α) and two non-catalytic subunits (β and γ) (Hardie, 2007). Two different isoforms exist for both the α and the β subunits (α1 and α2 or β1 and β2), while the γ subunits are encoded by three different genes (γ1, γ2 and γ3) (Hardie, 2007). The γ subunits can bind AMP or ATP in a competitive and mutually exclusive manner (Hardie, 2007). The binding of AMP increases the catalytic activity of the complex and enhances the phosphorylation of a key residue within the catalytic domain (Thr172), essential for activity. In contrast, the binding of ATP antagonizes the effects of AMP on enzymatic activity, transforming AMPK into a energy monitoring system of AMP:ATP ratio. The activation of AMPK turns on catabolic pathways to generate ATP, and switches off a number of processes that consume ATP, such as fatty acid, protein or cholesterol synthesis (Hardie, 2007). Interestingly, the activity of AMPK seems connected to that of SIRT1. AMPK enhances SIRT1 activity by increasing intracellular NAD+ levels (Canto et al., 2009; Costford et al., 2009; Fulco et al., 2008). This translates in the deacetylation of SIRT1 targets, such as PGC-1α in response to pharmacological or physiological AMPK activation (Canto et al., 2009). Whether AMPK is required for fasting-induced activation of SIRT1 and deacetylation of its targets remains unknown. Here, we provide evidence that AMPK acts as the primordial sensor of energy stress and that its activity is required to trigger SIRT1-dependent adaptations.
Results
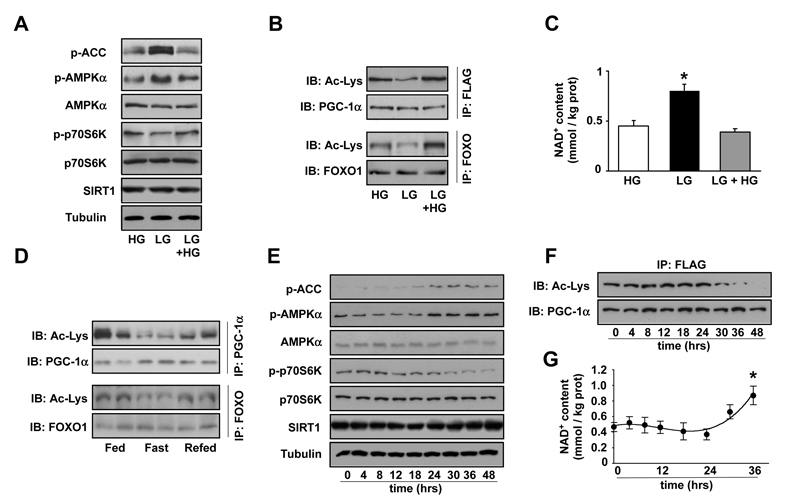
C2C12 myotubes display a number of adaptive changes after 48hrs incubation in glucose restricted medium (5mM). For example, AMPK is activated, as manifested by the phosphorylation on the Thr172 residue of the α subunit and the phosphorylation of ACC, an AMPK substrate (Fig.1A). This was in parallel to the downregulation of the mTOR pathway, as evidenced by p70S6K phosphorylation (Fig.1A), and the deacetylation of SIRT1 targets, PGC-1α and FOXO1 (Fig.1B). The deacetylation of SIRT1 targets was not due to higher SIRT1 protein levels (Fig.1A), but rather due changes in activity derived from increased NAD+ levels (Fig.1C), which reflected a metabolic rearrangement towards oxidative metabolism. Consequent to the changes in PGC-1α and FOXO activity through deacetylation, the expression of mitochondrial and lipid oxidation genes was increased after 48hrs in low glucose medium (Fig.S1). The deacetylation of these transcriptional regulators could also be observed in mouse gastrocnemius muscle after overnight fasting (Fig.1D).
Figure 1. Glucose restriction increases PGC-1α-dependent gene expression.
