Abstract
Background
National guidelines recommend that physicians discuss end-of-life (EOL) care planning with cancer patients whose life expectancy is less than one year.
Objective
To evaluate the incidence of EOL discussions for patients with stage IV lung or colorectal cancer, and where, when, and with whom discussions take place.
Design
Prospective cohort study of patients diagnosed with lung or colorectal cancer from 2003 to 2005.
Setting
Subjects lived in Northern California, Los Angeles County, North Carolina, Iowa, or Alabama, or received care in one of five large health maintenance organizations or one of fifteen Veteran’s Health Administration sites.
Patients
2155 patients with stage IV lung or colorectal cancer.
Measurements
EOL discussions reported in patient and surrogate interviews or documented in medical records through 15 months after diagnosis.
Results
73% of patients had EOL discussions identified by at least one source. Among patients who died during follow-up (N=1470), 87% had EOL discussions, versus 41% of patients who were alive at the end of follow-up (N=685). Among first EOL discussions documented in records (N=1081), 55% occurred in the hospital. Oncologists documented EOL discussions with only 27% of their patients. Among patients with documented EOL discussions who died during follow-up (N=959), discussions took place a median of 33 days before death.
Limitations
The depth and quality of EOL discussions was not evaluated. Much of the information about discussions came from surrogates of patients who died before baseline interviews could be obtained.
Conclusions
Although most patients with stage IV lung or colorectal cancer have discussions with physicians about EOL care planning before death, many discussions occur during acute hospital care, with non-oncology providers, and late in the course of illness.
Introduction
National guidelines recommend that physicians discuss end-of-life (EOL) care planning with patients who have incurable cancer and a life expectancy of less than one year (1, 2). Patients who have discussed their preferences for EOL care with a physician are more likely to choose palliation over aggressive measures at the EOL (3, 4), to die at home or under hospice care (4, 5), and to receive care that is consistent with their preferences (6). Less aggressive care at the EOL is associated with better quality of life near death (4, 7, 8).
We sought to assess the incidence, timing, location, and specialties of involved physicians for discussions about EOL care planning between physicians and patients with incurable cancer. Previous studies have estimated that fewer than 40% of advanced cancer patients have EOL discussions with physicians (4, 6). The cross-sectional nature of previous studies (9, 10), however, provides no information on the timing of discussions and could lead to underestimation of their incidence. We know little about which physicians have discussions and where discussions take place.
The Cancer Outcomes Research and Surveillance Consortium (CanCORS) (11) offers a unique opportunity to study conversations about EOL care planning. CanCORS is a multiregional, population- and health system-based cohort study of more than 10,000 incident lung and colorectal cancer patients that includes longitudinal data about EOL discussions starting at diagnosis. We interviewed patients with newly diagnosed metastatic lung or colorectal cancer or their surrogates at two time points after diagnosis and performed detailed medical record abstraction for 15 months after diagnosis in order to evaluate discussions in a comprehensive manner.
Methods
CanCORS enrolled approximately 10,000 patients aged >20 who were diagnosed with lung or colorectal cancer (any stage of disease) between 2003 and 2005. Subjects lived in one of five geographic regions (Northern California, Los Angeles County, North Carolina, Iowa, or Alabama) or received their care in one of five large health maintenance organizations (HMO) or one of fifteen Veterans Health Administration (VHA) sites from across the U.S.(11). Each geographically defined site identified incident cases within weeks of diagnosis by reviewing all pathology reports with a relevant cancer diagnosis obtained by a collaborating regional cancer registry as part of their rapid-case ascertainment protocol. The two other sites (HMO and VHA) identified, screened, and enrolled participants from hospital-based cancer registries within the provider organization in which the participant was a member. Additional information about the CanCORS study is available elsewhere (12). The study was approved by human subjects committees at all participating institutions.
