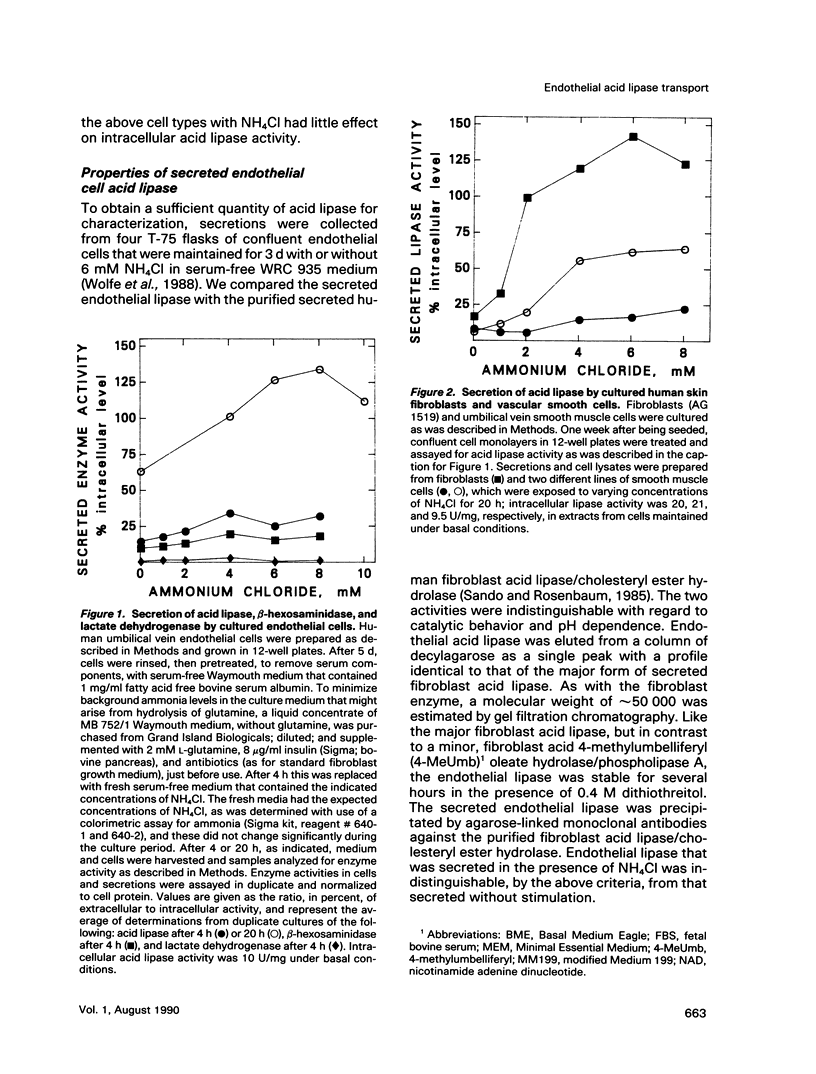
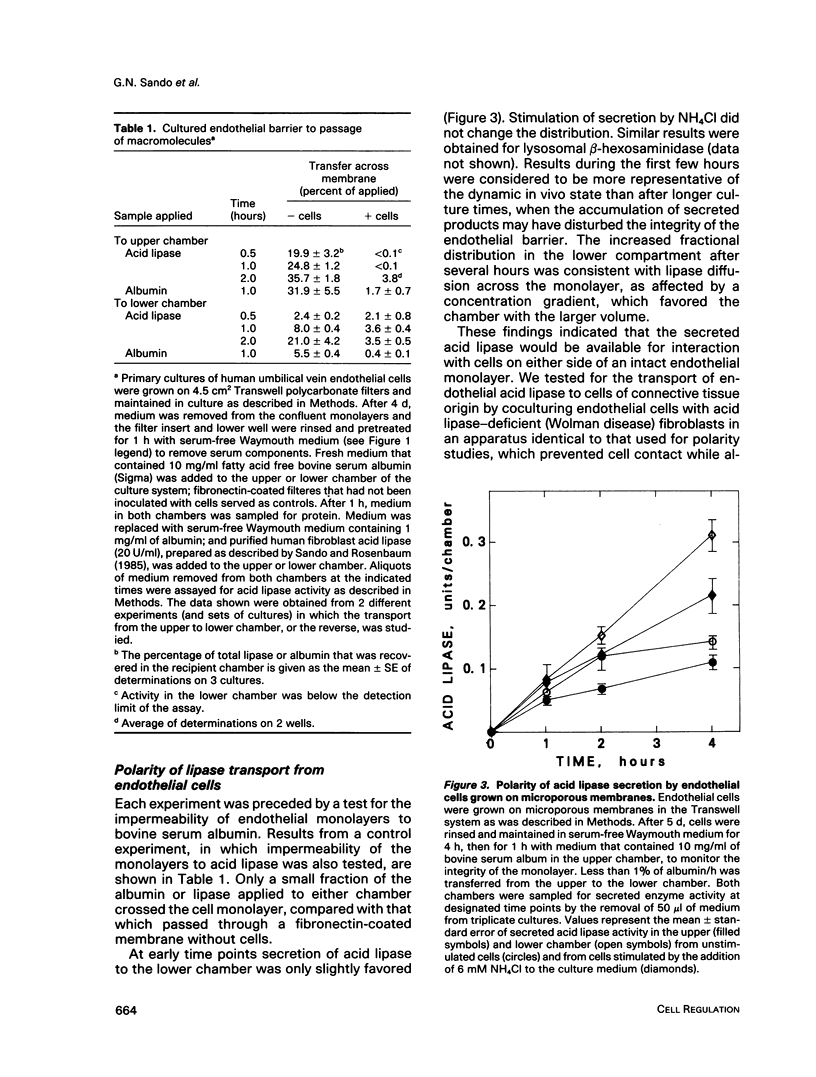
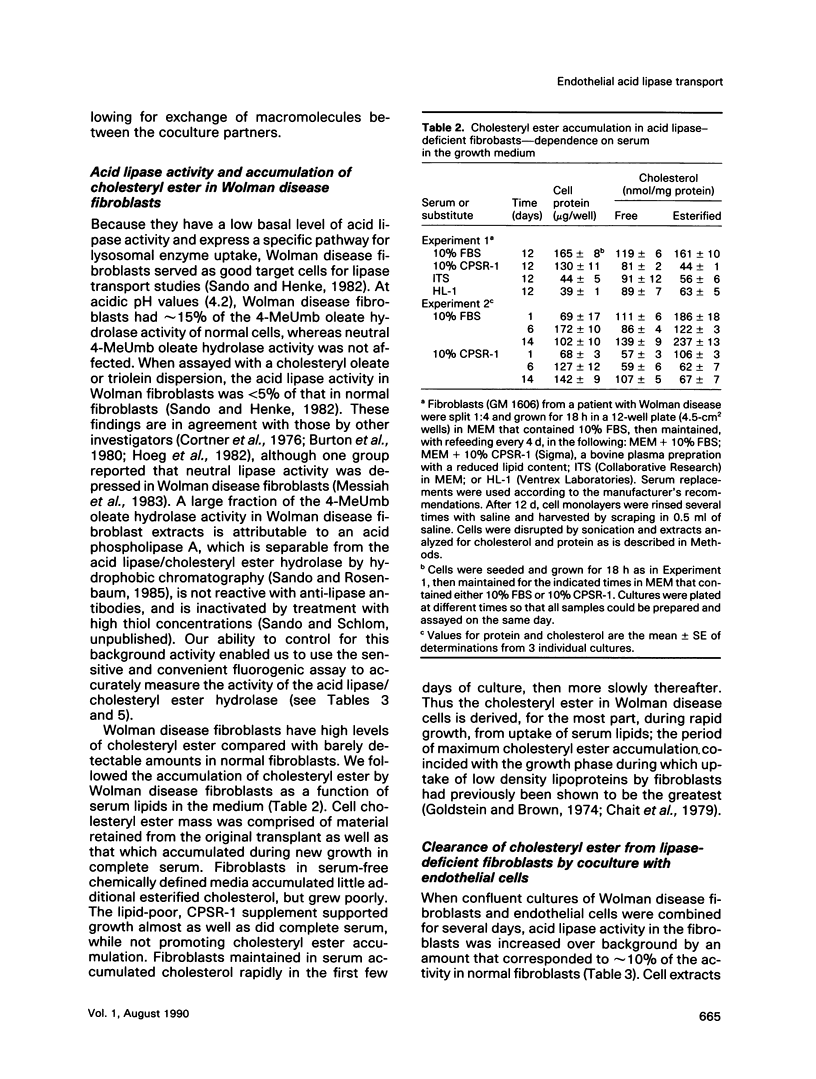
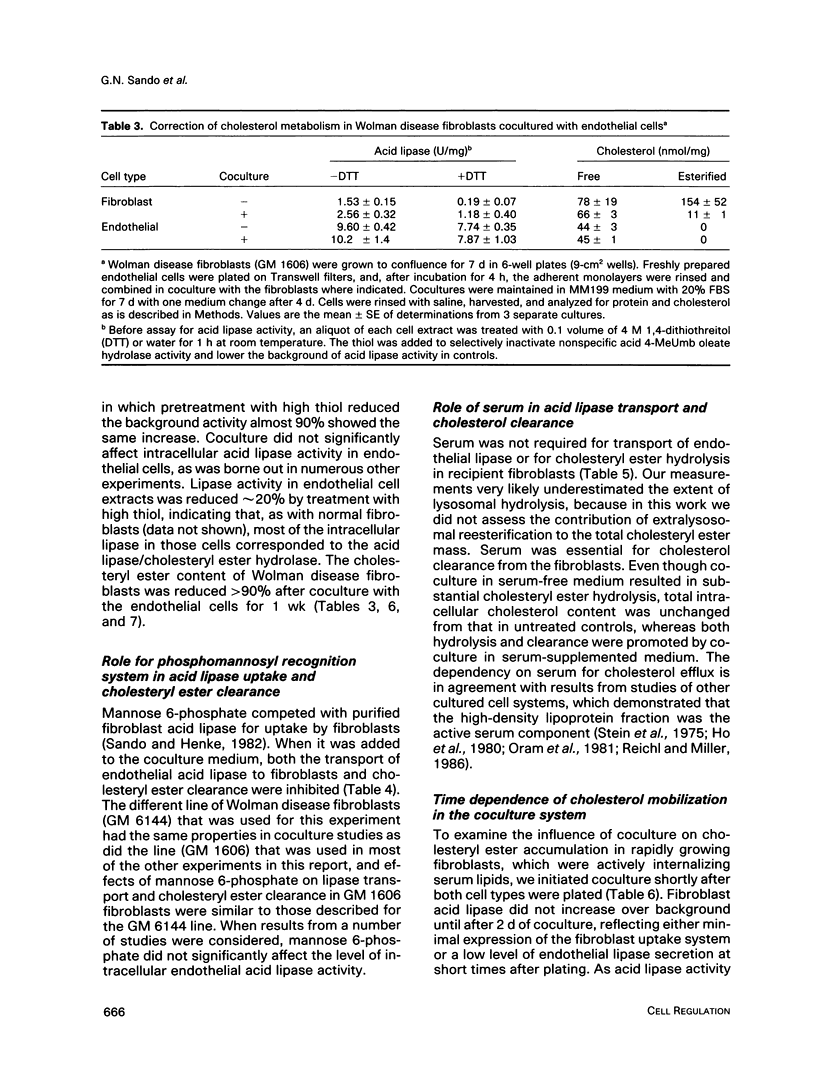
Abstract
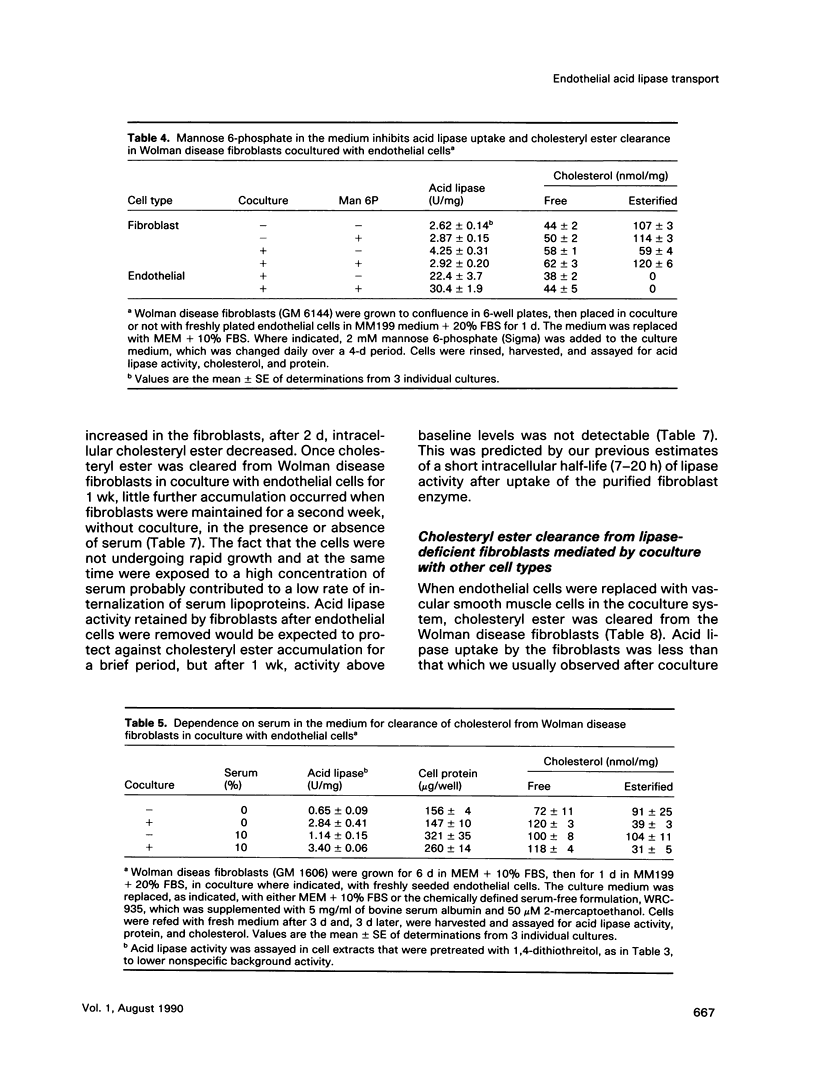
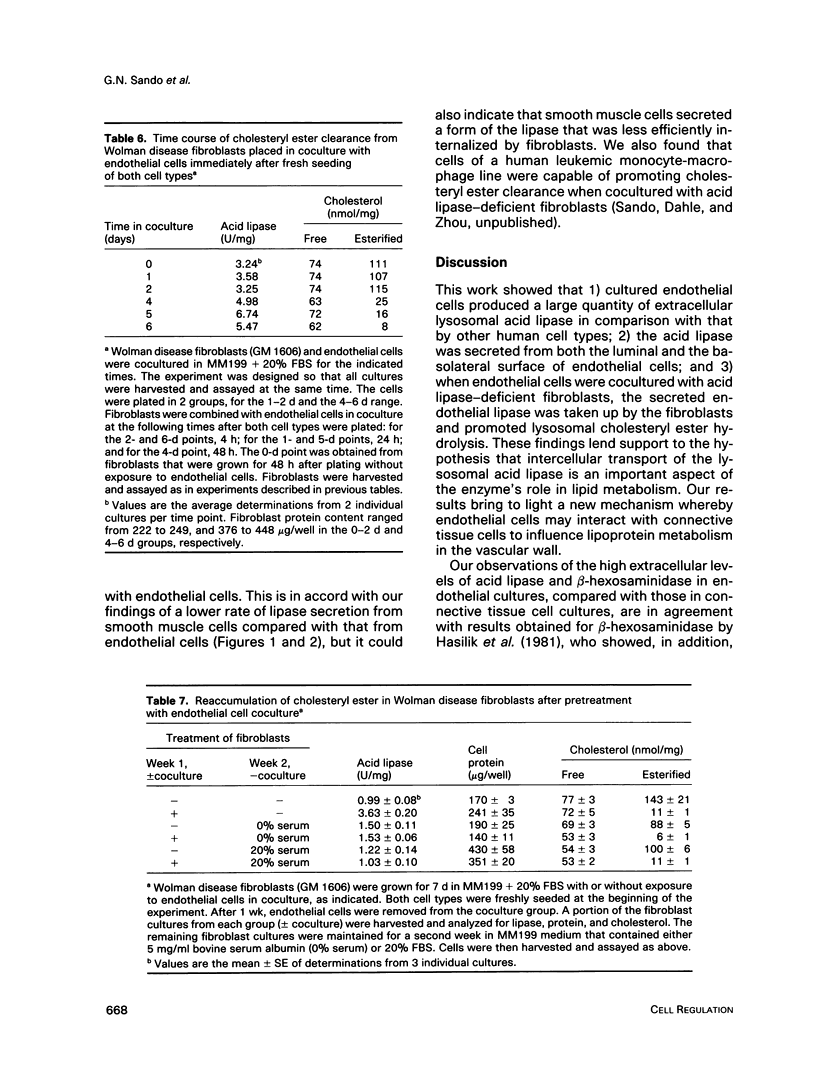
We present results from studies of human cell culture models to support the premise that the extracellular transport of lysosomal acid lipase has a function in lipoprotein cholesteryl ester metabolism in vascular tissue. Vascular endothelial cells secreted a higher fraction of cellular acid lipase than did smooth muscle cells and fibroblasts. Acid lipase and lysosomal beta-hexosaminidase were secreted at approximately the same rate from the apical and basolateral surface of an endothelial cell monolayer. Stimulation of secretion with NH4Cl did not affect the polarity. We tested for the ability of secreted endothelial lipase to interact with connective tissue cells and influence lipoprotein cholesterol metabolism in a coculture system in which endothelial cells on a micropore filter were suspended above a monolayer of acid lipase-deficient (Wolman disease) fibroblasts. After 5-7 d, acid lipase activity in the fibroblasts reached 10%-20% of the level in normal cells; cholesteryl esters that had accumulated from growth in serum were cleared. Addition of mannose 6-phosphate to the coculture medium blocked acid lipase uptake and cholesterol clearance, indicating that lipase released from endothelial cells was packaged into fibroblast lysosomes by a phosphomannosyl receptor-mediated pathway. Supplementation of the coculture medium with serum was not required for lipase uptake and cholesteryl ester hydrolysis by the