(A-C) C2C12 myotubes were infected with adenoviruses encoding FLAG-HA-PGC-1α. Then, cells were left for 48hrs in medium containing either 25mM glucose (HG) or 5mM glucose (LG). A third group was treated by adding 25mM glucose medium for 6hrs after 48hrs in LG (LG + 6hrs HG). (A) Total protein was obtained and 100μg were used for Western blot analysis to test the markers indicated. (B) 500μg of total protein extracts were used for immunoprecipitation (IP) against FLAG antibody or against FOXO to determine the acetylation levels of PGC-1α or FOXO1, respectively. (C) Acidic lysates were obtained for measurement of NAD+ levels. Data are presented as mean±S.E. from 6 different experiments. * Indicates statistical difference vs. HG group. (D) 300μg of nuclear extracts from gastrocnemius muscle from fed, fasted or re-fed C57BL/6J mice were used for IP against PGC-1α or FOXO1 to measure acetylation. (E-G) After infection with adenoviruses encoding FLAG-HA-PGC-1α C2C12 myotubes were left in 5mM glucose medium and samples were obtained at the times indicated. (E) 100μg of total protein extracts were used for Western analysis to test the markers indicated. (F) 500μg of total protein extracts were used for immunoprecipitation to detect the acetylation levels of PGC-1α (G) Acidic lysates were obtained for measurement of NAD+ levels. Data are presented as mean±S.E. from 5 different experiments. * indicates statistical difference vs. t=0. All images are representative of 3-6 independent experiments.
Remarkably, these changes were rapidly reversed by re-incubating myotubes in 25mM glucose or refeeding mice (Fig.1A-D and Fig.S1). Hence, the activity of metabolic sensors and the acetylation levels and activity of SIRT1 targets co-ordinately respond to external nutrient availability.
We then explored the sequential events leading to the adaptations in glucose restriction. AMPK and ACC phosphorylation increased 18-24hrs after incubation in low glucose, in parallel to a decrease in mTOR signalling (Fig.1E). Changes in PGC-1α acetylation levels took place 30-36hrs after incubation in low glucose medium (Fig.1B), in the absence of changes in SIRT1 protein levels (Fig.1E). Intracellular NAD+ levels increased 30-36hrs after glucose restriction, in line with the timing for PGC-1α deacetylation (Fig.1F). These results indicate that changes in AMPK activity during glucose restriction precede changes in NAD+ levels and SIRT1 activity.
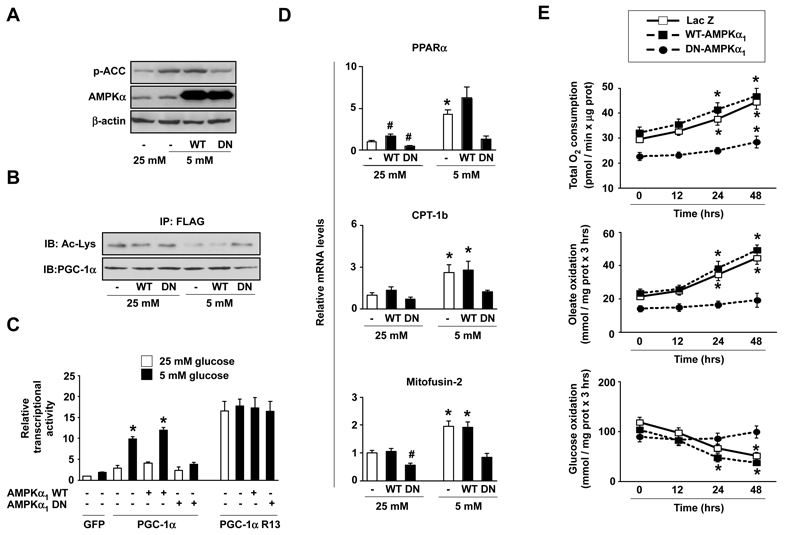
Based on the above results, we tested whether AMPK could act as the primordial sensor triggering SIRT1-dependent metabolic adaptations. For this purpose, we overexpressed lacZ (control), wild-type (WT) or dominant negative (DN) forms of AMPKα1 and examined the activation of PGC-1α in response to glucose deprivation. The DN-AMPKα1 robustly prevented AMPK activity (Fig.2A) and impaired PGC-1α deacetylation upon glucose restriction (Fig.2B). Consistent with the impaired deacetylation, studies on the mouse PGC-1α promoter reporter, which itself is activated in a PGC-1α-dependent manner, revealed that PGC-1α transcriptional activity was not significantly enhanced in glucose restricted cells when AMPK activity was blunted (Fig.2C). Interestingly, DN-AMPKα1 did not prevent the transcriptional activity of a PGC-1α mutant where the 13 acetylable lysines are mutated to arginine (R13) (Fig.2C). This latter observation suggests that the phosphorylation of PGC-1α by AMPK (Jager et al., 2007) might act as a signal for deacetylation, but does not contribute to the intrinsic activity of this transcriptional cofactor.