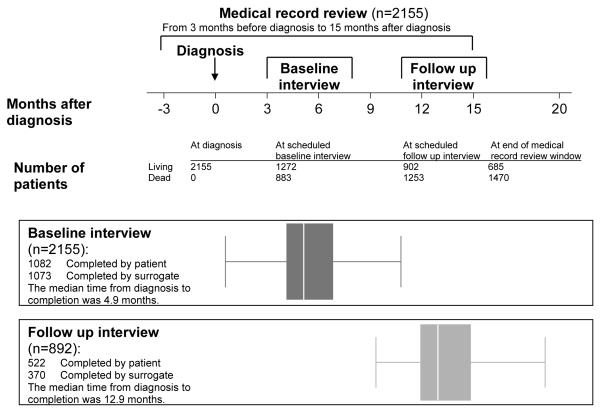
Figure 1 displays the data collection strategy for CanCORS. Patients, or surrogates of patients who were deceased or too ill to participate, were interviewed at baseline, approximately 4-6 months after diagnosis, using computer assisted telephone interviewing software after verbal informed consent was obtained from the interviewee. Bilingual interviewers used Spanish and Chinese instruments for patients who preferred those languages. Four versions of the baseline interview were available: a full patient interview; a brief patient interview, for patients unable to complete the full interview; a surrogate interview for surrogates of deceased patients; and a surrogate interview for living patients too ill to complete the interview themselves (13). A follow-up patient or surrogate interview was performed approximately 15 months after diagnosis after verbal patient or surrogate consent was obtained if the patient was alive at the time of the baseline interview.
Figure 1. Study design.
Box plots show the median time from diagnosis to completion of baseline and follow up interviews.
Medical records from hospitals, radiation treatment facilities, and offices of medical oncologists, surgeons, gastroenterologists, pulmonologists, and primary care physicians were abstracted for the time period beginning 3 months before diagnosis until death or 15 months after diagnosis. Written informed consent from patients or surrogates of living patients was required for medical record abstraction. When an appropriate institutional review board waiver had been obtained, medical records for deceased patients were reviewed without a request for consent; not all sites provided such a waiver.
Study cohort
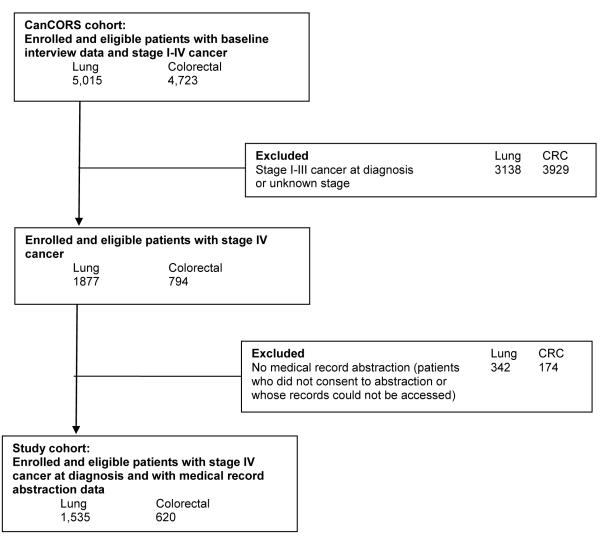
While CanCORS included patients with all stages of disease, for this study we focused on patients with stage IV disease at diagnosis. Such patients have incurable cancer and a limited life expectancy (median survival 4-8 months in metastatic lung cancer (14-16) and 12-24 months in metastatic colorectal cancer (17, 18)), making EOL discussions potentially appropriate. Figure 2 shows the selection of patients for this cohort, which included 1535 patients diagnosed with stage IV lung cancer and 620 with stage IV colorectal cancer who had a completed baseline interview and medical record data available. Among patients alive at the time of the baseline interview, follow-up interviews were completed by 69% and 72% of lung and colorectal cancer patients, respectively, or their surrogates. Characteristics of interview nonrespondents have been described previously (20).
Figure 2. Selection of analytic cohort.
The CanCORS study’s response rate, which accounts for both unsuccessful contacts and refusal/non-response, was 51.0%. The cooperation rate, which does not account for unsuccessful contacts, was 59.9% (12). Please see definitions provided by the American Association for Public Opinion Research for details of calculation of response and cooperation rates (19).