fibroblasts, but was necessary for cholesterol clearance. Results from our coculture model suggest that acid lipase may be transported from intact endothelium to cells in the lumen or the wall of a blood vessel. We postulate that delivery of acid hydrolases and lipoproteins to a common endocytic compartment may occur and have an impact on cellular lipoprotein processing.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMADOR E., DORFMAN L. E., WACKER W. E. SERUM LACTIC DEHYDROGENASE ACTIVITY: AN ANALYTICAL ASSESSMENT OF CURRENT ASSAYS. Clin Chem. 1963 Aug;12:391–399. [PubMed] [Google Scholar]
- Abraham D., Muir H., Olsen I., Winchester B. Direct enzyme transfer from lymphocytes corrects a lysosomal storage disease. Biochem Biophys Res Commun. 1985 Jun 14;129(2):417–425. doi: 10.1016/0006-291x(85)90167-6. [DOI] [PubMed] [Google Scholar]
- Baron R., Neff L., Brown W., Courtoy P. J., Louvard D., Farquhar M. G. Polarized secretion of lysosomal enzymes: co-distribution of cation-independent mannose-6-phosphate receptors and lysosomal enzymes along the osteoclast exocytic pathway. J Cell Biol. 1988 Jun;106(6):1863–1872. doi: 10.1083/jcb.106.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Sobhani M. K., Brunschede G. Y., Goldstein J. L. Restoration of a regulatory response to low density lipoprotein in acid lipase-deficient human fibroblasts. J Biol Chem. 1976 Jun 10;251(11):3277–3286. [PubMed] [Google Scholar]
- Burton B. K., Emery D., Mueller H. W. Lysosomal acid lipase in cultivated fibroblasts: characterization of enzyme activity in normal and enzymatically deficient cell lines. Clin Chim Acta. 1980 Feb 14;101(1):25–32. doi: 10.1016/0009-8981(80)90052-2. [DOI] [PubMed] [Google Scholar]