Figure 2. Disruption of AMPK activity renders PGC-1α activity and metabolic adaptations unresponsive to glucose restriction.
(A) C2C12 myotubes were infected with adenovirus encoding FLAG-HA-PGC-1α and either LacZ, a wild-type (WT) or a dominant negative (DN) form of AMPKα1. After infection cells were left for 48hrs in 25 or 5mM glucose medium. 100μg of total extracts were used for Western analysis. (B) As in (A), but 500 μg from total extracts were used for immunoprecipitation against FLAG to test PGC-1α acetylation levels. (C) C2C12 myoblasts were transfected with lacZ and either an empty vector (not shown) or a luciferase reporter construct linked to the mouse PGC-1α promoter. Simultaneously, cells were infected with adenovirus encoding for GFP (−), FLAG-HA-PGC-1α, FLAG-HA-PGC-1α R13, WT-AMPKα1 or DN-AMPKα1 as indicated. 36hrs later, cells were incubated for 48hrs in medium containing either 25 or 5mM glucose and luciferase activity was measured. A representative assay of 4 independent experiments is shown. Values are expressed as mean±SE. * indicates statistical difference vs. corresponding 25mM glucose group. (D) Similar to (A), but total mRNA was obtained for qRT-PCR analysis. Relative mRNA levels are shown as mean±SE from 4 experiments. * indicates statistical difference vs. corresponding 25mM glucose group. # indicates statistical difference vs. lacZ-infected 25mM group. (E) C2C12 myotubes were infected as in (A). Then, cells were left in 25 or 5mM glucose medium. At the indicated time points, O2 consumption, oleate and glucose oxidation rates were measured. Results are shown as mean±SE from 12, 6 and 4 independent experiments, respectively. * indicates statistical difference vs. time=0hrs.
In line with the defective PGC-1α activation during glucose restriction, the DN-AMPKα1 prevented the increase in the mRNA levels of mitochondrial and fatty acid oxidation genes, such as PPARα, CPT-1b, mitofusin-2 (Fig.2D), ERRα, PDK4 and COXIV (Fig.S2). We next evaluated the physiological impact of these changes by analyzing how oxidative metabolism responded to glucose restriction when AMPK activation is impaired. O2 consumption gradually increased 24 and 48hrs after glucose restriction in LacZ and WT-AMPKα1 expressing myotubes (Fig.2E). This response was largely attenuated in cells expressing DN-AMPKα1 (Fig.2E). While total O2 consumption in LacZ and WT-AMPKα1 expressing myotubes increased 50.9±9.7 and 45.5±9.9 %, respectively, after 48hrs in LG medium, only a 25.1±5.7 % increase was detected in the presence of DN-AMPKα1 (Fig.2E). These differences were more evident when lipid oxidation was specifically examined. Even 48hrs after the initiation of glucose restriction, fatty acid oxidation rates were unaltered in myotubes expressing DN-AMPKα1, in sharp contrast with myotubes expressing either lacZ or WT-AMPKα1 (Fig.2E). Furthermore, impaired AMPK activation prevented the decrease in glucose oxidation rates observed in LacZ and WT-AMPKα1 expressing myotubes (Fig.2E). Hence, AMPK activation during energy stress allows a rapid shift from glucose to lipid as the main substrate for oxidation.
