Abstract
Throughout life, neural stem cells (NSCs) in the adult hippocampus persistently generate new neurons that modify the neural circuitry. Adult NSCs constitute a relatively quiescent cell population but can be activated by extrinsic neurogenic stimuli. However, the molecular mechanism that controls such reversible quiescence and its physiological significance have remained unknown. Here, we show that the cyclin-dependent kinase inhibitor p57kip2 (p57) is required for NSC quiescence. In addition, our results suggest that reduction of p57 protein in NSCs contributes to the abrogation of NSC quiescence triggered by extrinsic neurogenic stimuli such as running. Moreover, deletion of p57 in NSCs initially resulted in increased neurogenesis in young adult and aged mice. Long-term p57 deletion, on the contrary, led to NSC exhaustion and impaired neurogenesis in aged mice. The regulation of NSC quiescence by p57 might thus have important implications for the short-term (extrinsic stimuli-dependent) and long-term (age-related) modulation of neurogenesis.
Keywords: adult neural stem cell, lifelong neurogenesis, p57, quiescence
Introduction
In the subgranular zone (SGZ) of the dentate gyrus (DG) of the mammalian hippocampus, new neurons are continuously generated throughout adult life. New neurons migrate to the granule cell layer (GCL) of the DG where they develop the morphological and functional properties of granule neurons, and then integrate into and modify the existing neural circuitry (van Praag et al, 2002; Zhao et al, 2008). Therefore, the neurogenesis in the adult hippocampus has been implicated in cognitive functions. Indeed, inhibition of neurogenesis in the DG of adult mice impaired pattern separation, learning and memory, recovery of stress response, and certain behavioural effects of antidepressants (Shors et al, 2001; Santarelli et al, 2002; Imayoshi et al, 2008; Zhao et al, 2008; Clelland et al, 2009; Snyder et al, 2011). Conversely, an increase in the number of newborn neurons induced by preventing their apoptosis resulted in improved pattern separation (Sahay et al, 2011). These properties of adult neurogenesis indicate its critical roles in the lifelong plasticity of brain functions. Adult neural stem cells (NSCs) provide the capacity for this continuous neurogenesis in the adult brain (Seri et al, 2001; Bonaguidi et al, 2011). Adult NSCs undergo self-renewal throughout adult life and continuously generate new neurons, although they constitute a relatively quiescent cell population (Ahn and Joyner, 2005; Lugert et al, 2010). Then, what is the physiological significance of NSC quiescence?
NSC quiescence may be significant in providing a reserved pool for supplying new neurons on demand in response to various extrinsic neurogenic stimuli. Promotion of NSC division has been observed in response to neurogenic stimuli that enhance production of new neurons, such as running and seizures (van Praag et al, 1999; Lugert et al, 2010). Once activated, NSCs differentiate into intermediate progenitor cells (IPCs), which then go through several rounds of cell division. The frequency of NSC division is thus expected to have a large impact on the number of new neurons. However, whether NSC quiescence indeed modulates the rate of neurogenesis and how NSC quiescence is abrogated in response to neurogenic stimuli have remained unknown.
The importance of lifelong NSC maintenance has been suggested by the observation that cognitive impairment of aged mammals occurs in parallel with a reduction in NSCs and neurogenesis (Kuhn et al, 1996; Kempermann et al, 1998; Santarelli et al, 2002; Bondolfi et al, 2004; Driscoll et al, 2006; Manganas et al, 2007; Ben Abdallah et al, 2010). The relative quiescence of diverse adult tissue stem cells is thought to contribute to lifelong stem cell maintenance, perhaps by preventing the exhaustion of their capacity for proliferation. In the haematopoietic system, for example, a highly quiescent population of haematopoietic stem cells (HSCs) appears to possess the highest self-renewal potential, and forced proliferation of HSCs might result in their depletion (Cheng et al, 2000; van Os et al, 2007; Foudi et al, 2009; Matsumoto et al, 2011; Zou et al, 2011). With regard to NSCs, several studies have suggested a correlation between abrogation of quiescence and impaired maintenance of NSCs (Kippin et al, 2005; Mira et al, 2010; Bonaguidi et al, 2011), although a direct causal relationship has not been demonstrated. For example, NSC quiescence has been implicated in lifelong NSC maintenance based on the experiments using p21-deficient mice, although the proliferation of NSCs in mice deficient in p21 was altered only under ischaemic or in vitro conditions (Qiu et al, 2004; Kippin et al, 2005). The identification of the cell-cycle machinery responsible for NSC quiescence in vivo and the modulation of this machinery are expected to provide insight into the potential role of NSC quiescence in lifelong NSC maintenance.
Cyclin-dependent kinase inhibitors (CKIs) play a primary role in the inhibition of cell-cycle progression. CKIs are categorized into two classes: the Ink4 family (p16Ink4a, p16Ink4b, p18Ink4c, and p19Ink4d) and the Cip/Kip family (p21Cip1, p27Kip1, and p57Kip2). Deletion of either p21 or p27 in mice results in increased progenitor proliferation in the hippocampus in normal or ischaemic condition. However, these CKIs appear to be expressed in differentiating cells rather than in quiescent NSCs (Qiu et al, 2004, 2009; Pechnick et al, 2008), suggesting the involvement of an unrecognized regulator in the cell-cycle regulation of NSCs. Recently, p57 was shown to be a key regulator of HSCs (Cheng et al, 2000; van Os et al, 2007; Foudi et al, 2009; Matsumoto et al, 2011; Zou et al, 2011), although its function in NSC quiescence has remained uncharacterized (Pateras et al, 2009).
We now show that, in the SGZ of the adult mouse hippocampus, p57 is expressed in quiescent radial NSCs, but not in rapidly dividing progenitors. Deletion of p57 in adult NSCs abrogates their quiescence and activates their proliferation. In addition, our results suggest that reduction of p57 protein in NSCs contributes to the abrogation of NSC quiescence triggered by running. We also found that the loss of p57 initially increased the number of new neurons, but subsequently leads to excessive reduction of NSCs and neurogenesis in the aged brain.
