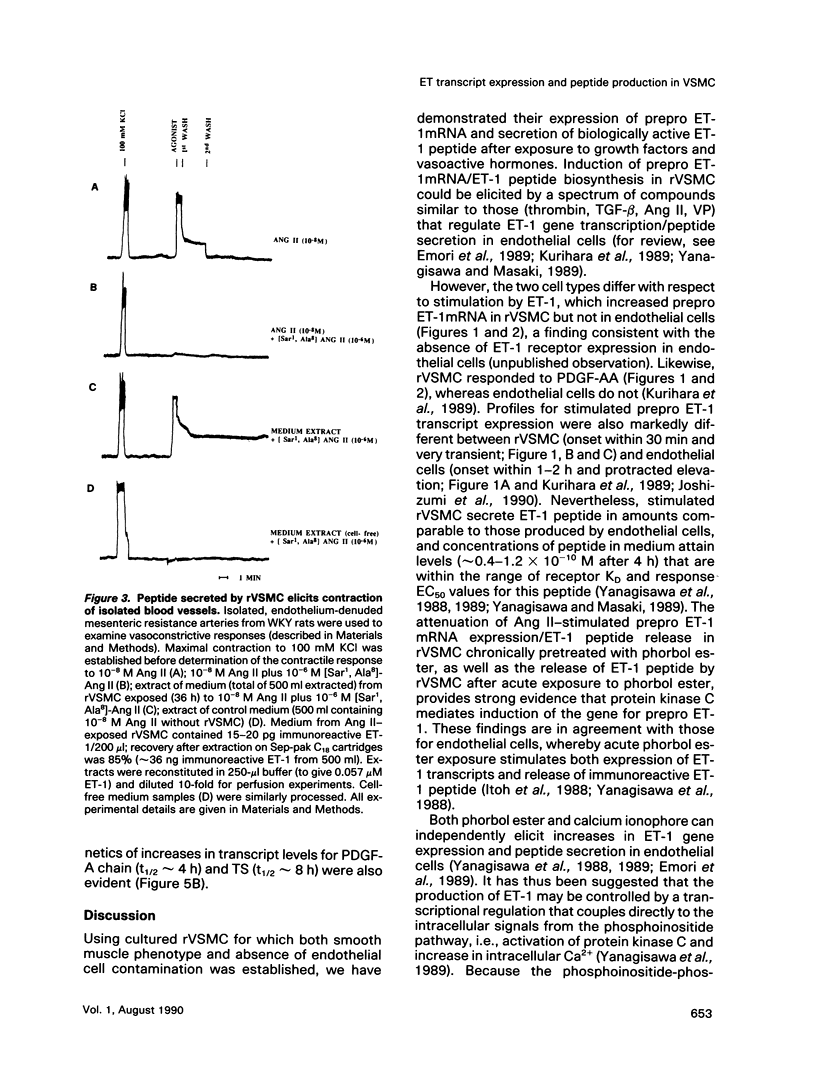
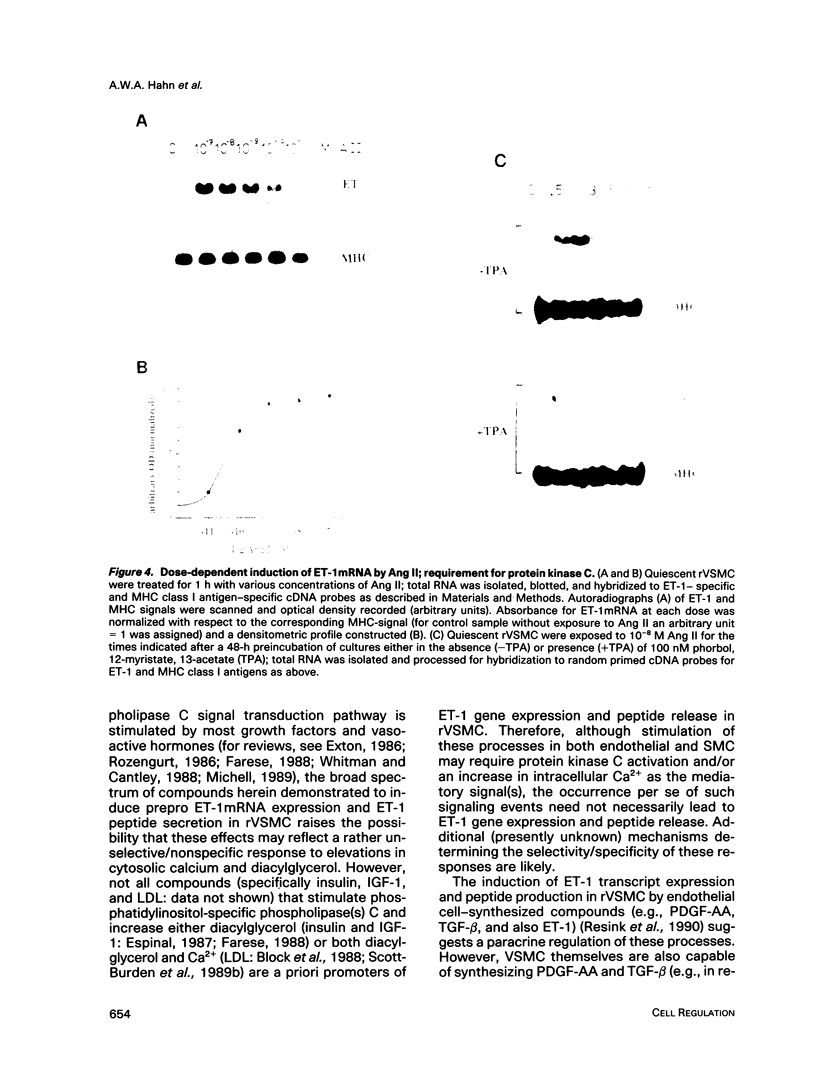
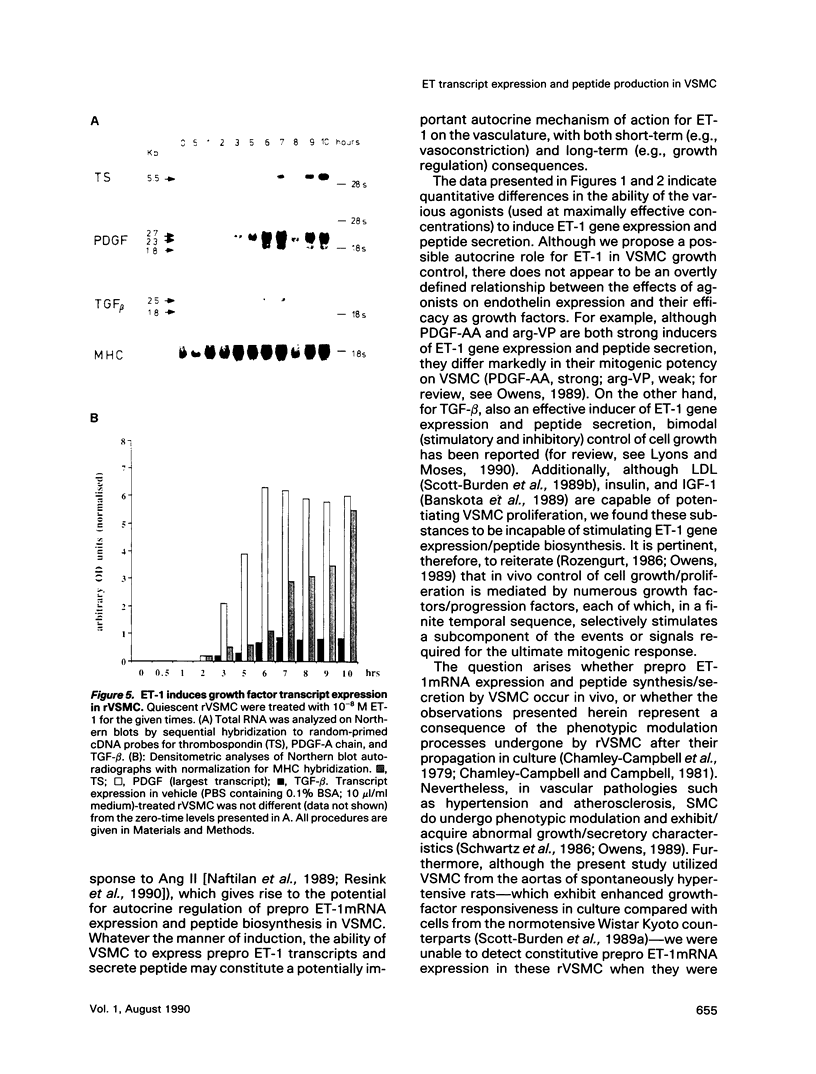
Abstract
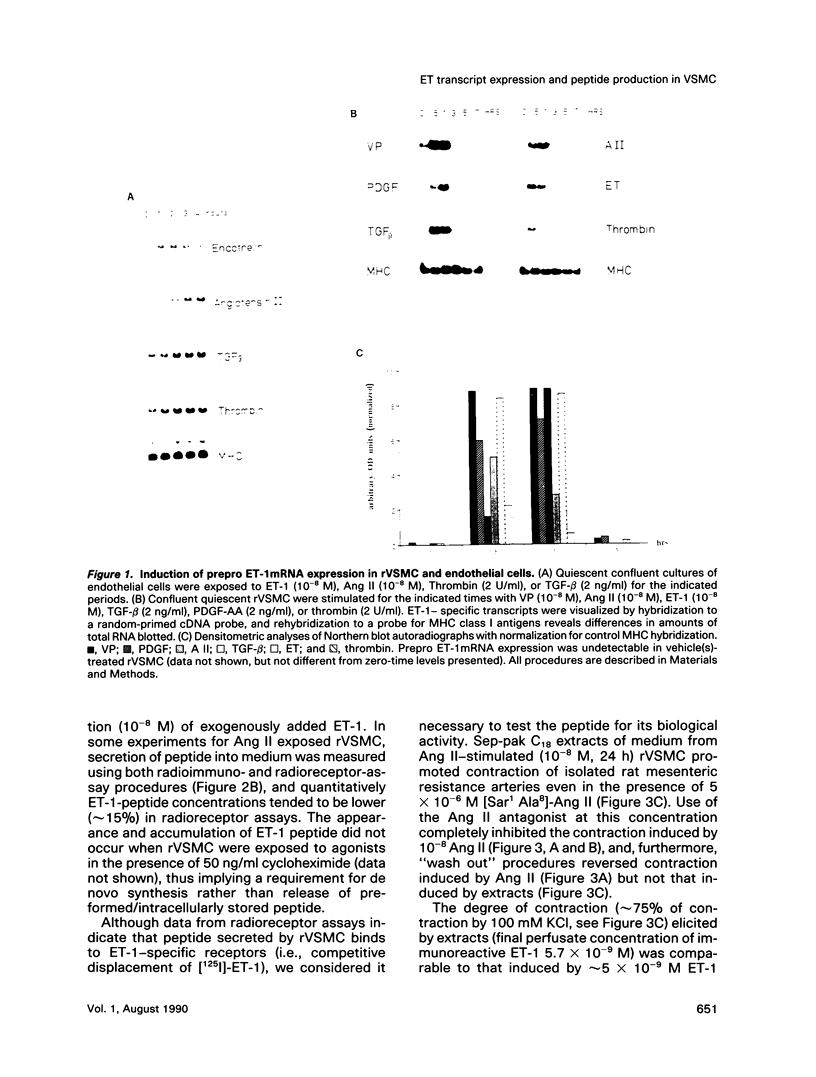
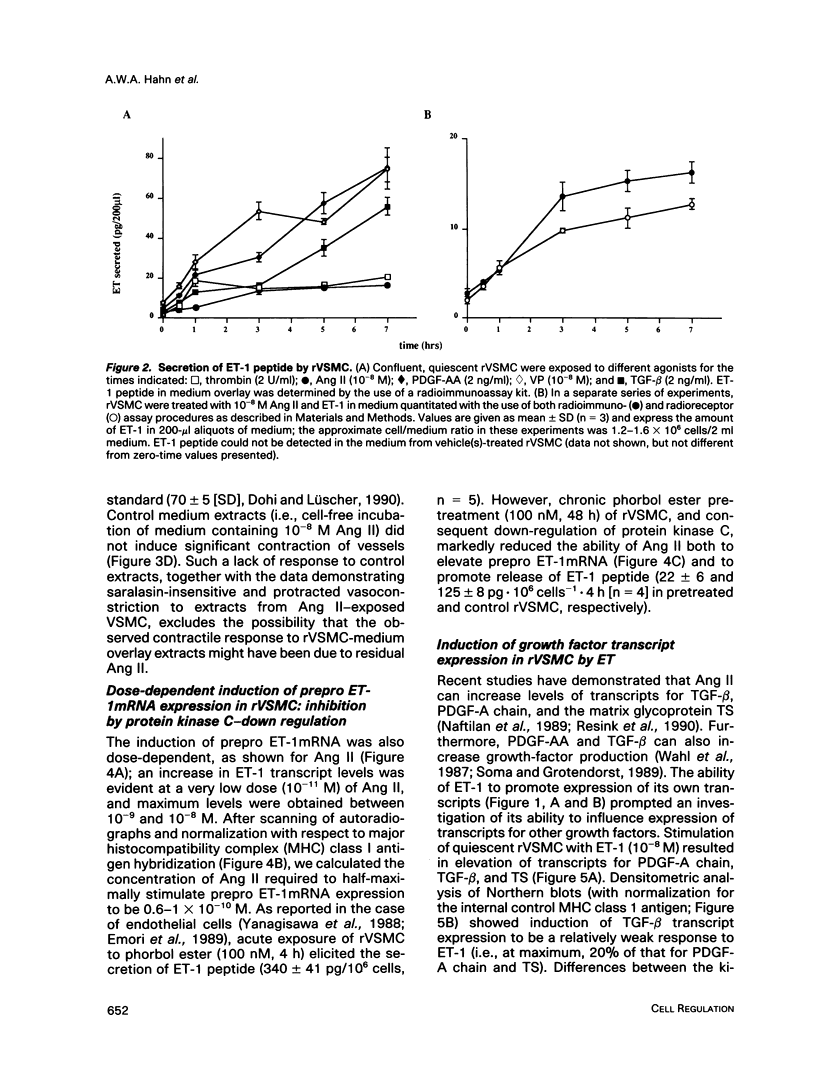
Endothelin (ET), a peptide originally isolated from the supernatants of cultured endothelial cells, exerts a wide variety of biological effects in different tissues. Endothelial-cell-synthesized ET-1 has been proposed to act in a paracrine manner on adjacent smooth muscle cells (SMC) in vivo, with effects that include both vascular reactivity (vasodilation/vasoconstriction) and mitogenesis. This study, by the use of immunocytochemically characterized SMC (rVSMC) isolated from the aortas of spontaneously hypertensive rats, has investigated a possible autocrine role for ET in regulation of the vasculature. Although quiescent cultures of rVSMC apparently did not constitutively express prepro ET-1mRNA, ET-specific transcripts could be induced by a variety of growth factors (transforming growth factor beta [TGF-beta]; platelet-derived growth factor-AA homodimer [PDGF-A chain]) and vasoactive hormones (angiotensin II [Ang II], arginine-vasopressin, and ET-1 itself). The kinetics for prepro ET-1mRNA induction in rVSMC were characteristically rapid in onset and transient. Down-regulation of protein kinase C by 48 h pretreatment of rVSMC with phorbol ester markedly reduced the subsequent ability of rVSMC to express ET-1 transcripts and secrete ET-1 peptide in response to Ang II. Inducible prepro ET-1mRNA expression was accompanied by a cycloheximide-inhibitable release of ET-1 peptide into the medium of rVSMC. ET-1 peptide was determined by both radioreceptor- and radioimmunoassay. Stimulated rVSMC accumulated ET-1 (approximately 200 pg.10(6) cells-1 x 4 h-1) at levels that attained biological