Definition of EOL discussion
Patients were classified as having an EOL discussion if a discussion about hospice or resuscitation was reported in the baseline or follow-up interview, or if there was medical record documentation of a discussion about advance care planning (do not resuscitate [DNR] order, hospice, palliative care, or not otherwise specified) or venue for dying (hospice, home, hospital, nursing home, or not otherwise specified). If the patient or surrogate did not answer the relevant interview questions (hospice, 3.2% baseline, 1.6% follow up, and 2.4% decedents’ surrogate interviews; resuscitation, 3.3% baseline interviews), then medical records were used to determine whether the patient had an EOL discussion.
Discussions about hospice were documented in medical record abstractions, in the baseline interview (“After your cancer was diagnosed, did any doctor or other health care provider discuss hospice care with you?” from all baseline interview types [patient, surrogate, and decedent]), and in the follow-up interview (“Was hospice ever recommended by any doctor or other health care provider?” from all follow-up interview types).
Discussions about resuscitation were evaluated in the medical record abstractions and the baseline interview (“Has a doctor ever talked to you about whether you would want to be revived or use life-sustaining machines?” asked in baseline patient and surrogate interviews for living patients, but not the decedent surrogate interview or any follow-up interviews). The follow-up and decedent interviews were shorter than the full baseline interview by design and did not assess resuscitation.
Palliative care and venues for dying other than hospice were evaluated in the medical record abstraction but not in interviews.
For each unique discussion in the medical record, we recorded the date of the discussion, topics discussed, provider(s) involved, and whether the discussion took place during a hospitalization. Discussions on separate dates were considered unique discussions, and the earliest recorded EOL discussion was considered the first EOL discussion for our analysis.
Statistical analyses
Descriptive statistics were used to describe patient characteristics and the incidence, details, and timing of EOL discussions. Fisher’s exact test was used to test differences between proportions, and linear regression was used to evaluate associations between continuous variables. Analyses were conducted using SAS (version 9.2; SAS Institute, Cary, NC) and Stata (version 11.1; StataCorp LP, College Station, TX).
Results
Patient characteristics are shown in Table 1. Nearly half of lung cancer patients were deceased at the time of the baseline interview, necessitating completion of the surrogate decedent interview, compared with 22% of colorectal cancer patients.
Table 1.
Patient characteristics according to cancer type. Data are given as frequency (percentage) unless otherwise specified.
| Metastatic Lung Cancer | Metastatic Colorectal Cancer | |
|---|---|---|
| Number of patients | 1535 | 620 |
| Sex3 | ||
| Male | 952 (62) | 368 (59) |
| Female | 583 (38) | 252 (41) |
| Race3 | ||
| White | 1210 (79) | 413 (67) |
| Black | 170 (11) | 121 (20) |
| Asian | 83 (5) | 37 (6) |
| Other | 69 (4) | 47 (8) |
| Missing | 3 (0.2) | 2 (0.3) |
| Ethnicity3 | ||
| Hispanic | 76 (5) | 47 (8) |
| Non-Hispanic | 1442 (94) | 562 (91) |
| Missing | 17 (1) | 11 (2) |
| Marital status3 | ||
| Married/living as married | 917 (60) | 369 (60) |
| Non-married | 607 (40) | 246 (40) |
| Missing | 11 (1) | 5 (1) |
| Age (years)3 | ||
| 21-54 | 193 (13) | 151 (24) |
| 55-59 | 187 (12) | 73 (12) |
| 60-64 | 204 (13) | 83 (13) |
| 65-69 | 245 (16) | 85 (14) |
| 70-74 | 276 (18) | 77 (12) |
| 75-79 | 214 (14) | 51 (8) |
| ≥ 80 | 216 (14) | 100 (16) |
| Comorbidity score at diagnosis*2 | ||
| None | 312 (20) | 194 (31) |
| Mild | 576 (38) | 240 (39) |
| Moderate | 328 (21) | 109 (18) |