Results
Adult hippocampal radial NSCs express p57
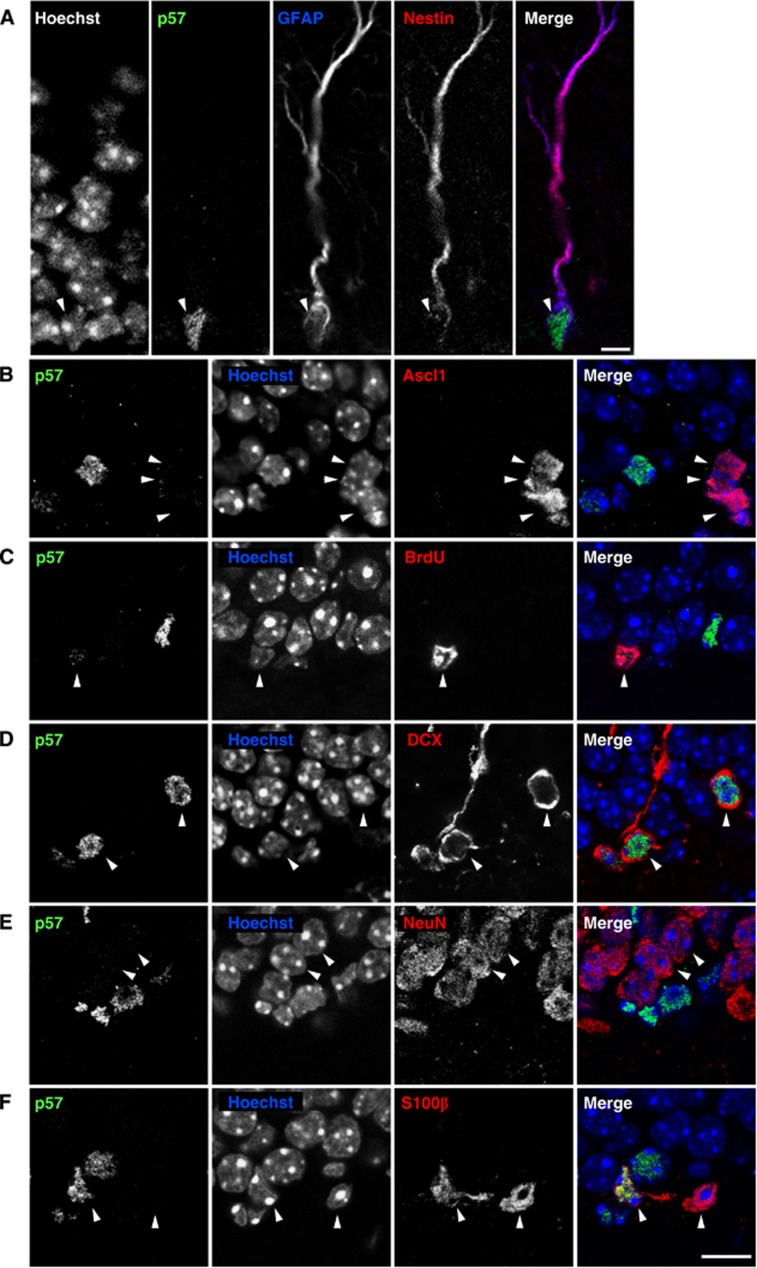
We first examined p57 expression in the DG of the adult mouse hippocampus using immunohistochemical analysis. Previous studies have suggested that radial glia-like cells, which are characterized by a radial fibre that spans the GCL and the expression of both glial fibrillary acidic protein (GFAP) and Nestin, are NSCs of the hippocampal SGZ (Bonaguidi et al, 2011). Significantly, we detected p57 signal in the nucleus of 74.4±0.6% (mean±s.e.m.) of radial NSCs (GFAP+ Nestin+ radial fibre+ cells) in the SGZ of the DG (Figure 1A). In contrast, only a small proportion of cells positive for Ascl1, a marker for IPCs in the neuronal lineage (Hodge et al, 2008; Roybon et al, 2009), expressed p57 (13.5±6.0% of Ascl1+ cells, Figure 1B). Moreover, cells positive for bromodeoxyuridine (BrdU, 2 h pulse) showed weak (48.3±5.7% of BrdU+ cells) or undetectable (51.7±5.7%) p57 signals (Figure 1C). Consistently, radial NSCs positive for proliferating cell nuclear antigen (PCNA) (GFAP+ Nestin+ radial fibre+ PCNA+ cells) expressed lower levels of p57 than PCNA− radial NSCs (GFAP+ Nestin+ radial fibre+ PCNA− cells) (Supplementary Figure S1). These results suggest that the expression of p57 is diminished in proliferating (active) radial NSCs. In addition to radial NSCs in the SGZ, most (98.2±0.5%) immature neurons positive for doublecortin (DCX) in the DG were also positive for p57 (Figure 1D). Furthermore, high levels of p57 staining were observed in mature astrocytes, identified by expression of S100β, with 68.7±4.7% of S100β+ cells positive for p57 (Figure 1F), but not in NeuN+ mature neurons in the GCL (Figure 1E). Together, these results indicate that p57 is expressed at high levels in hippocampal radial NSCs as well as in immature neurons and mature astrocytes, but not in IPCs and mature neurons in the SGZ/GCL.
Figure 1.
Radial NSCs in the adult hippocampus express p57. (A) p57 is expressed in adult hippocampal radial NSCs. Immunohistochemistry on brain sections of 3-month-old mice showing GFAP (blue), Nestin (red), and p57 (green) triple-labelled cells projecting radially from the SGZ of the DG (arrowhead). Nuclei were counterstained with Hoechst. Scale bar, 5 μm. (B–F) Expression of p57 in differentiated cell populations in the SGZ/GCL. Strong p57 staining was detected in DCX+ cells (D) and some S100β+ cells (F), but not in the majority of Ascl1+ IPCs (B), BrdU+ proliferating cells (2 h pulse, C) and NeuN+ mature neurons (E). Arrowheads indicate differentiated cell populations. Nuclei were counterstained with Hoechst (blue). Scale bar, 10 μm.
p57 regulates radial NSC quiescence
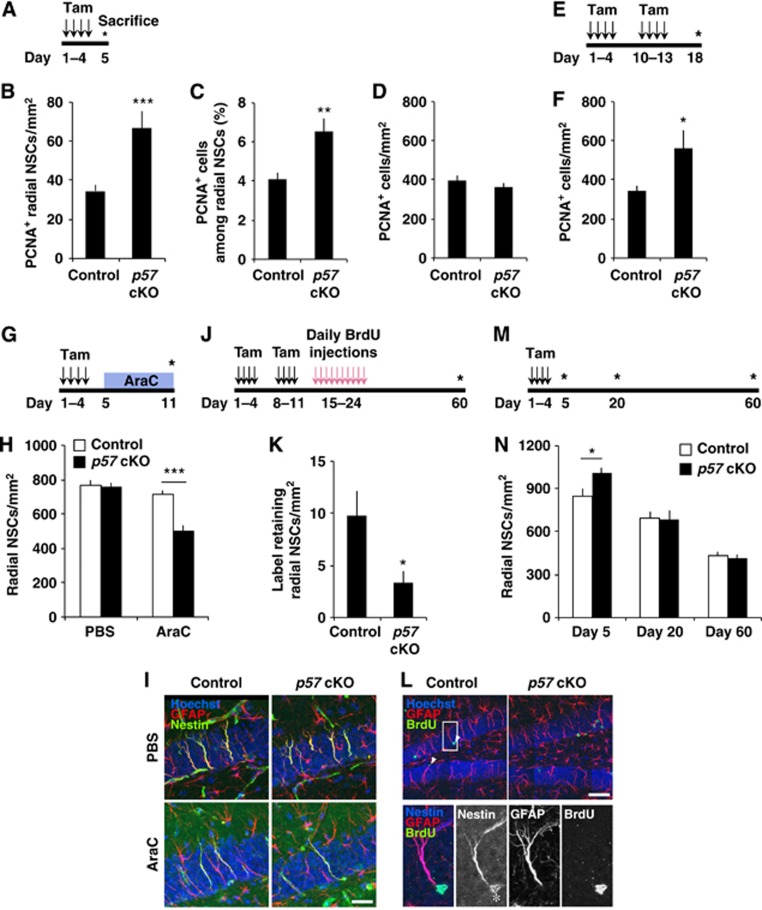
Given the expression of p57 in radial NSCs, we examined whether p57 plays a role in regulating the proliferation of these cells using conditional deletion of p57 in the NSCs. To this end, we crossed Nestin-CreERT2 mice, which express a tamoxifen (Tam)-sensitive gene for Cre recombinase in NSCs (Imayoshi et al, 2008), with mice harbouring a floxed allele of p57 (Matsumoto et al, 2011). Given that the paternal p57 locus undergoes genomic imprinting and only the maternal allele is expressed (Matsuoka et al, 1995), we used Netin-CreERT2 p57f/f mice and/or Nestin-CreERT2 p57+/f for p57 deletion in this study. Administration of tamoxifen in the resulting offspring resulted in a marked decrease in the number of p57+ cells in the SGZ (Supplementary Figure S2). The remaining p57+ cells in the SGZ were probably due to incomplete recombination of the floxed sequence induced by CreERT2/tamoxifen and to the existence of p57+ differentiated cells. Examination of 2-month-old animals 4 days after the first of 4 daily tamoxifen administration (Figure 2A) revealed a greater number of radial NSCs positive for PCNA, a marker for proliferating cells, in the hippocampal SGZ of the p57 conditional knockout (cKO) mice compared with control mice (Figure 2B). This increase could be an underestimation since the p57 gene deletion took place only in a partial radial NSC population (∼48%). In addition, the proportion of radial NSCs that were PCNA+ was also higher in p57 cKO mice than in control mice (Figure 2C). Although the total number of PCNA+ cells in the SGZ/GCL was not affected in p57 cKO mice at 4 days after the first tamoxifen administration (Figure 2D), examination of mice at 17 days after the first tamoxifen administration (day 18) revealed that the total number of PCNA+ cells in the SGZ/GCL was increased in p57 cKO mice as compared to controls (Figures 2E and F). These results suggest that p57 deletion induced the proliferation of NSCs, which subsequently increased the number of proliferating progeny in the young adult hippocampus.