- Burton B. K., Remy W. T., Rayman L. Cholesterol ester and triglyceride metabolism in intact fibroblasts from patients with Wolman's disease and cholesterol ester storage disease. Pediatr Res. 1984 Dec;18(12):1242–1245. doi: 10.1203/00006450-198412000-00003. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Chait A., Bierman E. L., Albers J. J. Low density lipoprotein receptor activity in fibroblasts cultured from diabetic donors. Diabetes. 1979 Oct;28(10):914–918. doi: 10.2337/diab.28.10.914. [DOI] [PubMed] [Google Scholar]
- Cornicelli J. A., Witte L. D., Goodman D. S. Inhibition of LDL degradation in cultured human fibroblasts induced by endothelial cell-conditioned medium. Arteriosclerosis. 1983 Nov-Dec;3(6):560–567. doi: 10.1161/01.atv.3.6.560. [DOI] [PubMed] [Google Scholar]
- Cortner J. A., Coates P. M., Swoboda E., Schnatz J. D. Genetic variation of lysosomal acid lipase. Pediatr Res. 1976 Nov;10(11):927–932. doi: 10.1203/00006450-197611000-00005. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Hoak J. C., Fry G. L. Effect of aspirin on thrombin-induced adherence of platelets to cultured cells from the blood vessel wall. J Clin Invest. 1978 Oct;62(4):847–856. doi: 10.1172/JCI109197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Truskey G. A., Warren H. B., O'Connor S. E., Eisenhaure B. H. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J Cell Biol. 1985 Sep;101(3):871–879. doi: 10.1083/jcb.101.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M. F., Martin J. C. Intracellular localization of beta-glucuronidase in fibroblasts after direct transfer from macrophages. Biochem J. 1988 Dec 1;256(2):335–341. doi: 10.1042/bj2560335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y. P., Storrie B. Animal cell lysosomes rapidly exchange membrane proteins. