Abstract
The zebrafish (Danio rerio) is a popular vertebrate model for biomedical research. The rapid development, transparency, and experimental accessibility of the embryo offer opportunities for assessing the developmental effects of anticancer treatment strategies. We therefore systematically investigated parameters for growing U251 human glioma cells expressing red fluorescent protein (U251-RFP) in zebrafish embryos. Factors optimized include injection volume, number of cells injected, anatomic site of injection, age of the embryo at the time of injection, and postinjection incubation temperature. After injection into the embryos, the U251-RFP cells proliferated and the resultant tumors, and even individual cells, could be visualized in real-time via fluorescence microscopy without the need for sacrifice. These tumors recruited host zebrafish vasculature, suggesting cancer cell–host tissue interactions. Having optimized parameters for introducing and growing these human cells in the zebrafish embryos, we exposed both embryos and transplanted cancer cells to ionizing radiation and temozolomide, either alone or in combination. The human tumors in each embryo were substantially diminished following exposure to ionizing radiation and the decrease was further enhanced by pretreatment with temozolomide. In contrast, temozolomide had no discernible effects on embryonic development. These results together support the relative safety of temozolomide during embryonic development, as well as its anticancer efficacy when combined with radiation. These results suggest the value of the zebrafish model for in vivo testing of the efficacy and safety of anticancer strategies, especially on the very young.
Introduction
Glioblastoma multiforme (GBM), the most common primary adult brain tumor, portends a poor prognosis for most patients. The median survival for patients with glioblastomas treated with the current standard of care remains less than 1 year (1). Glioblastoma multiforme is considered to be a relatively radioresistant malignancy, in part due to activation of the phosphoinositide 3-kinase signaling pathway (2, 3). Temozolomide (Temodar) is a DNA-methylating agent, with activity as monotherapy for the treatment of malignant gliomas (4–8). The cytotoxicity of temozolomide has been attributed to the perturbation of DNA repair through methylation of the O6 position of guanine (9). During replication, the O6 methylguanine incorrectly pairs with thymine, triggering the mismatch repair system (9). Repair of the mismatched bases leads to the preferential reinsertion of thymine, which is thought to cause repetitive and futile repair attempts leading to the generation of DNA strand breaks and, eventually, growth arrest and apoptosis (10). More encouragingly, temozolomide has been shown to improve treatment response and overall survival when combined with radiation therapy for treating adult glioblastoma multiforme (8). In contrast, data for combining temozolomide and radiation therapy for treating glioblastoma multiforme in the young remains sparse, including whether the combined treatment affects in utero development (11, 12). The availability of a vertebrate model system to help clarify such urgent questions would be welcome.
The zebrafish (Danio rerio) has attracted considerable attention in recent years as a model system for biomedical research because of compelling advantages such as high levels of physiologic and genetic homology with higher vertebrates such as mammals (13, 14). The aqueous environment of the zebrafish facilitates drug as well as studies using ionizing radiation (IR; immersion in water ensures radiation dose homogeneity), and the transparency of the progeny allows easy visualization of internal structures, including organs. Finally, rapid embryonic development facilitates investigations of effects on development. Recent reports have described the effects of radiation on viability and development in early zebrafish embryos, confirming anatomic effects similar to that described for humans (15, 16) and showed the usefulness of the model in delineating the toxicity of novel agents (17). The growth and interactions with the host tissues of human melanoma cells transplanted into zebrafish has been described (18–20). However, whether the zebrafish would be useful for studying the effects of the interactions of standard anticancer treatments on tumor growth, to our knowledge, has not been previously studied.
We therefore investigated the utility of the zebrafish embryonic system for testing the effects of radiation and chemotherapy on human glioblastoma cells by establishing parameters for the transplantation of U251 human glioma cells into zebrafish embryos. The transplanted human cancer cells recruited vasculature from the zebrafish host, confirming tumor effects on the tissue microenvironment. We determined that IR leads to dose-dependent suppression of the growth of human glioma cells, which was augmented by temozolomide. The radiosensitization of the human cancer cells by temozolomide was not accompanied by deleterious effects on development. These results together suggest the efficacy and safety of combined temozolomide and radiation, and further support the usefulness of zebrafish as a model system for studying the efficacy and safety of anticancer strategies.