relevance (approximately 10(-10) M). Sep-pak C18 extracts of medium from stimulated rVSMC elicited contraction of isolated endothelium-denuded rat mesenteric resistance vessels, and this response was characteristically protracted and difficult to "wash out." Synthetic (porcine) ET-1 promoted the expression of transcripts for PDGF-A chain, TGF-beta, and thrombospondin in quiescent rVSMC. Such effects of ET-1 on gene expression may be relevant to the mitogenic potential of ET-1 on VSMC. Our findings imply a role for ET-1 in the control of vascular function via both paracrine and autocrine regulatory mechanisms. The expression of prepro ET-1mRNA and peptide biosynthesis by rVSMC may have both short-term (e.g., vasoconstriction) and long-term (e.g., structural remodeling) consequences. A sustained loop of autocrine stimulation by ET-1 in SMC could contribute toward the pathogenesis of vasospasm and/or atherosclerosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baley P. A., Resink T. J., Eppenberger U., Hahn A. W. Endothelin messenger RNA and receptors are differentially expressed in cultured human breast epithelial and stromal cells. J Clin Invest. 1990 Apr;85(4):1320–1323. doi: 10.1172/JCI114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banskota N. K., Taub R., Zellner K., Olsen P., King G. L. Characterization of induction of protooncogene c-myc and cellular growth in human vascular smooth muscle cells by insulin and IGF-I. Diabetes. 1989 Jan;38(1):123–129. doi: 10.2337/diab.38.1.123. [DOI] [PubMed] [Google Scholar]