Figure 2.
Conditional deletion of p57 promotes radial NSC proliferation. (A) Experimental design employed for the selective deletion of p57. A p57-floxed mouse and a Nestin-CreERT2 mouse were crossed to conditionally knockout p57 in the adult hippocampal NSCs. Two-month-old mice were administered with tamoxifen for 4 days and then sacrificed at day 5. p57 f/f; Nestin-CreERT2 mice and/or p57 +/f; Nestin-CreERT2 mice were used for p57 deletion. (B–D) Acute activation of hippocampal radial NSCs after p57 conditional deletion. The numbers of PCNA+ radial NSCs (B), the proportion of PCNA+ cells among radial NSCs (C), and the PCNA+ cells in the SGZ/GCL (D) are shown. n=5 and 4 animals for control and p57 cKO, respectively. (E) Experimental design for quantification of the PCNA+ cells in the SGZ/GCL at 17 days after p57 deletion. (F) The number of PCNA+ cells in the SGZ/GCL was increased in p57 cKO mice at day 18 (n=5 and 4 animals for control and p57 cKO, respectively). (G) Experimental design for the quantification of quiescent radial NSCs. (H) Quantification of remaining radial NSCs immediately after AraC infusion. Four control and three p57 cKO mice were infused with AraC. As a control, three control and three p57 cKO mice were infused with PBS. (I) Representative images used in visualization and quantification of remaining radial NSCs after AraC treatment in (H) are shown. Scale bar, 25 μm. (J) Experimental design for the quantification of infrequently dividing radial NSCs. BrdU was injected 10 days after the administration of tamoxifen. Animals were sacrificed 36 days later. (K) Quantification of label-retaining radial NSCs. The number of BrdU+ radial NSCs (BrdU+ GFAP+ Nestin+ Radial fibre+) from SGZ in control (n=3) and p57 cKO mice (n=4) is shown. (L) Representative images used in visualization and quantification of label-retaining radial NSCs in (K) are shown. Arrowheads indicate BrdU+ radial NSCs. Close-ups are shown in the bottom. Asterisk indicates non-specific signal caused by cross-reaction of secondary antibody. Scale bar, 50 μm. (M) Experimental design for the quantification of the size of radial NSC pool at various time points after p57 deletion. (N) Transient expansion of radial NSCs after p57 deletion. The number of radial NSCs at various time points after p57 deletion is shown. Note that p57 deletion increased radial NSCs (left), but this expansion was transient (middle and right). Values represent mean±s.e.m. ***P<0.001; **P<0.01; *P<0.05; Student’s t-test.
To further examine whether the conditional deletion of p57 activates radial NSCs, we ablated mitotic cells by infusing the antimitotic drug cytosine-β-D-arabinofuranoside (AraC) into the brains of p57 cKO and control mice and then determined the number of remaining radial NSCs (Figure 2G). AraC infusion for 6 days has previously been shown to kill mitotic cells while leaving quiescent NSCs largely unaffected (Doetsch et al, 1999; Lugert et al, 2010). To verify the ablation of mitotic cells by AraC treatment, we injected BrdU 2 h before the animals were sacrificed for analysis. AraC treatment eliminated almost all of the BrdU+ cells in the hippocampal SGZ in both p57 cKO and control mice, demonstrating successful ablation of mitotic cells (unpublished observations). The number of radial NSCs in the SGZ of the control mice was not reduced immediately after AraC infusion (Figures 2H and I), suggesting that most radial NSCs are quiescent and therefore survive the antimitotic treatment. However, in p57 cKO mice, fewer radial NSCs remained in the SGZ after AraC treatment as compared to controls, supporting the notion that p57 is required for the maintenance of quiescence in hippocampal NSCs (Figures 2H and I). Next, we further confirmed our findings using S-phase label retention analysis. We investigated the number of relatively infrequently dividing radial NSCs by examining BrdU label-retaining radial NSCs (Figure 2J). Conditional deletion of p57 resulted in a significant decrease in the number of label-retaining radial NSCs (Figures 2K and L), also indicating that the loss of p57 reduces infrequently dividing quiescent radial NSCs in the DG. Together, these results indicate that p57 is required for the maintenance of radial NSC quiescence.
We next investigated the short-term impact of conditional p57 deletion in the size of the radial NSC pool (Figure 2M). Analysis performed 4 days after the first of 4 tamoxifen administration (day 5) revealed more radial NSCs in the SGZ of p57 cKO mice than in that of control mice (Figure 2N). In contrast, analysis performed 19 or 59 days after the first of 4 tamoxifen administration (day 20 or 60, respectively) showed no substantial difference in the number of radial NSCs between p57 cKO and control mice (Figure 2N; Supplementary Figure S3), suggesting that deletion of p57 results in a transient expansion of the radial NSC pool.
Reduction of p57 in radial NSCs contributes to NSC activation induced by running
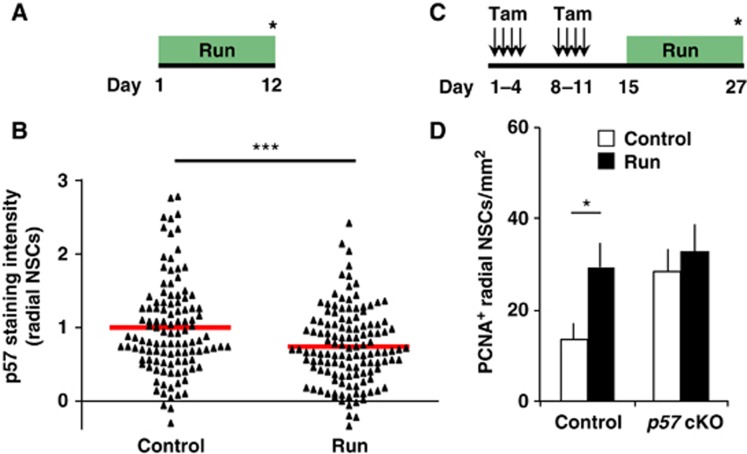
Previous studies have shown that NSC quiescence is abrogated by extrinsic neurogenic stimuli initiated by an animal’s experience, for example, by running (Hüttmann et al, 2003; Steiner et al, 2008; Lugert et al, 2010). To investigate the mechanism of this abrogation, we investigated whether p57 expression in the nucleus of radial NSCs is reduced in response to running and whether this reduction, if any, contributes to the activation of radial NSCs. Mice initially housed in small cages without a running wheel were exposed to spacious housing conditions with running wheels (run) or small housing (control) for 12 days, and p57 staining intensity in the nucleus of radial NSCs was quantified after this period (Figure 3A). We found that running reduced the staining intensity of p57 in the nucleus of radial NSCs in a statistically significant manner (Figure 3B; Supplementary Figure S4A). Given that deletion of p57 was found to be sufficient to promote radial NSC proliferation (Figure 2), the decrease in p57 levels induced by running may contribute to the recruitment of radial NSCs to the cell cycle. To investigate this, we examined the effects of running in p57 cKO mice. p57 cKO and control mice were housed in running and control conditions for 12 days, and then the animals were sacrificed (Figure 3C). Running resulted in significantly more PCNA+ radial NSCs in the SGZ of control mice (Figures 3C and D; Supplementary Figure S4B). On the other hand, it had a smaller effect on the number of these cells in p57 cKO mice, although running still tended to increase the number of these cells in p57 cKO mice probably due to incomplete loss of p57 (Figures 3C and 3D; Supplementary Figure S4B). These results support the notion that p57 reduction in runners contributes to the activation of radial NSCs, and therefore running has a smaller effect on NSC proliferation in p57 cKO mice. However, this lack of significant proliferation increase could also be due to a ceiling effect since there was already an increased level of PCNA+ radial NSCs in the p57 cKO mice without running. Moreover, we found that kainic acid (KA)-induced seizure also reduced the level of p57 in radial NSCs, and that p57 cKO dampened this KA-induced NSC activation (unpublished observations). Together, these results suggest that reduction of p57 protein in the nucleus of radial NSCs contributes to the activation of NSCs in response to extrinsic stimuli.