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3860–3864. doi: 10.1073/pnas.85.11.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diment S., Leech M. S., Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988 May 15;263(14):6901–6907. [PubMed] [Google Scholar]
- Diment S., Martin K. J., Stahl P. D. Cleavage of parathyroid hormone in macrophage endosomes illustrates a novel pathway for intracellular processing of proteins. J Biol Chem. 1989 Aug 15;264(23):13403–13406. [PubMed] [Google Scholar]
- Diment S., Stahl P. Macrophage endosomes contain proteases which degrade endocytosed protein ligands. J Biol Chem. 1985 Dec 5;260(28):15311–15317. [PubMed] [Google Scholar]
- Doherty J. J., 2nd, Kay D. G., Lai W. H., Posner B. I., Bergeron J. J. Selective degradation of insulin within rat liver endosomes. J Cell Biol. 1990 Jan;110(1):35–42. doi: 10.1083/jcb.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J. M., Sahagian G. G. Basis for low affinity binding of a lysosomal cysteine protease to the cation-independent mannose 6-phosphate receptor. J Biol Chem. 1990 Mar 15;265(8):4210–4217. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Ferris A. L., Brown J. C., Park R. D., Storrie B. Chinese hamster ovary cell lysosomes rapidly exchange contents. J Cell Biol. 1987 Dec;105(6 Pt 1):2703–2712. doi: 10.1083/jcb.105.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry G. L., Czervionke R. L., Hoak J. C., Smith J. B., Haycraft D. L. Platelet adherence to cultured vascular cells: influence of prostacyclin (PGI2). Blood. 