Abstract
EMBO J (2013) 32:11, 954–969 doi:; DOI: 10.1038/emboj.2013.27; published online February 12 2013
Neurotransmission in the brain critically depends on the maintenance of synapses as well as on regulated synaptic protein turnover. How synaptic proteostasis is held in check has remained largely enigmatic. A new paper in The EMBO Journal reports that the active zone proteins Piccolo and Bassoon put a brake on presynaptic protein turnover by restraining the activity of the E3 ubiquitin ligase Siah1, thereby preventing neurodegeneration.
To retain memories, neurons have evolved mechanisms of activity-dependent plasticity that involve remodelling of the synaptic proteome in response to internal or external cues. For example, the rapid re-organization of proteins at synaptic release sites, termed as active zones (AZ), may be utilized for the activity-dependent modulation of neurotransmission. Several mechanisms have been proposed for synaptic protein turnover, including local protein synthesis and degradation. Considerable attention has been paid to protein synthesis (Martin et al, 1997; Steward and Schuman, 2001), whereas the contributions of regulated protein turnover by the ubiquitin–proteasome system (UPS) or by the autophagy-lysosomal pathway to the regulation of synaptic efficacy are much less well understood. The current view suggests that the abundance of major synaptic proteins is controlled via a tightly controlled cascade of enzymes involved in the ubiquitination of protein substrates and their subsequent degradation by the 26S proteasome and/or by the autophagy-lysosomal pathway. Although a requirement for ubiquitin and the proteasome in neuronal function has been demonstrated (McNaught et al, 2001; Wilson et al, 2002), the molecular mechanisms underlying regulated synaptic protein turnover remain unclear.
Almost a decade ago, Speese et al (2003) identified Drosophila UNC-13 (Dunc13), one of the core components of the AZ, as an acutely regulated UPS substrate in the presynaptic terminal. In this issue, Waites et al (2013) now report that two other core components of the AZ, Piccolo and Bassoon, have a role in regulating presynaptic ubiquitination and proteostasis.
Piccolo and Bassoon are two giant vertebrate-specific AZ proteins whose precise role at the presynapse has remained somewhat enigmatic. Earlier work based on hypomorphic PiccoloΔExon14/Bassoon-deficient mice had suggested a regulatory role for Piccolo/Bassoon in synaptic vesicle (SV) organization (Mukherjee et al, 2010), though the defects reported were surprisingly mild. Garner and colleagues decided to re-visit Piccolo/Bassoon function using a tricistronic lentiviral vector that reduces the expression levels of both proteins in cultured neurons by >85%. Strikingly, they found dramatic alterations in Piccolo/Bassoon-deficient synapses including a profound loss of SVs and SV proteins, as well as multiple other presynaptic components including SNAP-25, and the AZ protein RIM1, eventually leading to synapse degeneration. These changes are reminiscent of, though much more dramatic than the reduced SV numbers reported by Südhof and colleagues in PiccoloΔExon14/Bassoon-deficient mice (Mukherjee et al, 2010). This discrepancy, likely, is explained by the fact that only three of the seven Piccolo isoforms were eliminated in PiccoloΔExon14 mice, suggesting that the phenotypes reported by Mukherjee et al (2010) reflect a mild version of the double knockdown (DKD) phenotype. Waites et al (2013), thus, identify the presynaptic AZ scaffolds Piccolo and Bassoon as components of the presynaptic UPS and as central regulators of presynaptic proteostasis.
What is the mechanism underlying the synaptic neurodegeneration in DKD boutons? The fact that the accumulation of tubulovesicular structures, containing insoluble protein aggregates and ubiquitin is one of the prominent features common to most neurodegenerative processes, prompted Garner and colleagues to test if presynaptic degradation in DKD neurons is a consequence of the enhanced activity of the ubiquitin-based proteolytic machineries. Using markers to visualize lysosomes and multivesicular bodies, degradative sorting stations for ubiquitinated membrane components and long-lived proteins, they show that these organelles were significantly upregulated in DKD neurons. Furthermore, pharmacological treatment of neurons with inhibitors of protein ubiquitination and degradation or overexpression of mutant ubiquitin, which is unable to form poly-ubiquitin chains and, thus, mark substrates for degradation, partially prevented SV protein loss in DKD terminals. These observations demonstrate that Piccolo/Bassoon DKD-induced protein degradation relies on poly-ubiquitin chain formation.