Materials and Methods
Animal care
Zebrafish were raised as previously described (15) and all procedures, including embryo collection and handling, were done in accordance with accepted standard operating procedures (21) approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania School of Medicine. Staging of the zebrafish embryos and larvae were performed as described by Kimmel et al. (22). The age of embryos is indicated throughout the manuscript as hours postfertilization (hpf) and days postfertilization (dpf) for all experimental data shown. Embryos were maintained at 30°C after transplantation procedures. Embryos raised beyond 24 hpf were treated with phenylthiourea (0.003%, w/v; Sigma) to prevent melanization. For each experiment, embryos containing the transplanted human cancer cells were then assessed daily through at least 9 dpf before the experiments concluded and animals were euthanized through exposure to MESAB (0.5 mmol/L 3-aminobenzoic acid ethyl ester, 2 mmol/L Na2HPO; Sigma) at the conclusion of the experimentation. Experiments involving visualization of zebrafish endothelium involved transgenic zebrafish embryos (fli1:EGFP; ref. 23) but husbandry protocols used were otherwise identical.
Labeling and characterization of the U251 human glioma cell line
U251 malignant glioma cells were purchased from the American Type Culture Collection and stably transfected with a RFP construct (pDsRed2-C1; Clontech). The expression of RFP did not affect the radiosensitivity of the cells (17), and the fluorescence emitted was linearly proportional to the number of cells, including those growing as tumor masses (data not shown). Cells were grown in DMEM (Life Technologies, Inc.) containing 10% fetal bovine serum (Life Technologies) and 0.4 mg/mL G418 (Life Technologies) and maintained in an incubator with 5% carbon dioxide and 21% oxygen. The clonogenicity of U251-RFP cells at 30°C and 37°C were established according to previously published methods (24). The radiosensitivity at these two temperatures was assessed using survival curves, and was found to be similar (Supplementary Fig. S1). Cells were plated at a density of 104 in 35-mm-diameter tissue culture plates containing complete medium over soft agar. On dpf 2, 7, and 14, phase contrast images of colonies were taken using a Nikon TE-200 microscope equipped with epifluorescence optics.
Preparation of cells for transplantation
Human U251-RFP cells were grown in cell culture to a confluence of ~50% before trypsinization and resuspension in HBSS (Invitrogen Life Technologies) at a concentration of 107 cells/mL. All cells were then strained and sorted via a fluorescence activated cell sorter (Becton Dickinson FACSCalibur). Fluorescence was measured by exciting cells at 594 nm and fluorescence-emitting cells were collected in a fresh, sterile container. Cells prepared using this procedure were ≥95% viable (as assessed by trypan blue exclusion) and fluorescent (as confirmed by fluorescence microscopy).
Transplantation procedure
The cell transplantation protocol was modified from the protocol described by Lee et al. (19). Briefly, needles were pulled using a P-97 Flaming/Brown Micropipette Puller (Sutter Instrument Co.). A Nanoject II microinjector (Drummond Scientific) was used to transplant 50 to 200 cells into chorionated blastula-stage zebrafish embryos at the oblong to sphere stage (~3.5–4.5 hpf) into the center of the embryonic yolk sac unless otherwise specified. After injection, embryos were maintained for 1 hour at 28°C before incubation at 31°C. At 1 dpf, transplanted embryos were examined by fluorescence microscopy to select for embryos that were morphologically normal and bearing a RFP-expressing U251 cell mass before manual dechorionation and placement into individual wells in standard 48-well polystyrene tissue culture plates (Costar). The embryonic yolk sac was the preferred injection target site because it offered an easily accessible target in young embryos that was associated with a high rate of embryo survival after microinjection of cells.