Figure 3.
Reduction of p57 contributes to radial NSC activation induced by running. (A) Two-month-old female mice initially housed in small cages without a running wheel were exposed to spacious housing with running wheels (run) or small housing (control) for 12 days and then sacrificed. (B) The staining intensity of p57 was measured in the nucleus of radial NSCs identified by their radial morphology and expression of GFAP and Nestin. The intensity of each nucleus was plotted for comparison. Red horizontal bar represents average staining intensity. n=3 for both control and run. (C) Two-month-old female mice initially housed in small cages without a running wheel were administered with tamoxifen and then exposed to spacious housing with running wheels (run) or small housing (control) for 12 days and then sacrificed. (D) Quantification of PCNA+ radial NSCs in the SGZ. Three control and p57 cKO mice were exposed to small housing and four control and three p57 cKO mice were exposed to spacious housing with running wheels. Values represent mean±s.e.m. ***P<0.001; *P<0.05; Student’s t-test.
Conditional deletion of p57 promotes neurogenesis in young adult mice
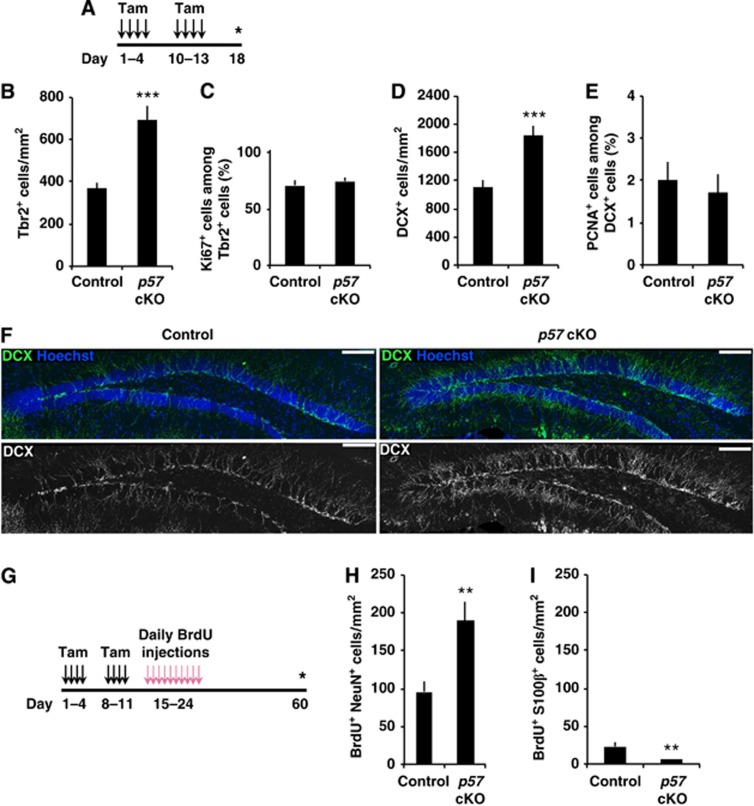
To test whether the activation of NSCs by the reduction of p57 indeed enhances neurogenesis, we next examined the impact of conditional p57 deletion on hippocampal neurogenesis. First, after administration of tamoxifen, we counted neuronal IPCs and immature neurons, identified by their positivity for Tbr2 and DCX, respectively (Figure 4A). We observed significantly more Tbr2+ neuronal IPCs in p57 cKO mice compared with control mice (Figure 4B). Moreover, conditional deletion of p57 resulted in significantly more DCX+ immature neurons compared with controls (Figures 4D and F). These results suggest that conditional deletion of p57 promotes hippocampal neurogenesis in young adult mice. In order to examine the possibility that the greater number of Tbr2+ cells and DCX+ cells induced by deletion of p57 in NSCs was due to activation of the proliferation of these cell populations, we determined the proportion of each made up of dividing cells. However, we did not detect a greater proportion of Ki67+ cells among Tbr2+ cells nor of PCNA+ cells among DCX+ cells in p57 cKO mice compared to controls (Figures 4C and E). It has previously been reported that the CDK inhibitors such as p21 and p27 are abundantly expressed in this population (Qiu et al, 2004, 2009; Pechnick et al, 2008) and they share with p57 a conserved CDK inhibitory domain in their NH2 terminus and therefore a similar CDK-binding activity (Pateras et al, 2009). Thus, the lack of p57 requirement in the cell-cycle regulation in DCX+ population, despite the high p57 expression in this population, may be due to functional compensation of p57 by p21 and p27. The higher numbers of these populations in p57 cKO mice were therefore not attributable to enhanced proliferation of Tbr2+ cells and DCX+ cells, although a detailed analysis to strictly examine the cell-cycle length of these populations in p57 cKO and control mice would be helpful in more fully supporting this conclusion (Artegiani and Calegari, 2012).
Figure 4.
Enhanced hippocampal neurogenesis by conditional deletion of p57. (A) Experimental design for the quantification of IPCs and neuroblasts/immature neurons after p57 deletion. Asterisk indicates time point of sacrifice. (B–E) Quantification of number of Tbr2+ IPCs (B) and DCX+ neuroblasts/immature neurons (D) in the SGZ/GCL of control (n=5) and p57 cKO mice (n=3). The proportion of Ki67+ cells among Tbr2+ cells (n=3, 3, C) and PCNA+ cells among DCX+ cells (n=5, 3, E) are shown. (F) Representative images of DCX+ cells in p57 cKO and control mice. Scale bar, 100 μm. (G) Experimental design for the quantification of newly generated mature neurons and astrocytes after p57 deletion. BrdU was injected 10 days after the administration of tamoxifen. Animals were sacrificed 36 days later. (H, I) Quantification of the number of BrdU+ cells expressing NeuN (H) and S100β (I) in the SGZ/GCL of control (n=3) and p57 cKO mice (n=4). Values represent mean±s.e.m. ***P<0.001; **P<0.01; Student’s t-test. See also Supplementary Figure S5.
We next examined whether p57 deletion in NSCs promotes the production of mature neurons in the hippocampus, given that substantial cell death occurs during hippocampal neurogenesis (Sun et al, 2004; Sierra et al, 2010). We used BrdU pulse-chase analysis to examine whether conditional deletion of p57 actually increases the number of mature neurons remaining at later time points compared with control mice (Figure 4G). Deletion of p57 resulted in two-fold more BrdU-labelled cells positive for neuron-specific nuclear protein (NeuN), a marker for mature neurons, in the GCL of the DG 36 days after the final BrdU administration (Figure 4H). In contrast, there were fewer BrdU+ S100β+ cells in p57 cKO mice compared with control mice (Figure 4I). Together, our data thus support the notion that p57 does not regulate the cell cycle of differentiated cell populations in the neuronal lineage and suggest that p57 deletion enhances adult neurogenesis through activation of radial NSCs.