1980 Feb;55(2):271–275. [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan P., St Clair R. W. Retroendocytosis of low density lipoprotein. Effect of lysosomal inhibitors on the release of undegraded 125I-low density lipoprotein of altered composition from skin fibroblasts in culture. J Biol Chem. 1984 Feb 10;259(3):1703–1713. [PubMed] [Google Scholar]
- Griffiths G., Hoflack B., Simons K., Mellman I., Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988 Feb 12;52(3):329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Griffiths G. The structure and function of a mannose 6-phosphate receptor-enriched, pre-lysosomal compartment in animal cells. J Cell Sci Suppl. 1989;11:139–147. doi: 10.1242/jcs.1989.supplement_11.11. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Marcus A. J., Hajjar K. A. Interactions of arterial cells. Studies on the mechanisms of endothelial cell modulation of cholesterol metabolism in co-cultured smooth muscle cells. J Biol Chem. 1987 May 25;262(15):6976–6981. [PubMed] [Google Scholar]
- Hasilik A., Voss B., Von Figura K. Transport and processing of lysosomal enzymes by smooth muscle cells and endothelial cells. Exp Cell Res. 1981 May;133(1):23–30. doi: 10.1016/0014-4827(81)90352-9. [DOI] [PubMed] [Google Scholar]
- Heider J. G., Boyett R. L. The picomole determination of free and total cholesterol in cells in culture. J Lipid Res. 1978 May;19(4):514–518. [PubMed] [Google Scholar]
- Ho Y. K., Brown M. S., Goldstein J. L. Hydrolysis and excretion of cytoplasmic cholesteryl esters by macrophages: stimulation by high density lipoprotein and other agents. J Lipid Res. 1980 May;21(4):391–398. [PubMed] [Google Scholar]