| Severe | 319 (21) | 77 (12) |
| Number of hospitalizations in 90 days before diagnosis2 |
||
| 0 | 963 (63) | 393 (63) |
| 1 | 497 (32) | 203 (33) |
| 2 to 4 | 75 (5) | 24 (4) |
| Speaks English in home1 | ||
| Yes | 1397 (91) | 541 (87) |
| No | 51 (3) | 30 (5) |
| Missing | 87 (6) | 49 (8) |
| Education1 | ||
| < High school | 352 (23) | 105 (17) |
| High school/some college | 902 (59) | 361 (58) |
| ≥ college degree | 252 (16) | 145 (23) |
| Missing | 29 (2) | 9 (1) |
| Income ($)1 | ||
| < 20,000 | 460 (30) | 161 (26) |
| 20,000-39,999 | 428 (28) | 142 (23) |
| 40,000-59,999 | 184 (12) | 78 (13) |
| ≥ 60,000 | 215 (14) | 118 (19) |
| Missing | 248 (16) | 121 (20) |
| Insurance3 | ||
| Medicare | 220 (14) | 82 (13) |
| Medicaid | 179 (12) | 78 (13) |
| Medicare + Private | 566 (37) | 166 (27) |
| Private | 383 (25) | 218 (35) |
| Other | 179 (12) | 73 (12) |
| Missing | 8 (1) | 3 (0.5) |
| HMO member4 | ||
| Yes | 412 (27) | 158 (25) |
| No | 1123 (73) | 462 (75) |
| PDCR Site4 | ||
| Cancer Research Network | 246 (16) | 92 (15) |
| Northern California | 324 (21) | 157 (25) |
| Alabama | 188 (12) | 99 (16) |
| Los Angeles | 248 (16) | 112 (18) |
| Iowa | 361 (24) | 0 (0) |
| North Carolina | 0 (0) | 97 (16) |
| Veterans Administration | 168 (11) | 63 (10) |
| Months from diagnosis to baseline interview |
||
| Median (interquartile range) | 5 (4, 7) | 5 (4, 6) |
| Range | 0, 25 | 2, 27 |
| Baseline interview completed | ||
| Patient | 522 (34) | 357 (58) |
| Brief | 130 (8) | 73 (12) |
| By surrogate: patient too sick | 134 (9) | 56 (9) |
| By surrogate: patient deceased | 749 (49) | 134 (22) |
| Followup interview completed** | ||
| Survivor | 252 (32) | 270 (56) |
| Decedent: by surrogate | 288 (37) | 82 (17) |
| Not completed: patient alive at baseline |
246 (31) | 134 (28) |
| Vital status at end of abstraction period | ||
| Alive | 322 (21) | 363 (59) |
| Dead | 1213 (79) | 257 (41) |
| Median survival, months | ||
| (25th percentile, 75th percentile) | 6 (2, 13) |
20 (7, 46) |
PDCR: Primary Data Collection and Research
Defined using the Adult Comorbidity Evaluation 27, a validated medical record-based system that assigns each patient a 4-category comorbidity score (none, mild, moderate, or severe) based on severity noted across multiple body systems, from 3 months prior to diagnosis to initial treatment.
Among patients alive at baseline interview and thus eligible for followup interview.
Data obtained from baseline or follow-up interviews
Data obtained from medical record abstraction
Data obtained primarily from baseline interview; if non-response to interview item, then data obtained secondarily from medical record abstraction; if both data sources are missing, then data are obtained from the administrative data (or tracking records)
Data obtained from administrative data (or tracking records)
Overall, 73% (1569/2155) of stage IV lung and colorectal cancer patients had evidence of EOL discussions from interviews and/or medical record abstraction. Of the 1569 patients with EOL discussions, 81% were reported by patients or surrogates and 69% were documented in medical records. Baseline interview reports of EOL discussions agreed with medical record documentation of discussions up to the date of the baseline interview for 65% of patients (1399/2155). This group included 640 patients with reported and documented EOL discussions, and 759 patients with no reported or documented EOL discussions. Discordant reports included 583 patients with EOL discussions reported in interviews but not documented in medical records, and 173 discussions documented in records but not reported by patients or surrogates.
The proportion of patients with documented or reported EOL discussions was higher for patients who died during the medical record review period (87%, 1285/1470) than for those alive at the end of follow-up (41%, 284/685, P<.001.) Results were similar when stratified by cancer type (Table 2).
Table 2.
