Abstract
Nature advance online publication February 27 2013; doi:; DOI: 10.1038/nature11993
Nature advance online publication February 27 2013; doi:; DOI: 10.1038/nature11928
Circular (circ)RNAs are to transcriptomics what the famous hidden musical theme is to Elgar’s Enigma Variations: woven into the entire composition but not unambiguously identified or understood by scholars to this day. Now, two studies (Hansen et al, 2013; Memczak et al, 2013) have made substantial progress in both, identifying thousands of circRNAs and beginning to crack the enigma of their cellular function, by demonstrating that the circRNAs CiRS-7/CDR1as and SRY function as natural and highly stable sponges for specific microRNAs.
Much work over decades has documented the complex ways in which genetic information is transcribed and processed into functional RNAs to ultimately shape phenotypes. Well-appreciated phenomena include alternative promoter choice, splicing, editing and 3′ end formation to generate surprisingly diverse mRNA populations, as well as the widespread, regulated and complex production of non-coding RNAs from genic as well as intergenic regions of the genome, reviewed in Mercer et al (2012). Occasional evidence for the formation of circular RNAs (circRNAs) had also been presented over the years and examples such as the circular testis-determining RNA SRY have achieved some prominence (Capel et al, 1993). Nevertheless, circRNAs have mostly been disregarded as rare, some form of transcriptional noise or RT–PCR artefacts. Thus, demonstrations of their widespread and substantial presence within transcriptomes have only recently come to the fore (Salzman et al, 2012; Wu et al, 2012; Jeck et al, 2013), although evidence as to their mechanism(s) of function has still been lacking.
Memczak et al (2013) devised a novel computational approach to detect evidence for circRNAs in RNA-seq data from ribosomal RNA-depleted samples. A hallmark of circRNAs is their unique ‘head-to tail’ splice junction that mediates circularization (Figure 1A). The authors found ∼2500 human, mouse and C. elegans circRNA candidates in a variety of RNA-seq libraries. circRNA sequences displayed some conservation suggesting biological function, and numerous examples showed cell type or developmental stage-specific expression suggestive of regulation. Eighty-five percent of human circRNAs aligned in sense orientation to known protein coding genes, typically overlapping with coding sequences and spanning across 1–5 exons. Importantly, around 50 circRNAs were validated in one or both of the following tests: verification of a head-to-tail junction by qPCR and northern blot demonstrating resistance to digestion with RNase R exonuclease to rule out linear variants formed by trans-splicing or genomic rearrangements. circRNAs were shown to be long-lived in vivo compared to their linear counterparts, as expected given that most RNA turnover involves exonucleases.
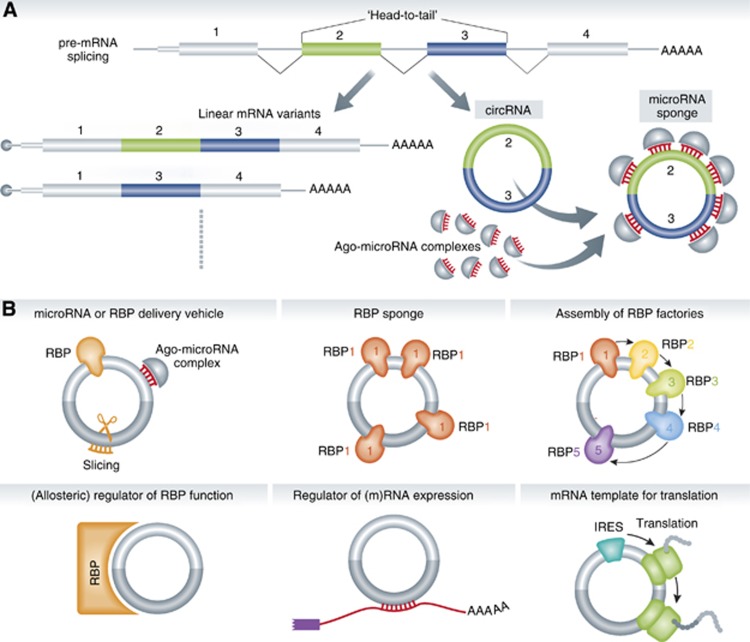
Figure 1.
Biogenesis and functions of circular RNAs. (A) A gene can be transcribed and spliced into linear and circular RNAs. Note the unique ‘head-to-tail’ splice junctions formed by an acceptor splice site at the 5′ end of an exon and a donor site at the 3′ end of a downstream exon. A demonstrated role for circRNAs is to act as a microRNA sponge. (B) Pictograms of additional plausible options for circRNA function. See main text for details.