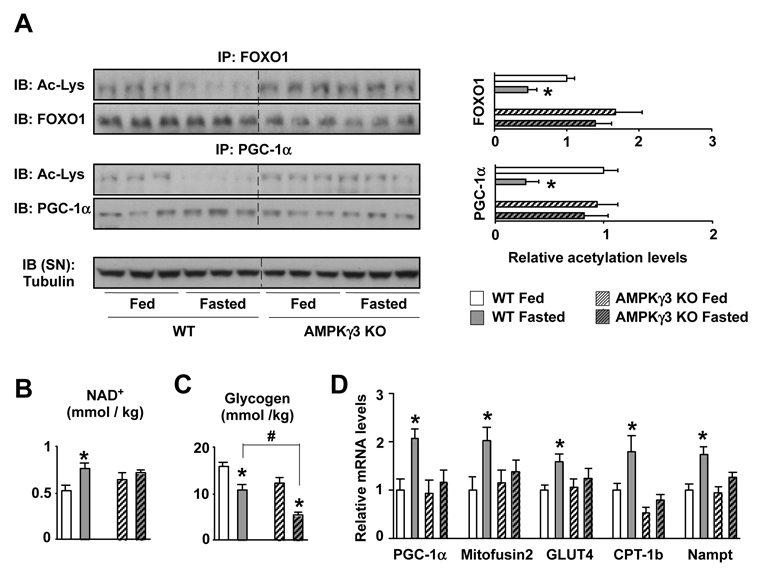
To provide in vivo evidence to support the above results, we used mice deficient in the AMPKγ3 subunit (Barnes et al., 2004) The γ3 is the predominant γ-isoform in glycolytic skeletal muscle (Mahlapuu et al., 2004), and is critical for effective adaptations of glycolytic muscle to metabolic challenges (Barnes et al., 2005b; Long et al., 2005). Fasting-induced PGC-1α and FOXO1 deacetylation was evident in EDL muscles from WT mice, but severely blunted in AMPKγ3 KO mice (Fig.3A). This defective SIRT1 activity could be consequent to the fact that intramuscular levels of NAD+ were unresponsive to fasting in AMPKγ3 KO mice (Fig.3B). Notably, muscle glycogen levels in WT mice dropped ~35% (~5 mmols x kg-1) after fasting, while ~55% (~7 mmols x kg-1) in the AMPKγ3 KOs (Fig.3C), suggesting that the AMPKγ3 KOs cannot shift to non-carbohydrate energy sources as efficiently as their WT littermates. Consequent to reduced PGC-1α activation, the expression of PGC-1α target genes was also impaired upon fasting in AMPKγ3 KO mice (Fig.3D). Interestingly, fasting induced Nampt expression, but this increase was blunted in the AMPKγ3 KOs (Fig.3D), providing an additional explanation for the lack of changes in NAD+ levels. Next, we tested whether administration of a claimed SIRT1 activator, such as resveratrol (Rsv), could rescue the defective reponse of PGC-1α acetylation to fasting in the EDL muscles of AMPKγ3 KOs. Interestingly, Rsv efficiently decreased PGC-1α acetylation levels in EDL muscles from WT mice (Fig.S3), but was unable to do so in muscles from the AMPKγ3 KOs (Fig.S3), indicating that the actions of Rsv on PGC-1α deacetylation require intact AMPK activity.
Figure 3. Impaired PGC-1α deacetylation and transcriptional response to fasting in AMPKγ3 KOs.