End of life discussions stratified by cancer type and vital status at the end of the abstraction period.
| Metastatic Lung Cancer | Metastatic Colorectal Cancer | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Overall | Dead at end of abstraction period |
Alive at end of abstraction period |
Overall | Dead at end of abstraction period |
Alive at end of abstraction period |
|
| Number of patients | 1535 | 1213 | 322 | 620 | 257 | 363 |
| End of life discussion | ||||||
| (1) Patient or surrogate reported | 980 (64) | 865 (71) | 115 (36) | 290 (47) | 169 (66) | 121 (33) |
| (2) Documented in medical record | 890 (58) | 808 (67) | 82 (25) | 191 (31) | 151 (59) | 40 (11) |
| (3) Any (1 or 2) | 1212 (79) | 1064 (88) | 148 (46) | 357 (58) | 221 (86) | 136 (37) |
For the 1569 patients with EOL discussions, topics included resuscitation (46%) and hospice care (82%). Other topics noted in medical records included palliative care (13%) and venues for dying other than hospice (3%, Table 3). Among first discussions recorded in the medical record (N=1081), such that information about the location, provider, and timing was available, the majority (55%) occurred in the inpatient hospital setting. Of first EOL discussions documented in medical records for which provider type was known (N=806), participating providers included medical oncologists (49%), general medical physicians (36%), palliative care physicians (6%), other medical specialists (7%), radiation oncologists (4%), surgeons (3%), and other providers (0.5%). Discussions with oncologists were divided evenly between inpatient and outpatient settings, but discussions with general medical physicians tended to take place in the inpatient setting (73%). Abstracted medical record data were available from visits with medical oncologists for 85% (1823/2155) of patients, with a median of 6 visits (interquartile range 2, 10). However, medical oncologists documented EOL discussions with only 27% of their patients (493/1823).
Table 3.
Details of end of life discussions. Data are given as frequency (percentage.)
| Any source |
Patient or surrogate reported |
Documented in medical record |
|||
|---|---|---|---|---|---|
|
|
|||||
| Number of patients | 1569 | 1270 | 1081 | ||
|
| |||||
| Topic discussed in any end of life discussion † | |||||
| Resuscitation1 | 714 (46) | 364 (29) | 437 (40) | ||
| Hospice | 1291 (82) | 1088 (86) | 786 (73) | ||
| Other EOL topic | |||||
| Palliative care | -- | -- | 143 (13) | ||
| Venue for dying (other than hospice) | -- | -- | 36 (3) | ||
| Other (advanced care planning, NOS) | -- | -- | 77 (7) | ||
|
| |||||
| Venue of first end of life discussion | |||||
| Inpatient | -- | -- | 590 (55) | ||
| Outpatient | -- | -- | 491 (45) | ||
|
| |||||
| Provider for first end of life discussion‡ (n=806) | Overall | Inpatient | Outpatient | ||
| General medicine | -- | -- | 294 (36) | 216 (73) | 78 (27) |
| Medical oncologist | -- | -- | 397 (49) | 200 (50) | 197 (50) |
| Palliative pain management, hospice | -- | -- | 48 (6) | 30 (63) | 18 (38) |
| Other medical specialist (gastroenterologist or pulmonologist) |
-- | -- | 60 (7) | 39 (65) | 21 (35) |
| Radiation oncologist | -- | -- | 30 (4) | 10 (33) | 20 (67) |
| Surgeon | -- | -- | 27 (3) | 17 (63) | 10 (37) |
| Other providers2 | -- | -- | 4 (0.5) | 0 (0) | 4 (100) |
Patients are represented once for each topic discussed.
Available for patients with end of life discussion documented in medical record abstraction, with known provider type. When records indicated that multiple providers were present for the discussion, patients are represented once for each provider type.
546 interviews included the item about resuscitation. Resuscitation was not asked about in the brief baseline interview, the surrogate deceased baseline interviews, or in any follow-up interviews.
Includes “other specialists” and key non-contact referrals.
