Abstract
EMBO J (2013) 32: 938–953 doi:; DOI: 10.1038/emboj.2013.31; published online March 08 2013
The transcription factor Oct4 plays a crucial role in the maintenance of the embryonic pluripotent state, but can also regulate early lineage commitment. In this issue of The EMBO Journal, Aksoy et al (2013) lend critical mechanistic insights into the ability of Oct4 to regulate and specify the primitive endodermal lineage. These regulatory actions are governed by alternative direct partnering of Oct4 with Sox17, instead of Sox2, that leads to global reprogramming of enhancer occupancy by Oct4 during primitive endoderm differentiation.
The process of how cell fates are acquired and maintained in multicellular organisms continues to be a major focus of biological research for over a century. The remarkable ability of progenitor cells to acquire a broad range of identities is mediated, in part, by the presence of distinct cis-regulatory elements throughout the genome, termed Enhancers. The latter control the spatial and temporal expression pattern of specific set of genes. Pluripotent embryonic stem cells (ESCs), which are derived from the inner cell mass (ICM) and have the ability to grow indefinitely while maintaining their differentiation capacity, constitute a unique tool for modelling cell fate choices in the Petri dish. The maintenance of pluripotency is governed by a network of transcription factors, including Oct4, Sox2 and Nanog, which repress genes that promote differentiation and activate genes that maintain pluripotency (Yamanaka et al, 2006; Hanna et al, 2010). Oct4 and Sox2 interact physically and cooperatively bind to DNA at genes enhancer and promoter sites, and simultaneously activate and repress pro-pluripotent and differentiation genes, respectively (Remenyi et al, 2003; Niwa, 2007).
Interestingly, apart from maintaining the pluripotent identity, Oct4 expression levels have been shown to influence early lineage commitment choices. Oct4 overexpression can promote ESC differentiation into a mixed population of primitive endoderm (PrE) and mesodermal cells (Niwa et al, 2000). Moreover, in response to defined extrinsic signalling cues, Oct4 can suppress neuroectodermal differentiation and promote mesendodermal fate, whereas Sox2 acts in an opposite manner (Thomson et al, 2011). While demonstrating that the same factors that maintain pluripotency can also control lineage choice in response to signalling cues that modulate their expression levels is well established, the molecular basis underlying Oct4 function in dictating somatic lineage fate choice, and in PrE fate determination in particular, remains vague.
In order to address these questions, Aksoy et al (2013) established a model of PrE specification by overexpression of Sox17 in murine ESCs, which is a major transcription factor specifically upregulated during early PrE differentiation. The authors examined the potential cooperation of Oct4 with Sox17 during PrE induction, and compared it to the well-established Oct4/Sox2 complex in pluripotent ESCs. By performing chromatin immunoprecipitation assays followed by high-throughput sequencing (Chip-Seq), they showed that Sox17 and Oct4 co-bind to enhancer elements enriched with a unique ‘compressed’ Oct4/Sox17 binding motif, which is distinct from the ‘canonical’ motif typically marking Oct4/Sox2 bound regions in ESCs. By performing Chip-Seq on mutant variants of Sox2 and Sox17 that harbour mutations at their protein interaction site with Oct4, and thus perturbs their biological function, Aksoy et al (2013) detected a redistribution of Oct4/Sox17 localization from compressed to canonical motif-containing enhancers. The latter findings suggest that the recruitment of Oct4 from the canonical to the compressed motif is dependent on specific cooperative binding with Sox2 and Sox17, respectively. Significant enrichment of Oct4/Sox17 compressed motif was found at gene enhancers that are upregulated following Sox17-induced endodermal differentiation, suggesting that Oct4/Sox17 co-binding contributes to the activation of these genes and can also help identify new regulators controlling adequate PrE specification. Further, Oct4 was found to directly interact with Sox17 in F9 embryonic carcinoma cell line model that can be induced into PrE-like cells by retinoic acid. Depletion of Oct4 interfered with RA-induced differentiation of F9 cells, and Chip-seq analysis in these cells revealed that endogenous Sox17 preferentially recruits Oct4 to the compressed motif-containing enhancers, and that this co-localization is functionally required for activation of selected PrE genes.
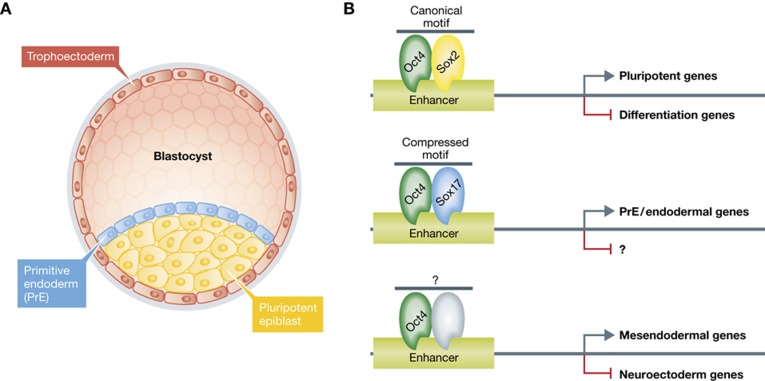
Aksoy et al (2013) provide evidence that this mechanism appears to be implemented in vivo. During early development, the mammalian late blastocyst consists of three cell types: the outer layer of trophectoderm, the pluripotent epiblast and the PrE (Yamanaka et al, 2006; Figure 1). Each cell population expresses specific profile of genes. The pluripotent epiblast cells express Oct4, Sox2 and Nanog, while PrE cell population expresses Oct4, Sox17 and Gata6/4 (Yamanaka et al, 2006; Plusa et al, 2008; Niakan et al, 2010). The authors integrated genome-wide single-cell RNA expression from pluripoitent epiblast and PrE cells, with the identified list of gene enhancers bound by Oct4/Sox17 complex at compressed motif-containing regions in ESCs. This analysis established that compressed motif is enriched among PrE-expressed genes, while the canonical motif was enriched among enhancers of genes upregulated in pluripotent epiblast cells in vivo. This suggests that Sox17 likely recruits Oct4 to enhancers of PrE genes and contributes to consolidation of PrE specification from the epiblast. However, conclusive genetic experiments will be required to functionally establish these correlative findings (e.g., genetic ablation of Oct4 specifically in Sox17+ PrE precursors in vivo).