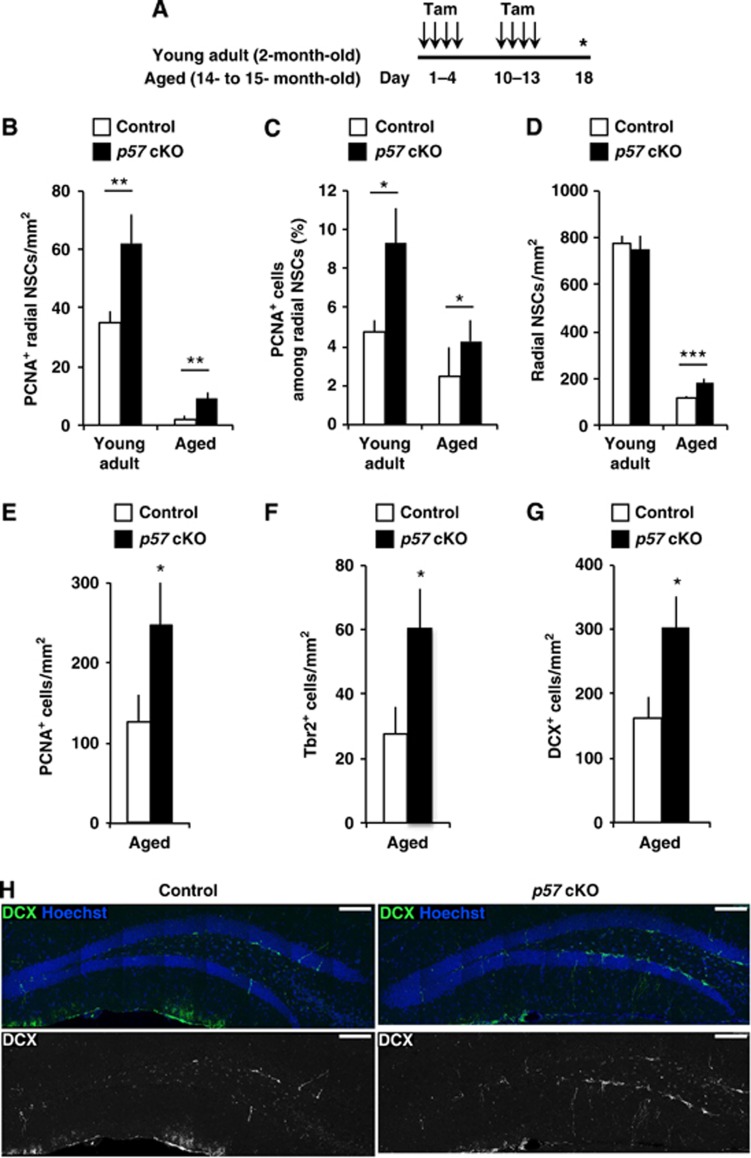
Conditional deletion of p57 in NSCs of aged mice reactivates radial NSC proliferation
We next examined whether the diminished proliferation of radial NSCs and decreased neurogenesis in aged mice might be reactivated by p57 deletion. We administered with tamoxifen repeatedly to mice at 2 months (young adult) and at 14–15 months (aged) of age and examined the proliferation of cells in the SGZ at 17 days after the first tamoxifen administration (day 18, Figure 5A). Interestingly, compared with aged controls, aged p57 cKO mice showed an increased number of PCNA+ radial NSCs like in young adult mice (Figure 5B). Consistent with this, the proportion of proliferating (PCNA+) cells among radial NSCs was greater in the aged p57 cKO mice compared to control aged mice (Figure 5C). Analysis performed using aged mice revealed more radial NSCs in the SGZ of p57 cKO mice than that of control mice (Figure 5D). These results indicate that conditional deletion of p57 in NSCs of aged mice reactivates radial NSC proliferation. In addition, the numbers of total PCNA+ cells (Figure 5E), Tbr2+ IPCs (Figure 5F), and DCX+ immature neurons (Figures 5G and H) were also greater in aged p57 cKO mice, suggesting that activation of radial NSCs by p57 deletion results in increased neurogenesis even in the aged brain. These results suggest that radial NSCs in the aged mouse brain retain more proliferative and neurogenic potential than is normally used and it can be reactivated by p57 deletion.
Figure 5.
Reactivation of radial NSC proliferation and hippocampal neurogenesis by conditional deletion of p57 in aged mice. (A) Tamoxifen was administered to young adult (2-month-old) and aged (14- to 15-month-old) mice. Animals were sacrificed 17 days after the first tamoxifen administration. (B–D) Quantification of the number of PCNA+ radial NSCs (B), the proportion of PCNA+ radial NSCs (C), and the number of radial NSCs (D) in the DG of young adult (left) and aged mice (right). n=5 and 3 for young adult control and p57 cKO, respectively, and n=4 and 4 for aged control and p57 cKO, respectively. (E–G) Quantification of PCNA+ cells (E), Tbr2+ IPCs (F), and DCX+ neuroblasts/immature neurons (G) in the SGZ/GCL of aged mice. (H) Representative images of DCX+ cells in aged control and p57 cKO mice. Scale bar, 100 μm. Values represent mean±s.e.m. *P<0.05; Mann–Whitney U-test in (C) and (E). Others, ***P<0.001; **P<0.01; *P<0.05; Student’s t-test.
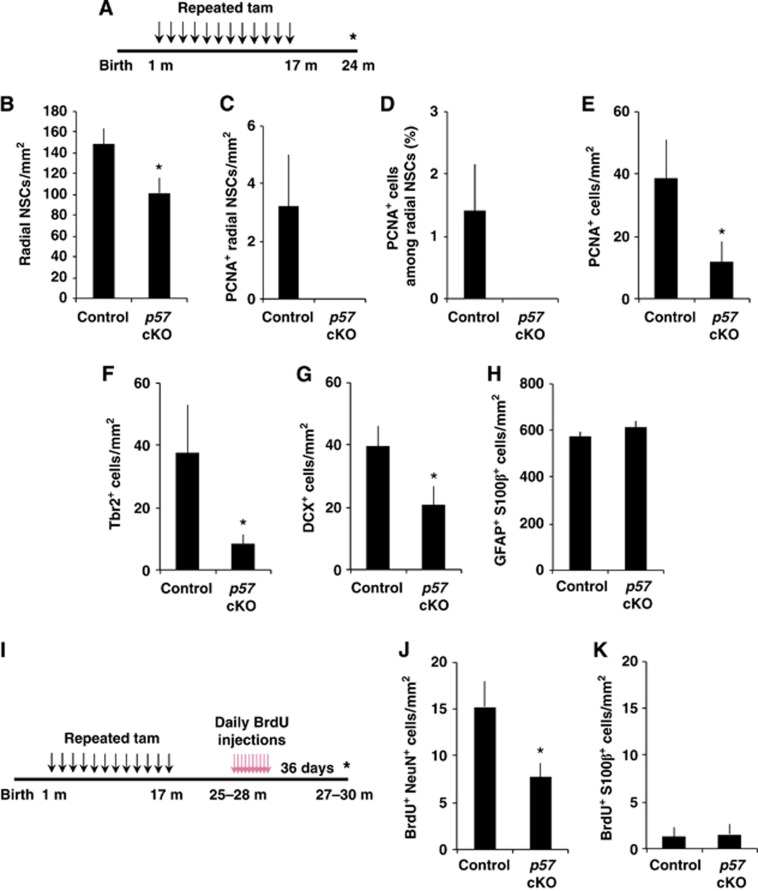
Long-term deletion of p57 results in excessive loss of radial NSCs and impaired neurogenesis
Lifelong neurogenesis requires lifelong maintenance of NSCs. To assess the physiological relevance of radial NSC quiescence in the lifelong maintenance of the radial NSC pool and neurogenesis in the adult hippocampus, we examined the consequences of long-term p57 deletion in adult NSCs. Beginning 1 month after birth, we repeatedly injected mice with tamoxifen to achieve a higher and continuous p57 deletion and then examined the brain 24 months later (Figure 6A). At this late time point, the number of radial NSCs was significantly reduced in p57 cKO mice compared with that in control mice (Figure 6B). We observed some PCNA+ proliferating radial NSCs in control mice but could not find any PCNA+ radial NSCs in long-term p57 cKO mice (Figures 6C and D). These losses of the radial NSC pool and its proliferating proportion were accompanied by marked decreases in the numbers of total proliferating (PCNA+) cells (Figure 6E), Tbr2+ IPCs (Figure 6F), and DCX+ immature neurons (Figure 6G) in the SGZ/GCL in long-term p57 cKO mice, suggesting impaired neurogenesis in the absence of p57. In contrast, long-term p57 deletion did not significantly affect the numbers of GFAP+ S100β+ astrocytes in the SGZ/GCL (Figure 6H). To determine whether long-term p57 deletion affected the number of newborn mature neurons which survived a substantial period in aged mice, we administered with BrdU once a day for 10 days to 25- to 28-month-old mice and examined BrdU+ neurons 36 days later (Figure 6I). We found that the number of BrdU+ NeuN+ neurons in the GCL of aged (27- to 30-month-old) mice was markedly fewer in long-term p57 cKO mice than in controls (Figure 6J). In contrast, we observed very few BrdU+ S100β+ cells in the SGZ/GCL of aged control mice and no difference in the number of these cells between aged control and long-term p57 cKO mice (Figure 6K). Together, these results suggest that p57 plays a substantial role in lifelong NSC maintenance as well as in preventing excessive loss of neurogenesis in the hippocampus during adulthood.