Imaging and analysis of tumor volumes and emitted fluorescence
Acquisition of images was performed as previously described (15). Following transplantation of the U251-RFP cells, embryos were serially examined under a ×100 PlanNeofluor objective mounted on a Nikon TE-200 microscope equipped with epifluorescence optics. The embryos of all treatment groups were handled identically and exposure to incidental light was minimized. Both bright field and fluorescent images were captured with a Hammamatsu CCD camera controlled with IP LabSpectrum v2.0.1 software (Scanalytics, Inc.). Images taken in the same focal plane in bright field and in transmitted light passing through RFP filters were merged via Adobe Photoshop CS2 (Adobe). The emitted fluorescent signal was analyzed via Kodak Molecular Imaging software v. 4.0.5 (Kodak) and found to be proportional to the number of viable cancer cells. Tumor size measurements were performed via manually outlining the area represented by tumor at the plane of imaging that shows the greatest dimensions of each tumor. This was designated as the region of interest (ROI) with the area represented by the ROI calculated by software.
Embryo irradiation and exposure to drug
Treatment (irradiation and exposure to temozolomide) of embryos was performed according to protocols approved by the Department of Environmental Health and Radiation Safety at the University of Pennsylvania. Embryos were either irradiated at 10 Gy or mock-irradiated as previously described (15). Embryos at 1 dpf were exposed to single fractions of 640 kVp γ-irradiation at room temperature using a J.L. Shepherd Mark I 137Cs irradiator. Temozolomide (Temodar, Schering Corp.) was dissolved in 10% DMSO and embryos were exposed to temozolomide for 12 hours at a final concentration of 100 μmol/L in embryo medium, which did not appreciably affect normal zebrafish embryonic development, before either irradiation or mock-irradiation at 1 dpf. All results are presented as arithmetic mean ± SE. Statistical analysis was performed using a Student’s t test.
Results
Optimizing parameters for transplanting human malignant glioma cells into zebrafish embryos
Recent success with growing human cells in zebrafish embryos (18, 20, 25) encouraged us to explore the possibility of developing a similar system using human glioma cells. To distinguish these cells against the background of zebrafish tissues, we prepared U251 cells stably expressing RFP. The expression of fluorescent protein did not significantly affect the radiosensitivity of the cells, which is consistent with results described for other human glioma cell lines expressing fluorescent protein (data not shown and ref. 26). We began by establishing parameters for optimally growing these cells, testing factors including final injection volume, number of U251-RFP cells injected per embryo, the anatomic site of injection, age of the embryo at transplantation, and incubation temperature. Having optimized these factors, we focused next on the microinjection location and first attempted to assess whether the U251-RFP cells could be successfully injected into and grow within the intracranial region of young embryos. We were able to successfully inject U251-RFP cells into the cranial junction of the animal pole and yolk sac of 2-day-old (2 dpf) embryos, which grew into a tumor mass that continued to grow throughout the duration of the experiment (to 7 days postfertilization; Supplementary Fig. S2A–C). However, microinjection into the cell mass was associated with a high embryo mortality rate (over 40% within 24 hours) even when the injected volume was reduced. In contrast, microinjecting into the yolk sac was associated with lower mortality rates of ~10% to 20%. We note that Lee et al. (19) have hypothesized that another potential advantage of injecting human cancer cells into the egg yolk may be less susceptibility to tissue microenvironment signaling and which may otherwise alter the cancer phenotype independently of cytotoxic agents.