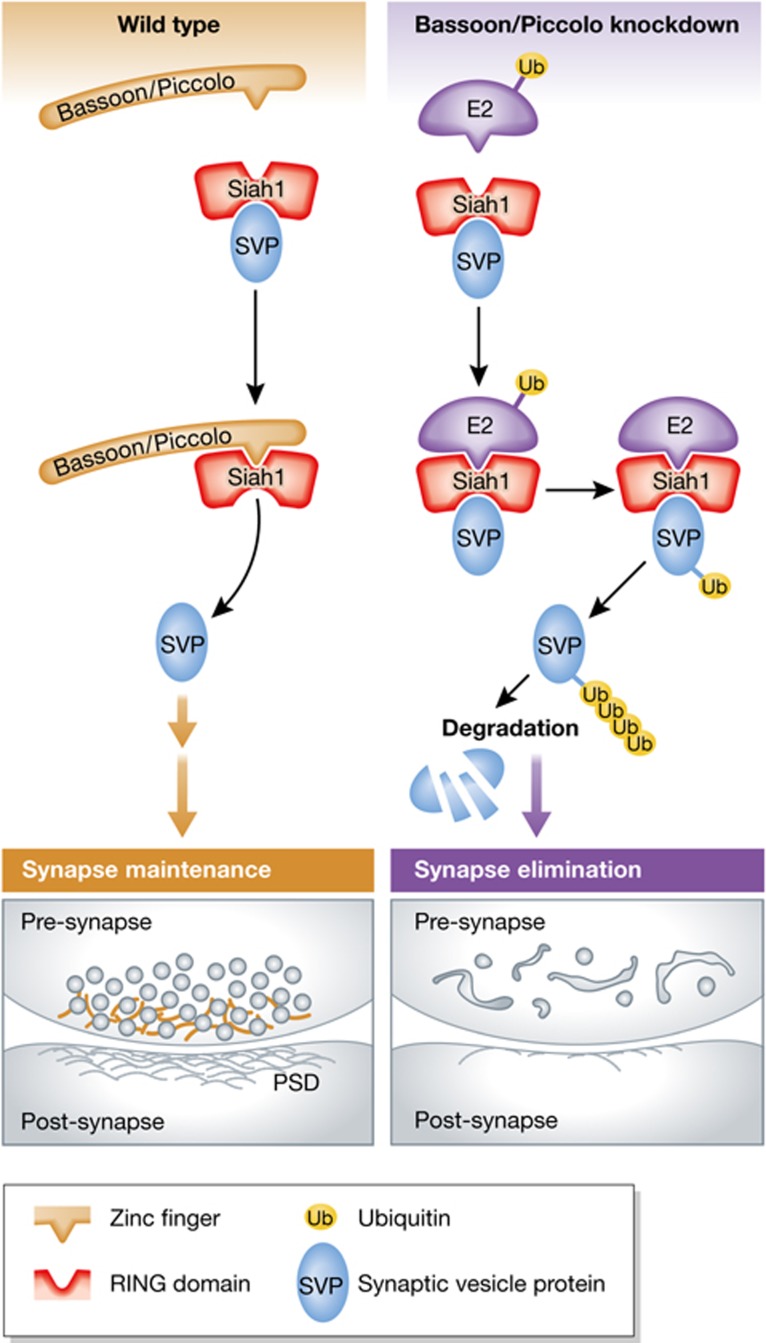
How does loss of Piccolo/Bassoon translate into elevated protein ubiquitination and degradation? One possibility is that Piccolo/Bassoon may directly regulate ubiquitin-conjugating or deconjugating enzymes. To investigate this, Waites et al performed a series of biochemical studies that reveal a direct interaction between Bassoon/Piccolo and the E3 ubiquitin ligase Siah1. Compellingly, this interaction competes with E2 ubiquitin-conjugating enzymes, which engage Siah1 via the same domain. As a result, Siah1-mediated ubiquitination is attenuated in Piccolo/Bassoon-deficient synapses, in agreement with a model wherein Piccolo/Bassoon serve as key regulators of presynaptic protein turnover and, thus, SV pool size (Figure 1). Consistent with this notion, Siah1 expression is inversely related to SV number and knockdown of Siah1 ameliorates the DKD phenotypes. Together, the data by Waites et al (2013) support the model that Siah1 as part of the ubiquitination machinery contributes to the loss of SVs as well as to the aberrant degradation of presynaptic proteins via the proteasome and/or the endolysomal pathway in Piccolo/Bassoon-deficient neurons.
Figure 1.
Piccolo/Bassoon as key regulators of presynaptic protein turnover. In control synapses, the AZ proteins Bassoon and Piccolo restrain the activity of the E3 ubiquitin ligase Siah1 via binding of their zinc finger domains to the Siah1 RING domain (left top). This interaction inhibits the presynaptic ubiquitination machinery, thereby maintaining synapse integrity (left bottom). Deletion of Bassoon and Piccolo triggers Siah1 poly-ubiquitination via recruitment of E2 ubiquitin-conjugating enzymes (right top). This leads to the aberrant degradation of SV proteins, and the accumulation of degradative organelles, finally resulting in synapse elimination (right bottom).