Wild type and AMPKγ3 KO mice in fed or fasted (20hrs) state were sacrificed and muscles were extracted and frozen (A) 2mg of total protein extracts from EDL muscles were used to immunoprecipitate FOXO1 and PGC-1α and check their acetylation. Relative quantifications of FOXO1 and PGC-1α acetylation levels are shown on the right as mean±SE of 6 muscles/group. (B) Acidic extracts from 50mg of gastrocnemius muscle were used to measure NAD+ content. Results are shown as mean±S.E. from 6 muscles measured in duplicate. (C) 20mg of quadriceps muscle were used to measure glycogen content. Results are shown as mean±S.E. from 5 muscles. # indicates statistical difference between the groups indicated (D) Total mRNA was extracted from quadriceps muscles and used for qPCR analysis. Results are shown as mean±SE from 10 muscles/group. Through the figure, * indicates statistical difference vs. respective fed group.
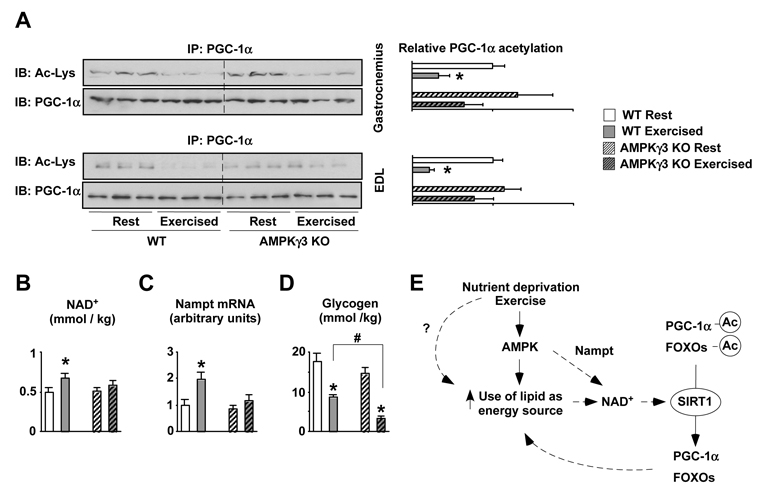
Finally, we evaluated whether AMPK was required for PGC-1α deacetylation in response to exercise, the most common energy challenge in skeletal muscle. PGC-1α and FOXO1 were robustly deacetylated 2.5hrs after swimming, an effect that was partially blunted in the AMPKγ3 KO mice (Fig.4A). As observed in fasted muscle, the increase in NAD+ observed during exercise recovery in WT mice was absent in AMPKγ3 KOs (Fig.4B). A recent report indicated that increases in NAD+ and SIRT1 activity after exercise could also derive from an increase in Nampt (Costford et al., 2009). Noteworthy, Nampt induction after exercise was impaired in AMPKγ3 KO mice (Fig.4C), further indicating that AMPK controls Nampt expression (Fig.4C). Also similar to what was observed in fasting, glycogen levels were markedly lower in the AMPKγ3 KO mice 2.5hrs after exercise (Fig.4D). This is in line with previous findings (Mu et al., 2003), indicating an impaired ability to rely on fat oxidation to recover glycogen levels.
Figure 4. Impaired PGC-1α deacetylation in response to exercise in AMPKγ3 KOs.