- Block L. H., Knorr M., Vogt E., Locher R., Vetter W., Groscurth P., Qiao B. Y., Pometta D., James R., Regenass M. Low density lipoprotein causes general cellular activation with increased phosphatidylinositol turnover and lipoprotein catabolism. Proc Natl Acad Sci U S A. 1988 Feb;85(3):885–889. doi: 10.1073/pnas.85.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C., Hendrickson H., Lorenz R. R., Vanhoutte P. M. Release of different relaxing factors by cultured porcine endothelial cells. Circ Res. 1989 Jun;64(6):1070–1078. doi: 10.1161/01.res.64.6.1070. [DOI] [PubMed] [Google Scholar]
- Campbell D. J. Circulating and tissue angiotensin systems. J Clin Invest. 1987 Jan;79(1):1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon R. O., 3rd Causes of chest pain in patients with normal coronary angiograms: the eye of the beholder. Am J Cardiol. 1988 Aug 1;62(4):306–308. doi: 10.1016/0002-9149(88)90229-9. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R. What controls smooth muscle phenotype? Atherosclerosis. 1981 Nov-Dec;40(3-4):347–357. doi: 10.1016/0021-9150(81)90145-3. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Dzau V. J., Safar M. E. Large conduit arteries in hypertension: role of the vascular renin-angiotensin system. Circulation. 1988 May;77(5):947–954. doi: 10.1161/01.cir.77.5.947. [DOI] [PubMed] [Google Scholar]
- Emori T., Hirata Y., Ohta K., Shichiri M., Marumo F. Secretory mechanism of immunoreactive endothelin in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1989 Apr 14;160(1):93–100. doi: 10.1016/0006-291x(89)91625-2. [DOI] [PubMed] [Google Scholar]
- Espinal J. Mechanism of insulin action. Nature. 1987 Aug 13;328(6131):574–575. doi: 10.1038/328574a0. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in calcium-mobilizing agonist responses. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:211–262. [PubMed] [Google Scholar]