Figure 6.
Excessive loss of radial NSCs by long-term p57 deletion. (A) Experimental design for the quantification of the number and the proliferation of radial NSCs and the generation of new neurons after long-term deletion of p57. Tamoxifen was administered repeatedly from 1 month to 17 months after birth, and then mice were sacrificed at 24 months after birth. (B–H) Quantification of the number of radial NSCs (B), the number of PCNA+ radial NSCs (C), the proportion of PCNA+ among radial NSCs (D), the number of PCNA+ cells (E), the number of Tbr2+ IPCs (F), and the number of DCX+ neuroblasts/immature neurons (G), and GFAP and S100β double-positive cells (H) in the SGZ/GCL of control (n=4) and p57 cKO (n=4). *P<0.05; Student’s t-test in (B). *P<0.05; Mann–Whitney test in (E–G). In (C, D), P=0.093; Mann–Whitney test. (I) Experimental design for the quantification of newly generated mature neurons and astrocytes after long-term p57 deletion. Tamoxifen was administered repeatedly from 1 month to 17 months after birth, and then animals were sacrificed at 27–30 months after birth (36 days after 10 days of BrdU injections). (J, K) Quantification of the number of BrdU+ cells expressing NeuN (J) and S100β (K) in the SGZ/GCL of control (n=7) and p57 cKO mice (n=4). *P<0.05; Student’s t-test. Values represent mean±s.e.m.
Discussion
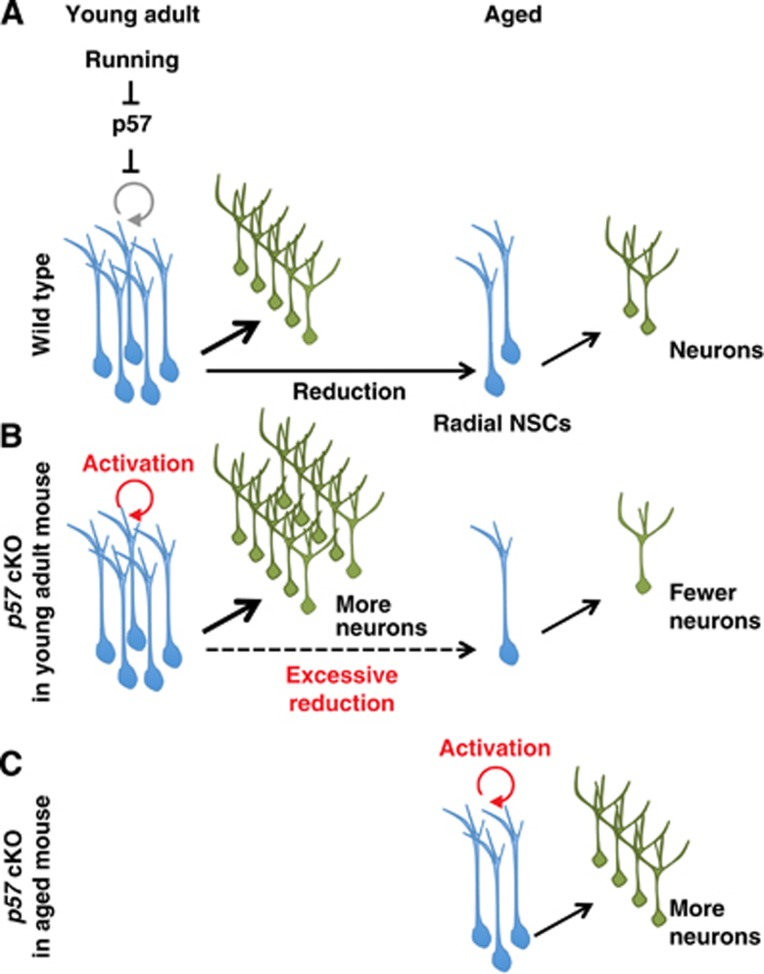
Our results have identified p57 as a key regulator of the quiescence of radial NSCs in the DG of the adult mouse hippocampus (Figure 7). While p57 is abundant in radial NSCs, it is reduced when these cells are activated to become IPCs and proliferate (Figure 1). These results are in contrast to other CKIs p27 and p21, which are expressed preferentially in DCX+ cells in the DG (Pechnick et al, 2008; Qiu et al, 2009). Such stem cell expression of p57, rather than p27 or p21, is also observed in the haematopoietic system (Yamazaki et al, 2006). Furthermore, we have now shown that deletion of p57 in the young adult CNS results in the proliferation of radial NSCs but not in that of IPCs or immature neurons. These results indicate that the cell cycle of radial NSCs is regulated by mechanisms distinct from those of their descendants. Together, our present results as well as recent findings in other adult tissue stem cells suggest a common function for p57 in the maintenance of adult stem cell quiescence (Matsumoto et al, 2011; Zacharek et al, 2011; Zou et al, 2011).
Figure 7.
A model for the regulation and significance of hippocampal NSC quiescence. (A) In wild-type mice, p57 restricts the proliferation of hippocampal radial NSCs. Reduction of p57 protein in NSCs contributes to the abrogation of NSC quiescence triggered by various extrinsic neurogenic stimuli. (B) Selective deletion of p57 in NSCs initially enhances NSC proliferation and neurogenesis in young adult mouse, but later leads to the NSC exhaustion and excessive reduction of neurogenesis in aged mice, suggesting that regulation of NSC quiescence by p57 is significant in modulating the pace of lifelong neurogenesis. (C) Acute p57 deletion in aged mice reactivates hippocampal NSC proliferation and neurogenesis.
Identifying p57 as a key regulator of NSC quiescence drove us to test whether p57 participates in the extrinsic stimuli-dependent abrogation of NSC quiescence. Our results now indicate that the reduction of p57 protein, a brake of NSC proliferation, in the nucleus contributes to trigger activation of NSC proliferation in response to running (Figures 3 and 7). Various external neurogenic stimuli may thus converge on the reduction of p57 protein in the nucleus of radial NSCs to activate NSC proliferation. Given that circulating insulin-like growth factor-1 (IGF-1) has been suggested to mediate an exercise-induced increase in the number of BrdU+ cells in the adult rat hippocampus and that p57 has been shown to be downregulated by IGF-1 treatment in the rat primary neuronal cell culture, IGF-1 is, for example, an possible candidate for the regulator of p57 reduction (Carro et al, 2000, 2001; Mairet-Coello et al, 2009).