Figure 1.
Oct4 regulates pluripotency and early development. (A) During early development the mouse late blastocyst comprises three distinct lineages: (1) the epiblast, which gives rise to the embryo proper, (2) primitive endoderm and (3) trophectoderm giving rise to extraembryonic cell types of placenta and yolk sac. (B) Model for cooperative action of Oct4 and Sox transcription factors in pluripotency and during lineage fate choice based on this study. In epiblast and ESCs, the cooperative binding of Oct4/Sox2 complex to canonical motif-containing enhancers facilitates pluripotency maintenance by up- and downregulation of pro-pluripotent and differentiation factors, respectively. Upon induction of PrE, Sox17 levels increase and directly recruit oct4 to compressed motif-containing enhancers, which positively regulate a set of PrE-specification genes (this study), and likely negatively regulate other yet to be defined genes. Notably, Oct4 can also drive commitment into the mesendodermal cell fate and repress neuroectodermal lineage by an undefined partner (Thomson et al, 2011).
Alltogether, these findings support a mechanism by which the expression levels of Sox2 and Sox17 endow Oct4 with alternative functions. In ESCs, Oct4/Sox2 complex regulates pluripotent and self-renewal genes. When PrE cells are specified following inductive signals, Sox17 levels increase and assmble Oct4/Sox17 complexes through direct protein–protein interaction. These interactions facilitate the redistribution of Oct4 to PrE-specific gene enhancers and triggers endodermal expression programme (Figure 1).
This exciting work raises many intriguing unsolved question. What is the molecular composition of Oct4/Sox17 containing protein complexes, and whether they also pertain a repressive function (in addition to gene activation capacity)? How do the Oct4/Sox17 complexes affect the chromatin signature at enhancers? What is the biological function of the newly identified genes during PrE specification in vivo that are activated through their compressed motif-containing enhancers? Oct4 was also implicated in mesodermal fate choice by directly binding and activating Brachyury promoter, and repressing Sox2 locus (Thomson et al, 2011). Thus, further investigation may be warranted to determine whether a similar cooperative mechanism involving a yet to be identified Sox partner recruits Oct4 to genes that promote the mesodermal fate (Figure 1b). Finally, given that posttranslational modifications (e.g., phosphorylation) are also known to modulate the functional outcome of key transcription factors during neural tube development (Ma et al, 2008), it will be of interest to investigate whether such modifications can also affect the partnership of Oct4 with other lineage commitment factors.
In conclusion, this study provides an elegant example of how cellular diversity during early development arises from the action of small number of regulatory molecules that are reused at different spatial and temporal patterns to achieve radically disparate outcomes. This switch allows transcriptional circuits to be rewired rapidly upon induction of differentiation in response to signalling cues. This may be particularly important in pluripotent stem cells, where a wide spectrum of differentiation choices needs to be rapidly, transiently and precisely executed. Given that Oct4 is at the heart of pluripotency and induction of early differentiation, further investigation of its biochemical partners in several cellular contexts is likely to open insightful avenues for understanding how distinct cellular fates are acquired during early development.
Acknowledgments
JHH is supported by a generous gift from Ilana and Pascal Mantoux; and research grants from the Leona M and Harry B Helmsley Charitable Trust, BIRAX inititative, The Sir Charles Clore Research Prize, an ERC starting investigator grant (StG-2011-281906), EMBO young investigator program, E-Rare program within the FP7 initiative, the Israel Science Foundation Regular and Bikura research grants, the ICRF Foundation, Fritz Thyssen Stiftung, the Alon Foundation scholar award, and a grant from Erica A and Robert Drake. AAM is supported by the Weizmann Dean fellowship award.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aksoy I, Jauch R, Chen J, Dyla M, Divakar U, Bogu GK, Teo R, Ng CKL, Herath W, Lili S, Hutchins AP, Robson P, Kolatkar PR, Stanton LW (2013) Oct4 switches partnering from Sox2 to Sox17 to reinterpret the enhancer code and specify endoderm. EMBO J 32: 938–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna JH, Saha K, Jaenisch R (2010) Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell 143: 508–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YC, Song MR, Park JP, Henry HoHY, Hu L, Kurtev MV, Zieg J, Ma Q, Pfaff SL, Greenberg ME (2008) Regulation of motor neuron specification by phosphorylation of neurogenin 2. Neuron 58: 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niakan KK, Ji H, Maehr R, Vokes SA, Rodolfa KT, Sherwood RI, Yamaki M, Dimos JT, Chen AE, Melton DA, McMahon AP, Eggan K (2010) Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev 24: 312–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H (2007) How is pluripotency determined and maintained? Development 134: 635–646 [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24: 372–376 [DOI] [PubMed] [Google Scholar]
- Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK (2008) Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development 135: 3081–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi A, Lins K, Nissen LJ, Reinbold R, Scholer HR, Wilmanns M (2003) Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev 17: 2048–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A, Ramanathan S (2011) Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell 145: 875–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Ralston A, Stephenson RO, Rossant J (2006) Cell and molecular regulation of the mouse blastocyst. Dev Dyn 235: 2301–2314 [DOI] [PubMed] [Google Scholar]