- Farese R. V. Calcium as an intracellular mediator of hormone action: intracellular phospholipid signaling systems. Am J Med Sci. 1988 Oct;296(4):223–230. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Giaid A., Gibson S. J., Ibrahim B. N., Legon S., Bloom S. R., Yanagisawa M., Masaki T., Varndell I. M., Polak J. M. Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7634–7638. doi: 10.1073/pnas.86.19.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman I. M., Castellot J. J., Jr Regulation of vascular smooth muscle cell growth by endothelial-synthesized extracellular matrices. Arteriosclerosis. 1987 Sep-Oct;7(5):463–469. doi: 10.1161/01.atv.7.5.463. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Yanagisawa M., Ohkubo S., Kimura C., Kosaka T., Inoue A., Ishida N., Mitsui Y., Onda H., Fujino M. Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Lett. 1988 Apr 25;231(2):440–444. doi: 10.1016/0014-5793(88)80867-6. [DOI] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Komuro I., Kurihara H., Sugiyama T., Yoshizumi M., Takaku F., Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988 Oct 10;238(2):249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- Kurihara H., Yoshizumi M., Sugiyama T., Takaku F., Yanagisawa M., Masaki T., Hamaoki M., Kato H., Yazaki Y. Transforming growth factor-beta stimulates the expression of endothelin mRNA by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1435–1440. doi: 10.1016/0006-291x(89)92270-5. [DOI] [PubMed] [Google Scholar]
- Langille B. L., O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986 Jan 24;231(4736):405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Moses H. L. Transforming growth factors and the regulation of cell proliferation. Eur J Biochem. 1990 Feb 14;187(3):467–473. doi: 10.1111/j.1432-1033.1990.tb15327.x. [DOI] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9050–9054. doi: 10.1073/pnas.83.23.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol. 1985 Sep;101(3):1059–1070. doi: 10.1083/jcb.101.