- Hoeg J. M., Demosky S. J., Jr, Brewer H. B., Jr Characterization of neutral and acid ester hydrolase in Wolman's disease. Biochim Biophys Acta. 1982 Apr 15;711(1):59–65. doi: 10.1016/0005-2760(82)90009-1. [DOI] [PubMed] [Google Scholar]
- Horrigan S., Campbell J. H., Campbell G. R. Effect of endothelium on beta-VLDL metabolism by cultured smooth muscle cells of differing phenotype. Atherosclerosis. 1988 May;71(1):57–69. doi: 10.1016/0021-9150(88)90302-4. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCoy K. L., Schwartz R. H. The role of intracellular acidification in antigen processing. Immunol Rev. 1988 Dec;106:129–147. doi: 10.1111/j.1600-065x.1988.tb00777.x. [DOI] [PubMed] [Google Scholar]
- McNamara A., Jenne B. M., Dean M. F. Fibroblasts acquire beta-glucuronidase by direct and indirect transfer during co-culture with macrophages. Exp Cell Res. 1985 Sep;160(1):150–157. doi: 10.1016/0014-4827(85)90244-7. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Messieh S., Clarke J. T., Cook H. W., Spence M. W. Abnormal neutral lipase activity in acid-lipase-deficient cultured human fibroblasts. Pediatr Res. 1983 Sep;17(9):770–774. doi: 10.1203/00006450-198309000-00018. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. G., Insull W., Jr Effect of regenerated endothelium on lipid accumulation in the arterial wall. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1724–1728. doi: 10.1073/pnas.74.4.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Gimbrone M. A., Jr Plasmalemmal proteins of cultured vascular endothelial cells exhibit apical-basal polarity: analysis by surface-selective iodination. J Cell Biol. 1986 Dec;103(6 Pt 1):2389–2402. doi: 10.1083/jcb.103.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E. F. Lessons from genetic disorders of lysosomes. Harvey Lect. 1979 1980;75:41–60. [PubMed] [Google Scholar]