(A) Wild-type and AMPKγ3 KO mice were sacrificed at rest or 2.5hrs after swimming (see methods) and muscles were frozen. 300μg of nuclear extracts from gastrocnemius or 2mg of total proteins from EDL muscles were used to immunoprecipitate PGC-1α and check its acetylation levels. (B) Acidic extracts from 50mg of gastrocnemius were used to measure NAD+. Results are shown as mean±S.E. from 3-4 muscles per group measured in duplicate. (C) 20mg of quadriceps were used to measure Nampt expression. (D) 20mg of quadriceps muscle were used to measure glycogen content. Results are shown as mean±S.E. from 3-4 muscles per group measured in duplicate. # indicates statistical difference between the groups indicated (E) Proposed scheme for the coordinated regulation of lipid utilization during energy stress. Upon low nutrient availability or increased energy demand, as during exercise, there is an increase in the AMP/ATP ratio, which activates AMPK, enhancing lipid oxidation in the mitochondria and inducing Nampt levels. These events will raise intracellular NAD+ levels, triggering SIRT1 activation, which deacetylates PGC-1α and FOXOs, both of which regulate genes that further favour mitochondrial respiration and lipid mobilization. Through the figure, * indicates statistical difference vs. respective fed group.
Discussion
We report that the acetylation levels of two SIRT1 targets, the transcriptional regulators PGC-1α and FOXO1, dynamically respond to energy stresses, such as exercise and fasting in skeletal muscle, or changes in glucose availability in C2C12 myotubes. Furthermore, our results indicate that AMPK activation precedes and determines the changes in SIRT1 activity in situations of energy stress. The regulation of the acetylation levels of transcriptional regulators through the AMPK/SIRT1 axis provides a mechanism by which mitochondrial and lipid oxidation genes can be rapidly and selectively controlled in response to energy levels.
Genetic ablation of the AMPKγ3 subunit largely prevents physiological and/or pharmacological activation of AMPK (Barnes et al., 2005a) and was enough to impair fasting- and exercise-induced PGC-1α deacetylation. This unequivocally proves that SIRT1-mediated deacetylation of PGC-1α requires functional AMPK. The unchanged NAD+ levels after fasting or during exercise recovery in the AMPKγ3 KO mice suggests that these mice are metabolically inflexible and, together with the defective induction of Nampt, might explain the defective SIRT1 activation. Along the same line, the AMPKγ3 KO mice showed higher glycogen depletion on fasting and after exercise. Our results reinforce previous observations showing that AMPK defective mice have impaired glycogen metabolism (Barnes et al., 2005a; Fujii et al., 2007; Mu et al., 2003). Additionally, AMPKγ3 KO mice highly rely on glucose metabolism during exercise recovery (Barnes et al., 2005a), explaining why glycogen levels hardly recover after exercise. Similarly, myotubes expressing a DN-AMPKα1 highly depend on glucose as oxidative energy source even during glucose restriction. Altogether, these data illustrate that AMPK is necessary to efficiently shift between different energy substrates in the muscle and optimize the use of glycogen stores.
Noteworthy, while several models of AMPK deficiency display decreased basal mitochondrial gene expression (Jorgensen et al., 2005), lower voluntary exercise activity (Mu et al., 2001) and exercise tolerance (Fujii et al., 2007), defective AMPK does not robustly affect exercise-induced gene expression (Barnes et al., 2005b; Jorgensen et al., 2005). In line with this, AMPK acts on mitochondrial gene expression through PGC-1α (Jager et al., 2007), which itself is dispensable for mitochondrial gene induction after exercise (Leick et al., 2008). Contrastingly, AMPK is required for mitochondrial biogenesis upon pharmacological energy depletion (Zong et al., 2002). This reflects how exercise triggers more pathways than those derived from energy depletion, which redundantly regulate exercise-induced transcriptional changes (Jensen et al., 2009).
The clear effect of the ablation of AMPKγ3 on fasting-induced PGC-1α responses is striking, as AMPK activation during fasting is controversial (de Lange et al., 2006). AMPK activity, however, was estimated to be ~50% lower in the AMPKγ3 KOs than in WT mice after fasting (Barnes et al., 2005a). An additional explanation for the defects observed lies in the fact that NAD+ levels remain stable during fasting/feeding transitions in the AMPKγ3 KO mice, probably due to the inability of their mitochondria to shift between substrates (Barnes et al., 2005a), which would prevent activation of SIRT1. This link between AMPK and mitochondrial fitness might also explain why AMPK participates in the adaptations to other situations such as calorie restriction (Apfeld et al., 2004; Schulz et al., 2007).