Considering that adult neurogenesis acts as a base for plasticity that allows modulation of neural circuits in the hippocampus, the rate of neurogenesis could be relevant to the extent of the brain’s plasticity. We have now shown that p57 plays a key role in determining that rate. Conditional deletion of p57 resulted in an initial increase in the number of DCX+ immature neurons followed (∼2 months later) by an increase in the number of NeuN+ mature neurons located in the GCL (Figure 4). However, these findings lead to the question of how p57 deletion affects adult neurogenesis. Whereas p57 deletion may increase hippocampal neurogenesis simply through activation of NSC proliferation, it is possible that p57 regulates the cell cycle of populations other than radial NSCs in the DG. Expression of p57 in DCX+ immature neurons may support this hypothesis. However, since most DCX+ immature neurons rarely divide under normal conditions, and more importantly, since we did not observe a significant increase in the number of proliferating DCX+ cells after p57 deletion (Figure 4E), the cell cycle of DCX+ cells does not appear to be a major target for p57 in the regulation of adult neurogenesis. It remains possible, however, that p57 controls adult neurogenesis in part through its cell cycle-independent functions, given that p57 has been shown to promote glial fate determination through direct suppression of Ascl1 function during embryonic development (Joseph et al, 2009). However, this hypothesis does not fit well since the expression patterns of p57 and Ascl1 proteins appear mutually exclusive in the DG (Figure 1B). It thus appears likely that the impaired quiescence of p57-deficient radial NSCs contributes to increased neurogenesis. Together, these conclusions further support the notion that NSC quiescence is significant in providing a reserved pool or a backup which can be activated on demand (short-term modulation of the rate of neurogenesis) in response to an animal’s experience (Figure 7).
The mechanism that regulates NSC pool size has not been fully elucidated. We found that deletion of p57 resulted in a transient increase in the number of radial NSCs (Figure 2N). This finding is intriguing, in particular because it suggests that cell-cycle regulation might control the NSC pool size. However, fate analysis of these expanded NSCs will be necessary to examine whether these expanded GFAP+ Nestin+ radial fibre+ cells are bona fide NSCs. In addition, it will be of interest to investigate the basis of the transient nature of NSC expansion. However, the increasing rate of divisions at 17 days post tamoxifen (Figure 5B) does not produce an increase in NSC number at 19 days (Figure 2N), suggesting that some other mechanisms may be involved in regulation of NSC number, for example, a stem cell niche that is able to support only a limited number of NSCs.
Our data also reveal the role of NSC quiescence in the long-term (age-related) modulation of neurogenesis. This is based on the observation that, although deletion of p57 initially resulted in the recruitment of quiescent NSCs into the cell cycle and stimulation of neurogenesis (Figures 2 and 4), it later led to excessive reduction in NSC pool and impaired neurogenesis (Figure 6). The regulation of NSC quiescence by p57 might thus have important implications for the modulation of the pace of lifelong neurogenesis (Figure 7). Excessive reduction in NSC pool size after long-term p57 deletion might be explained by the recently proposed ‘disposable model’, which stipulates that radial NSCs that have been activated to proliferate undergo several rounds of asymmetric cell division to produce IPCs and then irreversibly differentiate into mature astrocytes (Encinas et al, 2011). However, p57 deletion in the present study resulted in a reduction, rather than an increase, in the number of S100β+ mature astrocytes (Figure 4). Our results are thus more consistent with the classical view of stem cell exhaustion where cells achieve the limit of their self-renewal capacity (Kippin et al, 2005).
In this study, we have shown that acute deletion of the p57 reactivates neurogenesis not only in young adult mice but also in aged mice, suggesting that even the aged brain retains a certain level of plasticity and that the reduced level of neurogenesis apparent in the aged brain can be increased through inhibition of p57 (Figures 5 and 7). Moreover, these results raise a possibility that pharmacological p57 inhibition may be beneficial in the medical treatment of neurodegenerative disease, mood disorders, and ageing-related cognitive impairment. Previous studies have suggested that increased neurogenesis in the DG resulting from inhibition of apoptosis led to improved cognitive function such as pattern separation (Sahay et al, 2011). However, inhibition of apoptosis might not be appropriate for clinical application since it may result in inhibition of physiologically important cell death (Mouret et al, 2009). Transient abrogation of NSC quiescence and promotion of neurogenesis through inhibition of p57 is thus a potential therapeutic approach to amelioration of mental disorders and age-related impairment of cognition.
Materials and methods
Mice
All mice used in the study were backcrossed to the C57BL6 background. p57 cKO mice were obtained by crossing p57-floxed mice (Matsumoto et al, 2011) and Nestin-CreERT2 mice (kindly provided by R Kageyama and I Imayoshi). The resulting mice were treated with tamoxifen to activate CreERT2. Mice were kept in standard laboratory cages (31 cm × 19 cm × 12 cm) and maintained in a 12-h light/dark cycle with free access to food and water ad libitum and according to the Animal Care and Use Committee of the University of Tokyo.
Tamoxifen administration
Stock solutions of tamoxifen (Sigma) were prepared at a concentration of 20 mg/ml in corn oil (Nacalai tesque). Young adult (2-month-old, Figures 2A, E, M, 3, 4A; 4-month-old, Figures 2G, J, and ) and aged (14- to 15-month-old; Figure 5) mice were orally administered with 5–6 mg of tamoxifen once per day for 4 consecutive days (Figures 2A, G). For a higher degree of Cre-mediated recombination, a second round of administration was performed after the initial tamoxifen treatment (Figures 2E and J, Supplementary Figures S2–S5). For long-term p57 deletion, a total of five rounds of administration (for 2–3 consecutive days per round, 12 administration total) were carried out beginning at 1 month after birth with roughly 4 months between administrations (Figure 6).
Immunofluorescent analysis
Animals were anaesthetized and transcardially perfused with ice-cold phosphate-buffered saline (PBS) followed by ice-cold 4% paraformaldehyde (PFA) solution in PBS. Brains were post-fixed with 4% PFA at 4°C for 2 h, cryoprotected in a 10% sucrose solution in PBS at 4°C for 2 h, then in a 20% solution for 10 h and 30% for 24 h, and finally embedded and frozen in OCT (TissueTEK). Immunofluorescence staining of colonal brain sections (20 μm) was performed. Antigen retrieval was performed by autoclave treatment of sections for 10 min at 105°C in Target Retrieval Solution (DAKO) for BrdU, Nestin and PCNA staining. Sections were exposed to Tris-buffered saline (TBS) containing 0.1% Triton X-100 and 2% donkey serum (blocking buffer), and incubated first for 48 h at 4°C with primary antibodies in blocking buffer and then 3 h at room temperature with Alexa-Fluor-conjugated secondary antibodies in blocking buffer. Fluorescence images were obtained with a laser confocal microscope (Leica TCS-SP5).
Antibodies used for immunostaining included mouse monoclonal antibodies to BrdU (BD Bioscience) at 1:500 dilution, to Nestin (BD Pharmingen) at 1:200, to PCNA (Calbiochem) at 1:1000, to GFAP (Millipore) at 1:1000, to Ascl1 (BD Pharmingen) at 1:500, to S100β (Sigma, SH-B4) at 1:500 and to NeuN (Millipore) at 1:500; rat monoclonal antibodies to BrdU (Abcam) at 1:200 and to Ki67 (DAKO) at 1:500; rabbit monoclonal antibodies to Sox2 (Cell Signaling) at 1:200; rabbit polyclonal antibodies to GFAP (DAKO) at 1:2000, to p57 (SIGMA) at 1:500, to S100β (DAKO) at 1:100 and to Tbr2 (Abcam) at 1:1000; and goat polyclonal antibodies to DCX (Santa Cruz Biotechnology) at 1:500. Alexa-Fluor-labelled secondary antibodies and Hoechst 33342 (for nuclear staining) were obtained from Molecular Probes. Images of immunostaining were processed using Photoshop CS4 software (Adobe).
Administration of thymidine analogues
To label dividing cells, mice were injected once with BrdU (Sigma, 50 mg/kg body weight) intraperitoneally (i.p.) 2 h before sacrifice (Figures 1C and 2G). For identification of label-retaining NSCs and newly generated cells, BrdU was injected for 10 consecutive days (Figures 2J, 4G and 6I). Cells positive for BrdU were detected by immunostaining.
AraC infusion
AraC treatment was performed as described previously (Doetsch et al, 1999). In brief, AraC (Sigma, 2%) in PBS or a vehicle alone was infused into the brain for 6 days using a mini osmotic pump (Alzet, model 1007D). Cannulas (Alzet, Brain infusion kit III) were implanted stereotaxically in the left lateral ventricle (anterior, 0 mm; lateral, 1.1 mm; depth, 2 mm relative to bregma and the surface of the brain). Immediately after pump removal, mice were sacrificed. Brains were processed and analysed by immunostaining as described above.