What could be a function of circRNAs? A smoking gun led the investigations by both teams: a previously reported human circRNA running antisense to the Cerebellar Degeneration-Related protein 1 (CDR1) locus (Hansen et al, 2011) harbours ∼70 conserved matches to the miR-7 seed and was termed as CDR1as (antisense) (Memczak et al, 2013) or CiRS-7 (Circular RNA Sponge for miR-7) (Hansen et al, 2013), respectively. This striking feature suggested a possible function as microRNA sponge (Figure 1A), a term that was first used for linear transcripts with concatenated microRNA target sites which were artificially expressed in cells to ‘sponge up’ or inhibit an endogenous microRNA. Native linear non-coding RNAs with a limited number of microRNA seed matches carrying out the same function were subsequently discovered in plants and mammals, as reviewed in Ebert and Sharp (2010). CiRS-7 (or CDR1as) is an abundant, largely cytoplasmic RNA, suggesting that it could sponge up much of the available miR-7 population in cells (Memczak et al, 2013). Dense Argonaute (Ago) protein footprints across CiRS-7 were revealed by PAR-CLIP (Memczak et al, 2013) and HITS-CLIP experiments (Hansen et al, 2013). Together with direct evidence of an association between Ago, miR-7 and CiRS-7 (Hansen et al, 2013), this demonstrated occupancy of the miR-7 target sites. Importantly, no linear form of CiRS-7 was detectable in human HEK293 cells, and limited central and 3′ base pairing between miR-7 and CiRS-7 excluded miR-7 directed slicing of the circRNA.
CiRS-7 knockdown or overexpression in HEK293 cells led to marked changes in transcriptome composition, prominently including changes to the levels of known miR-7 targets (Memczak et al, 2013). A multifaceted transfection approach in HeLa and HEK293 cells demonstrated that the presence of CiRS-7 reduced the effect of miR-7 on both reporter constructs and endogenous miR-7 targets (Hansen et al, 2013). Analogous experiments with SRY uncovered its function as a miR-138 sponge (Hansen et al, 2013). Since miR-7 and CiRS-7 share expression domains in the mouse brain Memczak et al (2013) reasoned that miR-7 depletion and CiRS-7 overexpression could elicit a similar phenotype. They chose zebrafish as their model as it has lost the CDR1 locus but miR-7 is conserved and highly expressed in the brain. Indeed, zebrafish embryo injection studies showed that both, morpholino knockdown of miR-7 and introduction of linear or circular versions of CiRS-7 caused specific reduction in midbrain size, suggesting that CiRS-7 acts as a miR-7 sponge in this setting.
Taken together, the two studies show that two examples, CiRS-7 and SRY, have all the attributes of potent naturally occurring microRNA sponges offering a strong paradigm for circRNA function. Thinking about additional plausible roles of circRNAs, additional attractive possibilities come to mind (Figure 1B). Instead of acting as repository for microRNAs, circRNAs could be involved in their intracellular transport, and the ability of another microRNA, miR-671, to trigger slicing of CiRS-7 (Hansen et al, 2011) suggests a possible mechanism for the timed release of the circRNA cargo. Obviously, circRNAs could also function more broadly to store, sort or localize RNA-binding proteins (RBPs). Would it not be fascinating if circRNAs served in the organized assembly or regulation of ‘RBP factories’, such as the recently recognized enzymes of metabolic pathways with RBP activity (Castelló et al, 2012), or served as their allosteric regulators? circRNAs could also directly target (m)RNA by partial base pairing. Finally, the ability of circRNAs to function as templates for protein synthesis should also not be dismissed since internal ribosome entry site (IRES)-mediated translation can in principle occur on circRNAs (Chen and Sarnow, 1995).
Footnotes
The authors declare that they have no conflict of interest.
References
- Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R (1993) Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 73: 1019–1030 [DOI] [PubMed] [Google Scholar]
- Castelló A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, Krijgsveld J, Hentze MW (2012) Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149: 1393–1406 [DOI] [PubMed] [Google Scholar]
- Chen CY, Sarnow P (1995) Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 268: 415–417 [DOI] [PubMed] [Google Scholar]
- Ebert MS, Sharp PA (2010) Emerging roles for natural microRNA sponges. Curr Biol 20: R858–R861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J (2013) Natural RNA circles function as efficient miRNA sponges. Nature (advance online publication 27 February 2013; doi:; DOI: 10.1038/nature11993) [DOI] [PubMed] [Google Scholar]
- Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J (2011) miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J 30: 4414–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19: 141–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature (advance online publication 27 February 2013; doi:; DOI: 10.1038/nature11928) [DOI] [PubMed] [Google Scholar]
- Mercer TR, Gerhardt DJ, Dinger ME, Crawford J, Trapnell C, Jeddeloh JA, Mattick JS, Rinn JL (2012) Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat Biotechnol 30: 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO (2012) Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 7: e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wang Y, Cao M, Pantaleo V, Burgyan J, Li W-X, Ding S-W (2012) Homology-independent discovery of replicating pathogenic circular RNAs by deep sequencing and a new computational algorithm. Proc Natl Acad Sci USA 109: 3938–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]