Although the new work by Waites et al (2013) clearly provides stunning and unexpected insights into the role of Piccolo/Bassoon in presynaptic proteostasis a number of questions remain. For example, both Piccolo and Bassoon are expressed during neuronal differentiation and are among the first proteins to arrive at nascent synapses, suggesting a possible role in synapse formation. Once a synapse is established, new components must continue to arrive to replace aged proteins and organelles destined for degradation. The balance between arrival and departure of components may determine whether a synapse is strengthened, weakened or eliminated. In line with this hypothesis, decreased synaptic levels of Bassoon and Piccolo could affect axonal transport of SV precursors and, therefore, impair the biogenesis of SVs at presynaptic terminals. Interestingly, kinesin-3 motors are implicated in the axonal transport of both SV precursors and of Piccolo/Bassoon transport vesicles. The neuromuscular junctions of imac (a Drosophila kinesin-3 family member) mutant flies, in addition to lacking SVs, display greatly reduced levels of the AZ protein Brp/ELKS/CAST/ERC (Pack-Chung et al, 2007) and the absence of synaptic boutons in these flies resembles the neurodegeneration phenotype seen in Piccolo/Bassoon DKD neurons. Thus, if and how deletion of AZ proteins may relate to changes in axonal transport of SV precursors remains an interesting subject for future studies. A second open question pertains to the physiological regulation of presynaptic protein turnover at the level of individual synapses. Sensory experience and learning stabilize or eliminate synapses and thereby dynamically sculpture mature neuronal networks. The molecular mechanisms that underlie the selective stabilization or elimination of synapses are largely unknown. While the novel and unexpected finding by Waites et al (2013) that AZ proteins regulate synapse loss and elimination contributes a valuable piece to our understanding of how neuronal networks reshape, several questions remain as to how this mechanistically translates into the regulation of SV pool size and synapse degeneration. AZ proteins orchestrate SV exo- and endocytosis and are, thus, an important element in coupling SV fusion and clearance of release sites with endocytic retrieval of SV membranes. Are Piccolo and Bassoon part of a presynaptic machinery regulating SV turnover via sensing activity-dependent exo- and endocytic cycling of SVs? Another intriguing question is whether the pathological alterations in brain levels of these proteins trigger neurodegeneration and affect neuronal survival over time. These issues remain to be explored in future studies.
Finally, the identity of the multivesicular endolysosomal structures observed in Bassoon/Piccolo DKD neurons is unclear. Apart from the UPS, which preferentially targets short-lived soluble proteins, neuronal degradation of membrane and long-lived proteins, as well as defective organelles are mediated by the autophagy–lysosomal pathway. Impairment of either of these systems may lead to the accumulation and aggregation of proteins, resulting in cellular toxicity and eventually in neurodegeneration. Future work will need to determine whether and to which degree the neurodegeneration observed in Piccolo/Bassoon DKD terminals is dictated by a failure in UPS only, or more likely is a combination of alterations in the UPS and in the autophagy–lysosomal pathway as suggested by the accumulation of multivesicular endolysosomal structures. Nonetheless, with the identification of Piccolo/Bassoon as important players of ubiquitin-mediated proteostasis at the presynapse, Waites et al (2013) set the stage for future studies aimed at addressing the detailed mechanisms governing activity-dependent SV turnover and synapse elimination.
Footnotes
The authors declare that they have no conflict of interest.
References
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER (1997) Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91: 927–938 [DOI] [PubMed] [Google Scholar]
- McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P (2001) Failure of the ubiquitin-proteasome system in Parkinson's disease. Nat Rev Neurosci 2: 589–594 [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Yang X, Gerber SH, Kwon HB, Ho A, Castillo PE, Liu X, Sudhof TC (2010) Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proc Natl Acad Sci USA 107: 6504–6509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack-Chung E, Kurshan PT, Dickman DK, Schwarz TL (2007) A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci 10: 980–989 [DOI] [PubMed] [Google Scholar]
- Speese SD, Trotta N, Rodesch CK, Aravamudan B, Broadie K (2003) The ubiquitin proteasome system acutely regulates presynaptic protein turnover and synaptic efficacy. Curr Biol 13: 899–910 [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM (2001) Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci 24: 299–325 [DOI] [PubMed] [Google Scholar]
- Waites CL, Leal-Ortiz SA, Okerlund N, Dalke H, Fejtova A, Altrock WD, Gundelfinger ED, Garner CC (2013) Bassoon and Piccolo maintain synapse integrity by regulating protein ubiquitination and degradation. EMBO J 32: 954–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Bhattacharyya B, Rachel RA, Coppola V, Tessarollo L, Householder DB, Fletcher CF, Miller RJ, Copeland NG, Jenkins NA (2002) Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nat Genet 32: 420–425 [DOI] [PubMed] [Google Scholar]