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Goodman L. V., Dixit V. M. Cell surface thrombospondin is functionally essential for vascular smooth muscle cell proliferation. J Cell Biol. 1988 Feb;106(2):415–422. doi: 10.1083/jcb.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Post-receptor signalling pathways. Lancet. 1989 Apr 8;1(8641):765–768. doi: 10.1016/s0140-6736(89)92582-8. [DOI] [PubMed] [Google Scholar]
- Naftilan A. J., Pratt R. E., Dzau V. J. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaki T., Nakayama M., Yamamoto S., Kato R. Endothelin-mediated stimulation of DNA synthesis in vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Feb 15;158(3):880–883. doi: 10.1016/0006-291x(89)92804-0. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Geisterfer A. A., Yang Y. W., Komoriya A. Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol. 1988 Aug;107(2):771–780. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza N. B., Schwartz J. H., Goud H. D., Levinsky N. G. Rat aortic smooth muscle cells in culture express kallikrein, kininogen, and bradykininase activity. J Clin Invest. 1990 Feb;85(2):597–600. doi: 10.1172/JCI114479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. W., van Deurs B. Growth factor control of myoepithelial-cell differentiation in cultures of human mammary gland. Differentiation. 1988 Dec;39(3):197–215. doi: 10.1111/j.1432-0436.1988.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Pohla H., Kuon W., Tabaczewski P., Doerner C., Weiss E. H. Allelic variation in HLA-B and HLA-C sequences and the evolution of the HLA-B alleles. Immunogenetics. 1989;29(5):297–307. doi: 10.1007/BF00352839. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Sarzani R., Brecher P., Chobanian A. V. Growth factor expression in aorta of normotensive and hypertensive rats. J Clin Invest. 