The final factor assessed was the ideal post-microinjection temperature for the human cancer cells. Human cells are typically incubated at 37°C, reflecting human body temperature. Wild zebrafish live in tropical environments typically cooler than human body temperature; that is, the embryos develop best at temperatures below 30°C. Given this temperature differential, we first established that our U251-RFP cells in cell culture were able to proliferate and form colonies despite being maintained for long periods of time at temperatures ranging from 28°C to 31°C (Supplementary Fig. S2D–F), results consistent with that observed for the melanoma cell line C8161 by Lee et al. (19). To formally test whether a range of incubation temperature may affect the growth of the U251-RFP, we injected these cells into zebrafish embryos and subsequently maintained the embryos at 28°C, 30°C, 32°C, or 34°C. We found that the embryos survived best at ≤32°C, whereas there were no significant differences in the survival of the U251-RFP cells maintained at any of temperatures above 28°C. There were no significant differences in the radiosensitivity of U251-RFP cells at 30°C compared with 37°C (Supplementary Fig. S1). These results suggest that the U251-RFP cells can tolerate and proliferate within a range of temperatures that include those suitable for the zebrafish host.
Durability of human glioma cells proliferating in the zebrafish embryonic host: tracking individual cells
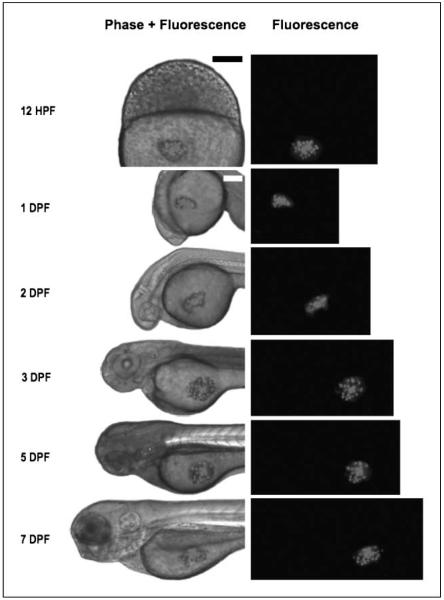
Having optimized the parameters for microinjecting and detecting U251-RFP cells in the zebrafish embryos, we wished to document the proliferation and durability of the injected human cells over a longer interval. Many experiments involving zebrafish embryos conclude at 7 days postfertilization, because the yolk sac provides sufficient nutrition during this interval such that supplemental feeding is not required. Because of these considerations, we sought to confirm that the human glioma cells survive to at least 7 dpf in the zebrafish embryonic host. We found that the transplanted human cells survived and proliferated within the embryo over 7 days, throughout the duration of the experiment (serial imaging of representative embryos containing transplanted U251-RFP cells is shown in Fig. 1). It was also notable that the presence of the transplanted human cells did not seem to jeopardize the normal phenotypic development of the embryo during the time frame of the experiment. This would be seem to be analogous to a xenografted tumor in a mouse not impairing its ability of the mouse to thrive, whereas the tumor remains small, which then allows the effects of treatment on the tumor to be investigated.
Figure 1.
Persistence of human malignant glioma cells within, and lack of developmental effects on, the developing zebrafish embryo. U251-RFP cells growing within the developing zebrafish embryo for at least 7 d at 30°C do not perturb development. Lateral views of a representative zebrafish embryo after transplantation of U251-RFP human glioma cells, imaged under fluorescence alone (right column images) or merged bright field and fluorescent images (left column images). Leftmost column of text, embryonic age at time of imaging. 12 hpf, embryo imaged at 2 h after transplantation. Bar, 300 μm. The remainder of embryos were imaged at 1, 2, 3, 5, or 7 dpf. Bar, 200 μm.