Among patients with documented EOL discussions who died during the medical record review period (N=959), the first EOL discussion took place a median of 33 days before death (IQR 13, 75 days). Table 4 shows the timing of first EOL discussions relative to death, stratified by survival time. Patients who lived longer were more likely to have had earlier EOL discussions (mean increase in time between discussion and death of 0.29 months for each 1 month increase in survival, 95% CI 0.26, 0.32, P<0.001). The first EOL discussion took place a median of 34 days before death among patients who lived 1-3 months after diagnosis and a median of 69 days before death among patients who lived more than 12 months. The median time between diagnosis and the first EOL discussion also increased with survival time, from 21 days among patients who lived 1 to 3 months after diagnosis, to 353 days among patients who lived more than a year after diagnosis. Overall, among all patients who died during the medical record review period but who lived at least a month after diagnosis (N=943), 16% had no EOL discussions before death, 33% had discussions in the last month of life, and 51% had discussions before the last month of life.
Table 4. Timing of first end of life discussion for patients who died.
Includes only the discussions reported in the medical record abstraction; patients with no documented EOL discussions are excluded.
| Months between diagnosis and death |
N | Days between EOL discussion and death Median (IQR) |
Proportion for whom discussion occurred < 1 month prior to death |
|---|---|---|---|
| <1 | 165 | 14 (7, 23) | N/A |
| 1 to 3 | 258 | 34 (14, 54) | 47 |
| 3 to 6 | 222 | 53 (19, 97) | 34 |
| 6 to 9 | 126 | 47 (16, 162) | 42 |
| 9 to 12 | 99 | 54 (15, 223) | 36 |
| > 12 | 89 | 69 (23, 244) | 29 |
| Overall | 959 | 33 (13, 75) | N/A |
Discussion
We evaluated the incidence, timing, location, and involved providers of EOL discussions between patients with incurable lung or colorectal cancer and their physicians. Overall, most patients had EOL discussions documented in medical records or reported by patients or surrogates, including 87% of patients who died during the study period. In contrast with national guidelines that recommend early EOL discussions, conversations took place a median of about one month before death. Oncologists cared for a majority of the patients, but documented EOL discussions with only 27% of the patients they saw. More than half of EOL discussions took place during acute hospital admissions rather than during periods of stable outpatient care.
Previous literature, based on an English-language MEDLINE search to August 2011, has reported that fewer than 40% of patients with advanced cancer have EOL discussions with their physicians (4, 6). Our study demonstrated nearly twice that rate. Existing work has relied on select populations of patients, such as those who receive care at specific cancer centers and/or patients whose cancer has progressed after initial chemotherapy. Previous work has also evaluated EOL discussions using patient or surrogate reports, without medical record review, and in a cross-sectional manner. CanCORS used population-based sampling in multiple regions and health care systems, a strategy designed to create a cohort more representative of the general population. CanCORS has lower response rates than previous studies, an important limitation, but without biases toward patients who receive care at large centers, who receive cancer-directed therapy, or who live long enough to become eligible for such studies. Longitudinal assessment also enabled us to detect conversations that occurred near death, which accounted for a substantial proportion of EOL discussions in our study.
Previous work has suggested indirectly that discussions occur late in the course of illness, leading, for example, to hospice referrals that occur within days of death (21, 22). Our study shows directly that most EOL conversations begin in the final weeks of life, long after decisions about initial cancer treatments are likely to have taken place. Few patients had documented conversations about palliative care, even though early palliation offers important benefits to patients with incurable lung cancer throughout the disease trajectory (23), including better quality of life and mood and longer survival. Early discussions about EOL care may also help patients with the psychological work of the EOL period, including acceptance of one’s life situation, grief over the losses inherent in death, and growth in relationships (24, 25). Conversations about EOL care may therefore best take place near the time patients are diagnosed with advanced cancer, rather than in the last weeks or days of life.
Existing literature asserts that many physicians avoid EOL discussions until death is imminent (26-29). The late timing of EOL discussions is one possible manifestation of avoidance; our findings suggest other ways that this tendency may play out. Guidelines recommend that EOL discussions take place during periods of relative medical stability (30, 31), but we found that most EOL discussions occurred in the inpatient hospital setting. This finding raises the possibility that acute medical deterioration, and not the diagnosis of incurable cancer, triggers physicians to talk about EOL care. Existing literature has also shown that physicians who have close long-term relationships with patients often wish to avoid EOL discussions (32). We found that oncologists have EOL discussions with only about one-quarter of their patients. Primary care physicians may also have important roles in EOL decision-making, but most discussions with general medicine physicians occurred in the inpatient setting, suggesting that these were hospital-based physicians and not those providing longitudinal primary care. Physicians involved in longitudinal care, however, may be best informed about the patient’s prognosis and disease trajectory and best equipped to have meaningful discussions about the patient’s values and goals.