From a pharmacological perspective, our results on incubated muscle show that Rsv, initially believed to be a direct SIRT1 activator, requires AMPK to promote PGC-1α deacetylation in glycolytic muscle. Together with recent data (Um et al., 2009), our results suggest that SIRT1 activation in response to Rsv, rather than being a direct effect on SIRT1, might be an indirect consequence of AMPK activation (Canto et al., 2009). Another explanation is that phosphorylation by AMPK might be required for PGC-1α deacetylation by SIRT1 (Canto et al., 2009). Consequently, PGC-1α would remain acetylated when AMPK activity is defective even when SIRT1 is active. Altogether, these observations indicate that Rsv requires AMPK for its metabolic effects.
Based on our data, we propose that AMPK acts as an initial sensor of energy stress and allows the cell to efficiently shift between different energetic substrates. This, together with the ability of AMPK to regulate Nampt expression, impacts on intracellular NAD+ levels, which modulate SIRT1 action on its downstream targets such as PGC-1α and FOXO1 (Fig.4D). Consequently, SIRT1 activation constitutes an indirect consequence of the metabolic and transcriptional rearrangements induced by AMPK activation.
Experimental procedures
Reagents and Materials
A full list of Reagents and Materials is provided as Supplemental Experimental Procedures.
Animal studies
For the fasting experiment in Fig.1, animals were purchased from Charles River (L’Arbresle, France). 10 week-old C57Bl/6J male mice were maintained in a temperature-controlled (23°C) facility with a 12hrs light/dark cycle. To study the effects of fasting, food was removed for 16hrs, including the overnight period. The AMPKγ3 KO mice have been described previously (Barnes et al., 2004). WT or AMPKγ3 KO mice (6-9 months old) had free access to water and standard rodent chow. When examining the effects of fasting, food was removed 20hrs prior to the extraction of hindlimb muscles, which were cleaned of fat and blood, and quickly frozen in liquid nitrogen. For exercise experiments, mice swam in 45cm Ø plastic containers for four 30-min intervals separated by 5-min rest periods for a total swimming time of 2hrs. Then, mice were dried and allowed to recover from exercise for 2.5 hrs. In the recovery period mice had free access to food and water.
Cell culture and adenoviral infection
C2C12 skeletal muscle cells were grown and differentiated as described (Canto et al., 2009). C2C12 were considered as myotubes after 96hrs of differentiation. Differentiation medium was supplemented with BSA-conjugated oleic acid (0.2mM). Adenoviral infections of C2C12 myocytes were performed after 48hrs of differentiation. Cells were washed with PBS and left for 1hr in serum-free DMEM 4.5g/l glucose containing the appropriate amount of viral particles (MOI=100 per each virus used, using GFP (for the different PGC-1α forms) or LacZ (for AMPKα1 WT and DN forms) as control to make even the final viral amount). Media was replaced with fresh differentiation media for additional 48hrs before any treatment took place. All the adenoviruses used in this study have been described (Rodgers et al., 2005; Woods et al., 2000)
Metabolic measurements
Oxygen consumption was measured using the XF24 equipment (Seahorse Bioscience Inc., North Billerica, MA) as described (Watanabe et al., 2006). Oleate and glucose oxidation rates were estimated as described previously (Pich et al., 2005).
NAD+ and NADH measurements
NAD+ intracellular levels in muscle (30mg) and cultured myotube samples (1mg) were estimated as described (Rodgers et al.,2005)
Statistics
Differences between two groups were assessed using two-tailed t-tests. Analysis of variance, assessed by Bonferonni’s multiple comparison test, was used when comparing more than two groups.