- Olsen I., Dean M. F., Harris G., Muir H. Direct transfer of a lysosomal enzyme from lymphoid cells to deficient fibroblasts. Nature. 1981 May 21;291(5812):244–247. doi: 10.1038/291244a0. [DOI] [PubMed] [Google Scholar]
- Oram J. F., Albers J. J., Cheung M. C., Bierman E. L. The effects of subfractions of high density lipoprotein on cholesterol efflux from cultured fibroblasts. Regulation of low density lipoprotein receptor activity. J Biol Chem. 1981 Aug 25;256(16):8348–8356. [PubMed] [Google Scholar]
- Owada M., Neufeld E. F. Is there a mechanism for introducing acid hydrolases into liver lysosomes that is independent of mannose 6-phosphate recognition? Evidence from I-cell disease. Biochem Biophys Res Commun. 1982 Apr 14;105(3):814–820. doi: 10.1016/0006-291x(82)91042-7. [DOI] [PubMed] [Google Scholar]
- Reichl D., Miller N. E. The anatomy and physiology of reverse cholesterol transport. Clin Sci (Lond) 1986 Mar;70(3):221–231. doi: 10.1042/cs0700221. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Sando G. N., Henke V. L. Recognition and receptor-mediated endocytosis of the lysosomal acid lipase secreted by cultured human fibroblasts. J Lipid Res. 1982 Jan;23(1):114–123. [PubMed] [Google Scholar]
- Sando G. N., Rosenbaum L. M. Human lysosomal acid lipase/cholesteryl ester hydrolase. Purification and properties of the form secreted by fibroblasts in microcarrier culture. J Biol Chem. 1985 Dec 5;260(28):15186–15193. [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Stein Y., Glangeaud M. C., Fainaru M., Stein O. The removal of cholesterol from aortic smooth muscle cells in culture and Landschutz ascites cells by fractions of human high-density apolipoprotein. Biochim Biophys Acta. 1975 Jan 24;380(1):106–118. doi: 10.1016/0005-2760(75)90049-1. [DOI] [PubMed] [Google Scholar]