Our study has several limitations. We focused on whether EOL discussions took place but did not evaluate the depth or quality of discussions. We evaluated a limited number of topics of discussion, primarily hospice and resuscitation, even though EOL discussions may touch on much broader topics. Not all interviews included questions about resuscitation. We also relied on surrogate rather than patient reports of discussions in many cases, even though surrogates may not have been present for all discussions. This issue is especially notable for patients with lung cancer, many of whom died before the baseline interview could be performed.
The concordance rate between patient or surrogate reports and medical record documentation was 65%. The majority of discordant reports reflected reported but not documented discussions. The lower rates of documented EOL discussions could be explained by incomplete retrieval of records, inadequate abstraction of available records, or incomplete documentation of EOL discussions. Our use of combined interview and medical record data was designed to provide the most complete possible assessment, and our finding of nearly twice the rate of EOL discussions as that historically reported strongly supports the sensitivity of our method.
Future studies should consider the content and quality of EOL discussions. Previous work suggests that a complex set of interactions between patients and physicians leads to avoidance of EOL discussions until death is near (26-29); further research should consider reasons for avoidance and ways to facilitate early discussions.
We have reported the incidence, timing, location and involved providers of EOL discussions. Although most patients have conversations with physicians about EOL care before they die, these discussions tend to take place in the hospital, with non-oncology providers, and when death is imminent. Future efforts should focus on ways to initiate these conversations earlier in the disease trajectory and with continuity providers, so that decisions about care of incurable cancer include the full spectrum of options, including palliation.
Acknowledgements
The work of the CanCORS Consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (U01 CA093344) and the NCI supported Primary Data Collection and Research Centers (Dana-Farber Cancer Institute/Cancer Research Network U01 CA093332, Harvard Medical School/Northern California Cancer Center U01 CA093324, RAND/UCLA U01 CA093348, University of Alabama at Birmingham U01 CA093329, University of Iowa U01 CA093339, University of North Carolina U01 CA093326) and by a Department of Veteran’s Affairs grant to the Durham VA Medical Center CRS 02-164. Dr. Mack was funded by an American Cancer Society Mentored Research Scholar Grant and by the National Palliative Care Research Center.
Primary Funding Sources National Cancer Institute and Department of Veteran’s Affairs.
Footnotes
Availability of study materials: Protocol: available on request at https://www.cancors.org/public/pub
Statistical Code: available from Dr. Mack at jennifer_mack@dfci.harvard.edu
Data: not available
References
- 1.National Comprehensive Cancer Network [Accessed 8/30/11];Practice Guidelines in Oncology. Palliative care. http://www.nccn.org/professionals/physicians_gls/PDP/palliative.pdf.