Running
Six female C57BL6 mice initially housed in small cages (19 cm × 12 cm × 10 cm) without a running wheel were used for the experiment (three mice per cage). Three mice were selected randomly and moved to a spacious cage (41 cm × 25 cm × 10 cm) with unlimited access to a running wheel for 12 days. Three controls were moved to a small cage without a running wheel. Brains were processed and analysed by immunostaining as described above.
Quantification of labelled cells and statistical analysis
Projections of 12 Z-stacks (total 14.7 μm thick) and 16 X-Y tiles (2 × 8 covering whole DG) were used for all quantifications. Images were obtained with a confocal microscope (Leica TCS-SP5) equipped with a 63 × /1.20 water objective lens (Leica, HCX Plan Apo CS) and a motorized XYZ stage (Leica), and reconstructed with LAS-AF software (Leica). The numbers of labelled cells were normalized by the GCL area (mm2). The GCL area was measured via ImageJ software (National Institutes of Health). Quantifications were made blindly. For each animal, every eighth section from bregma −1.82 mm to −2.80 mm (DG) was assessed. More than three adult mice per age group were analysed in each experiment. Quantitative data represent mean±s.e.m. Data were compared between groups with the unpaired Student’s t-test or Mann–Whitney U-test. A P-value of <0.05 was considered statistically significant.
Quantification of p57 intensity
The nuclei (labelled by Hoechst 33342) of radial NSCs (GFAP+ Nestin+ radial fibre+ cells) were outlined manually using Photoshop CS4 software (Adobe). Next, the average fluorescence intensity of counterstained p57 within each outlined area was measured using Metamorph software (Molecular Devices). More than 100 nuclei from 3 brains were observed. After subtraction of the background signal, the p57 fluorescence intensity was compared between groups.
Supplementary Material
Acknowledgments
We thank R Kageyama and I Imayoshi for Nestin-CreERT2 mice; K Shimizu for technical advice about AraC treatment; and members of the Gotoh Laboratory for helpful discussion. This work was supported by Grants-in-Aid for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, by Innovative Areas ‘Neural Diversity and Neocortical Organization’ of MEXT, by Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Agency, by the Japan Society for the Promotion of Science, and in part by the Global COE Program ‘Integrative Life Science Based on the Study of Biosignaling Mechanisms’ of MEXT.
Author contributions: SF carried out the experiments and analysed the data. AM and KIN provided the p57-floxed mice. SF and YG designed the study and wrote the manuscript. YG supervised the study.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahn S, Joyner AL (2005) In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437: 894–897 [DOI] [PubMed] [Google Scholar]
- Artegiani B, Calegari F (2012) Age-related cognitive decline: can neural stem cells help us? Aging (Albany NY) 4: 176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP (2010) Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging 31: 151–161 [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H (2011) In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145: 1142–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M (2004) Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging 25: 333–340 [DOI] [PubMed] [Google Scholar]
- Carro E, Nuñez A, Busiguina S, Torres-Aleman I (2000) Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci 20: 2926–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I (2001) Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci 21: 5678–5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT (2000) Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287: 1804–1808 [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325: 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, García-Verdugo JM, Alvarez-Buylla A (1999) Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA 96: 11619–11624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ (2006) The aging hippocampus: a multi-level analysis in the rat. Neuroscience 139: 1173–1185 [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G (2011) Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8: 566–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, Hock H (2009) Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol 27: 84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, Kowalczyk TD, Wolf SA, Encinas JM, Rippey C, Enikolopov G, Kempermann G, Hevner RF (2008) Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci 28: 3707–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhäuser C, Gray WP (2003) Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci 18: 2769–2778 [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R (2008) Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 11: 1153–1161 [DOI] [PubMed] [Google Scholar]
- Joseph B, Andersson ER, Vlachos P, Södersten E, Liu L, Teixeira AI, Hermanson O (2009) p57Kip2 is a repressor of Mash1 activity and neuronal differentiation in neural stem cells. Cell Death Differ 16: 1256–1265 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1998) Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 18: 3206–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Martens DJ, van der Kooy D (2005) p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev 19: 756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16: 2027–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, Haas CA, Kempermann G, Taylor V, Giachino C (2010) Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6: 445–456 [DOI] [PubMed] [Google Scholar]
- Mairet-Coello G, Tury A, DiCicco-Bloom E (2009) Insulin-like growth factor-1 promotes G(1)/S cell cycle progression through bidirectional regulation of cyclins and cyclin-dependent kinase inhibitors via the phosphatidylinositol 3-kinase/Akt pathway in developing rat cerebral cortex. J Neurosci 29: 775–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M (2007) Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science 318: 980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Takeishi S, Kanie T, Susaki E, Onoyama I, Tateishi Y, Nakayama K, Nakayama KI (2011) p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell 9: 262–271 [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ (1995) p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev 9: 650–662 [DOI] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortigüela R, Marqués-Torrejón MA, Nakashima K, Colak D, Götz M, Fariñas I, Gage FH (2010) Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7: 78–89 [DOI] [PubMed] [Google Scholar]
- Mouret A, Lepousez G, Gras J, Gabellec MM, Lledo PM (2009) Turnover of newborn olfactory bulb neurons optimizes olfaction. J Neurosci 29: 12302–12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateras IS, Apostolopoulou K, Niforou K, Kotsinas A, Gorgoulis VG (2009) p57KIP2: ‘Kip’ing the cell under control. Mol Cancer Res 7: 1902–1919 [DOI] [PubMed] [Google Scholar]
- Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V (2008) p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc Natl Acad Sci USA 105: 1358–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Takagi Y, Harada J, Rodrigues N, Moskowitz MA, Scadden DT, Cheng T (2004) Regenerative response in ischemic brain restricted by p21cip1/waf1. J Exp Med 199: 937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Takagi Y, Harada J, Topalkara K, Wang Y, Sims JR, Zheng G, Huang P, Ling Y, Scadden DT, Moskowitz MA, Cheng T (2009) p27Kip1 constrains proliferation of neural progenitor cells in adult brain under homeostatic and ischemic conditions. Stem Cells 27: 920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybon L, Hjalt T, Stott S, Guillemot F, Li JY, Brundin P (2009) Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS ONE 4: e4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472: 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Blier P, Hen R (2002) Behavioral and physiologic effects of genetic or pharmacologic inactivation of the substance P receptor (NK1). J Clin Psychiatry 63(Suppl 11): 11–17 [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21: 7153–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372–376 [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7: 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476: 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Zurborg S, Hörster H, Fabel K, Kempermann G (2008) Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience 154: 521–529 [DOI] [PubMed] [Google Scholar]
- Sun W, Winseck A, Vinsant S, Park OH, Kim H, Oppenheim RW (2004) Programmed cell death of adult-generated hippocampal neurons is mediated by the proapoptotic gene Bax. J Neurosci 24: 11205–11213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os R, Kamminga LM, Ausema A, Bystrykh LV, Draijer DP, van Pelt K, Dontje B, de Haan G (2007) A limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells 25: 836–843 [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270 [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Iwama A, Takayanagi S, Morita Y, Eto K, Ema H, Nakauchi H (2006) Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J 25: 3515–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek SJ, Fillmore CM, Lau AN, Gludish DW, Chou A, Ho JW, Zamponi R, Gazit R, Bock C, Jäger N, Smith ZD, Kim TM, Saunders AH, Wong J, Lee JH, Roach RR, Rossi DJ, Meissner A, Gimelbrant AA, Park PJ et al. (2011) Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell 9: 272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132: 645–660 [DOI] [PubMed] [Google Scholar]
- Zou P, Yoshihara H, Hosokawa K, Tai I, Shinmyozu K, Tsukahara F, Maru Y, Nakayama K, Nakayama KI, Suda T (2011) p57(Kip2) and p27(Kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell 9: 247–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.