- Stoll L. L., Spector A. A. Interaction of platelet-activating factor with endothelial and vascular smooth muscle cells in coculture. J Cell Physiol. 1989 May;139(2):253–261. doi: 10.1002/jcp.1041390206. [DOI] [PubMed] [Google Scholar]
- Stoll L. L., Spector A. A. Lipid transfer between endothelial and smooth muscle cells in coculture. J Cell Physiol. 1987 Oct;133(1):103–110. doi: 10.1002/jcp.1041330113. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Enzymic diagnosis of sphingolipidoses. Methods Enzymol. 1978;50:456–488. doi: 10.1016/0076-6879(78)50049-9. [DOI] [PubMed] [Google Scholar]
- Unemori E. N., Bouhana K. S., Werb Z. Vectorial secretion of extracellular matrix proteins, matrix-degrading proteinases, and tissue inhibitor of metalloproteinases by endothelial cells. J Biol Chem. 1990 Jan 5;265(1):445–451. [PubMed] [Google Scholar]
- Werdelin O., Mouritsen S., Petersen B. L., Sette A., Buus S. Facts on the fragmentation of antigens in presenting cells, on the association of antigen fragments with MHC molecules in cell-free systems, and speculation on the cell biology of antigen processing. Immunol Rev. 1988 Dec;106:181–193. doi: 10.1111/j.1600-065x.1988.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Wolfe R. A., Braatz J. A., Miller D. A., Heifetz A. H. A new serum-free medium for monoclonal antibody production. Biotechniques. 1988 Jan;6(1):62–67. [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]