Additional experimental procedures can be found in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgements
This work was supported by grants of the Ecole Polytechnique Fédérale de Lausanne, Swiss National Science Foundation, NIH (DK59820), Swedish Research Council, Swedish Foundation for Strategic Research, EU FP6 (EUGENE2; LSHM-CT-2004-512013), and the European Research Council Ideas programme (Sirtuins; ERC-2008-AdG23118 for JA, and ICEBERG; ERC-2008-AdG23285 for JRZ). CC is supported by an EMBO fellowship. The authors thank Pere Puigserver, Fabienne Foufelle and Pascal Ferré for providing materials and the members of the Auwerx and Zierath labs for support and discussion.
References
- Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BR, Glund S, Long YC, Hjalm G, Andersson L, Zierath JR. 5′-AMP-activated protein kinase regulates skeletal muscle glycogen content and ergogenics. Faseb J. 2005a;19:773–779. doi: 10.1096/fj.04-3221com. [DOI] [PubMed] [Google Scholar]
- Barnes BR, Long YC, Steiler TL, Leng Y, Galuska D, Wojtaszewski JF, Andersson L, Zierath JR. Changes in exercise-induced gene expression in 5′-AMP-activated protein kinase gamma3-null and gamma3 R225Q transgenic mice. Diabetes. 2005b;54:3484–3489. doi: 10.2337/diabetes.54.12.3484. [DOI] [PubMed] [Google Scholar]
- Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, et al. The 5′-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem. 2004;279:38441–38447. doi: 10.1074/jbc.M405533200. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Cahill GF, Jr., Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA, Jr., Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966;45:1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church T, Jubrias SA, Conley KE, Smith SR. Skeletal Muscle NAMPT is Induced by Exercise in Humans. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00318.2009. doi:10.1152/ ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange P, Farina P, Moreno M, Ragni M, Lombardi A, Silvestri E, Burrone L, Lanni A, Goglia F. Sequential changes in the signal transduction responses of skeletal muscle following food deprivation. Faseb J. 2006;20:2579–2581. doi: 10.1096/fj.06-6025fje. [DOI] [PubMed] [Google Scholar]
- Fujii N, Seifert MM, Kane EM, Peter LE, Ho RC, Winstead S, Hirshman MF, Goodyear LJ. Role of AMP-activated protein kinase in exercise capacity, whole body glucose homeostasis, and glucose transport in skeletal muscle -insight from analysis of a transgenic mouse model. Diabetes Res Clin Pract. 2007;77(Suppl 1):S92–98. doi: 10.1016/j.diabres.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. Embo J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TE, Wojtaszewski JF, Richter EA. AMP-activated protein kinase in contraction regulation of skeletal muscle metabolism: necessary and/or sufficient? Acta Physiol (Oxf) 2009;196:155–174. doi: 10.1111/j.1748-1716.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. Faseb J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E463–474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- Long YC, Barnes BR, Mahlapuu M, Steiler TL, Martinsson S, Leng Y, Wallberg-Henriksson H, Andersson L, Zierath JR. Role of AMP-activated protein kinase in the coordinated expression of genes controlling glucose and lipid metabolism in mouse white skeletal muscle. Diabetologia. 2005;48:2354–2364. doi: 10.1007/s00125-005-1962-5. [DOI] [PubMed] [Google Scholar]
- Mahlapuu M, Johansson C, Lindgren K, Hjalm G, Barnes BR, Krook A, Zierath JR, Andersson L, Marklund S. Expression profiling of the gamma-subunit isoforms of AMP-activated protein kinase suggests a major role for gamma3 in white skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E194–200. doi: 10.1152/ajpendo.00147.2003. [DOI] [PubMed] [Google Scholar]
- Mu J, Barton ER, Birnbaum MJ. Selective suppression of AMP-activated protein kinase in skeletal muscle: update on ‘lazy mice’. Biochem Soc Trans. 2003;31:236–241. doi: 10.1042/bst0310236. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr., Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, Palacin M, Zorzano A. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet. 2005;14:1405–1415. doi: 10.1093/hmg/ddi149. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMPK-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2009 doi: 10.2337/db09-0482. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.