1989 Apr;83(4):1404–1408. doi: 10.1172/JCI114029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Bühler F. R. Regulation of smooth muscle proliferative phenotype by heparinoid--matrix interactions. Trends Pharmacol Sci. 1988 Mar;9(3):94–98. doi: 10.1016/0165-6147(88)90175-7. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Hahn A. W., Baur U., Box R. J., Bühler F. R. Induction of growth-related metabolism in human vascular smooth muscle cells by low density lipoprotein. J Biol Chem. 1989 Jul 25;264(21):12582–12589. [PubMed] [Google Scholar]
- Simonson M. S., Wann S., Mené P., Dubyak G. R., Kester M., Nakazato Y., Sedor J. R., Dunn M. J. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J Clin Invest. 1989 Feb;83(2):708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma Y., Grotendorst G. R. TGF-beta stimulates primary human skin fibroblast DNA synthesis via an autocrine production of PDGF-related peptides. J Cell Physiol. 1989 Aug;140(2):246–253. doi: 10.1002/jcp.1041400209. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Takuwa Y., Yanagisawa M., Takuwa N., Masaki T. Endothelin, its diverse biological activities and mechanisms of action. Prog Growth Factor Res. 1989;1(4):195–206. doi: 10.1016/0955-2235(89)90011-2. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Masaki T. Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci. 1989 Sep;10(9):374–378. doi: 10.1016/0165-6147(89)90011-4. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M., Kurihara H., Morita T., Yamashita T., Oh-hashi Y., Sugiyama T., Takaku F., Yanagisawa M., Masaki T., Yazaki Y. Interleukin 1 increases the production of endothelin-1 by cultured endothelial cells. Biochem Biophys Res Commun. 1990 Jan 15;166(1):324–329. doi: 10.1016/0006-291x(90)91948-r. [DOI] [PubMed] [Google Scholar]