- 2.National Consensus Project for Quality Palliative Care . Clinical Practice Guidelines for Quality Palliative Care. Pittsburgh, PA: [Accessed 8/30/11]. http://www.nationalconsensusproject.org. [Google Scholar]
- 3.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709–14. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 4.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. Jama. 2008;300(14):1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prigerson HG. Determinants of hospice utilization among terminally ill geriatric patients. Home Health Care Serv Q. 1991;12(4):81–112. doi: 10.1300/j027v12n04_07. [DOI] [PubMed] [Google Scholar]
- 6.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–8. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright AA, Keating NL, Balboni TA, Matulonis UA, Block SD, Prigerson HG. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol. 2010;28(29):4457–64. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallston KA, Burger C, Smith RA, Baugher RJ. Comparing the quality of death for hospice and non-hospice cancer patients. Med Care. 1988;26(2):177–82. doi: 10.1097/00005650-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann L, Shaykevich S, Weeks JC. Communication and understanding of prognosis among stage IV lung and GI cancer patients. 2007 ASCO Annual Meeting Proceedings Part I; Jun 20, 2007. p. 9024. 2007. [Google Scholar]
- 10.Jenkins V, Solis-Trapala I, Langridge C, Catt S, Talbot DC, Fallowfield LJ. What oncologists believe they said and what patients believe they heard: an analysis of phase I trial discussions. J Clin Oncol. 2011;29(1):61–8. doi: 10.1200/JCO.2010.30.0814. [DOI] [PubMed] [Google Scholar]
- 11.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22(15):2992–6. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Catalano P, Ayanian JZ, Weeks JC, Kahn K, Landrum MB, Zaslavsky AM, Lee J, Pendergast J. Harrington DP Representativeness of participants in the Cancer Care Outcomes Research and Surveillance Consortium relative to the Surveillance, Epidemiology, and End Results Program. Medical Care. 2011 doi: 10.1097/MLR.0b013e318222a711. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients’ experience and outcomes: development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14(8):837–48. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 14.Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: surveillance, epidemiology, and end results data. Cancer. 2001;92(8):2211–9. doi: 10.1002/1097-0142(20011015)92:8<2211::aid-cncr1565>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Blackstock AW, Herndon JE, 2nd, Paskett ED, et al. Outcomes among African-American/non-African-American patients with advanced non-small-cell lung carcinoma: report from the Cancer and Leukemia Group B. J Natl Cancer Inst. 2002;94(4):284–90. doi: 10.1093/jnci/94.4.284. [DOI] [PubMed] [Google Scholar]
- 16.Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117(5):1239–46. doi: 10.1378/chest.117.5.1239. [DOI] [PubMed] [Google Scholar]
- 17.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–83. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 19.American Association for Public Opinion Research Standard definitions: Final dispositions of case codes and outcome rates for surveys. Accessed at http://www.aapor.org/uploads/Standard_Definitions_04_08_Final.pdf on 8/30/11.
- 20.Keating NL, Beth Landrum M, Arora NK, et al. Cancer patients’ roles in treatment decisions: do characteristics of the decision influence roles? J Clin Oncol. 2010;28(28):4364–70. doi: 10.1200/JCO.2009.26.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315–21. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 22.Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. N Engl J Med. 1996;335(3):172–8. doi: 10.1056/NEJM199607183350306. [DOI] [PubMed] [Google Scholar]
- 23.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 24.Block S. Psychological considerations, growth, and transcendence at the end of life. JAMA. 2001;285:2898–2905. doi: 10.1001/jama.285.22.2898. [DOI] [PubMed] [Google Scholar]
- 25.Lamont EB, Christakis NA. Complexities in prognostication in advanced cancer: “to help them live their lives the way they want to”. JAMA. 2003;290(1):98–104. doi: 10.1001/jama.290.1.98. [DOI] [PubMed] [Google Scholar]
- 26.The AM, Hak T, Koeter G, van Der Wal G. Collusion in doctor-patient communication about imminent death: an ethnographic study.[see comment] BMJ. 2000;321(7273):1376–81. doi: 10.1136/bmj.321.7273.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyaji NT. The power of compassion: truth-telling among American doctors in the care of dying patients. Social Science & Medicine. 1993;36(3):249–64. doi: 10.1016/0277-9536(93)90008-r. [DOI] [PubMed] [Google Scholar]
- 28.Ruddick W. Hope and deception. Bioethics. 1999;13(3-4):343–57. doi: 10.1111/1467-8519.00162. [DOI] [PubMed] [Google Scholar]
- 29.Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Archives of Internal Medicine. 1998;158(21):2389–95. doi: 10.1001/archinte.158.21.2389. [DOI] [PubMed] [Google Scholar]
- 30.Quill TE. Perspectives on care at the close of life. Initiating end-of-life discussions with seriously ill patients: addressing the “elephant in the room”. JAMA. 2000;284(19):2502–7. doi: 10.1001/jama.284.19.2502. [DOI] [PubMed] [Google Scholar]
- 31.Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ. 2005;330(7498):1007–11. doi: 10.1136/bmj.330.7498.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christakis N, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: A prospective cohort study. BMJ. 2000;320:469–72. doi: 10.1136/bmj.320.7233.469. [DOI] [PMC free article] [PubMed] [Google Scholar]