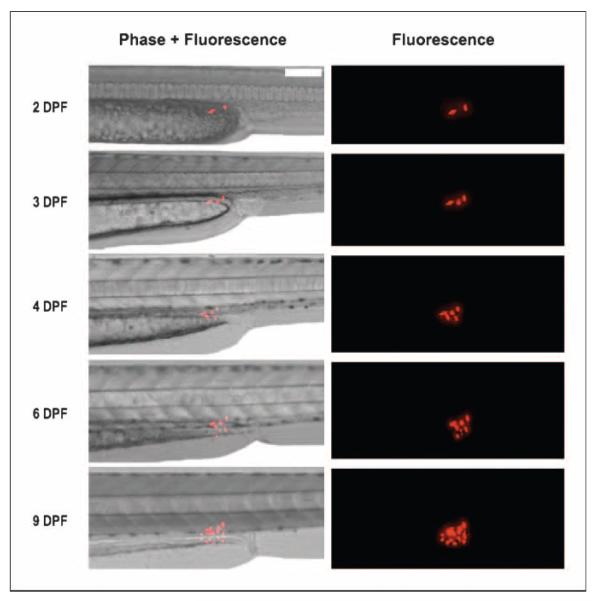
A compelling advantage of the zebrafish embryonic system is that it is transparent throughout the embryonic period. The optical clarity of the zebrafish therefore allows the tracking of individual human cancer cells. For example, in the experiment shown in Fig. 2, what seem to be two U251-RFP cells were injected into the zebrafish embryo at 2 dpf (Fig. 2, 2 dpf). These two cells were then observed to divide and proliferate over successive days, as far as out as 9 dpf (Fig. 2, 9 dpf). For example, from the initial two cells injected at 2 dpf, three cells could be observed by 3 dpf, and then five cells by 4 dpf (Fig. 2, 3 dpf and 4 dpf). These experiments together therefore indicate the usefulness of the zebrafish embryo for growing and tracking human glioma cancer cells, either individually or as a tumor mass.
Figure 2.
Visualization of individual human glioma cells proliferating within a live zebrafish embryo. Lateral views of a single, live zebrafish embryo over a 9-d period following transplantation of individual U251-RFP cells. At baseline (2 dpf), two individual U251-RFP cells are observed. At 3 dpf, three individual U251-RFP cells observed. At 4 dpf, five individual U251-RFP cells are noted. At 6 dpf, at least seven individual U251-RFP cells are notable. At 9 dpf, there is a further increase in the number of U251-RFP cells. Scale bar, 200 μm.
Human glioma cells implanted within zebrafish embryos recruit blood vessels from the host tissues
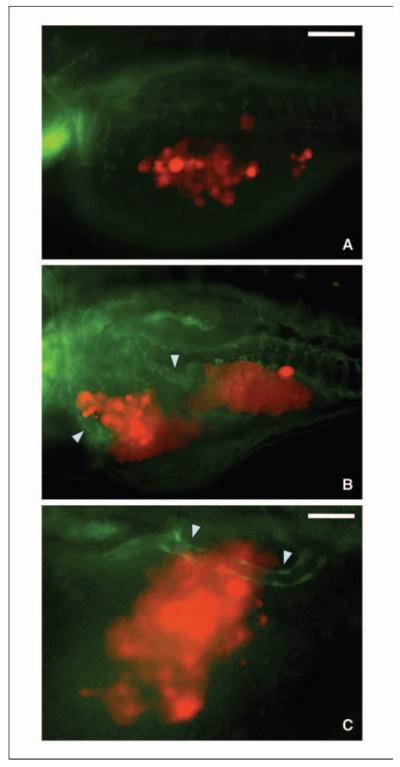
The proliferation and durability of the injected human glioma cells within the embryos suggested that the human cells were deriving sufficient “nutrition” from the zebrafish host tissues. Although individual cells may be able to do so via passive diffusion, larger tumor masses may need to recruit blood vessels or stimulate de novo angiogenesis. The availability of a transgenic zebrafish strain that has its vasculature outlined with green fluorescent protein (fli1:EGFP) has facilitated investigations of blood vessel development (23). We therefore microinjected U251-RFP cells into fli1:EGFP embryos. The human glioma cells were injected into the yolk sac and were located away from the developing cell mass. Therefore, the human tumor mass was initially distinct from the developing zebrafish endothelium (Fig. 3A). Over time, however, aspects of the developing zebrafish vasculature were noted to extend toward and directly contact the human tumor mass (Fig. 3B–C). These results suggest that human glioma cells may be able to stimulate angiogenesis and recruit blood vessels from the zebrafish host tissues, and which in turn may support the continuing growth of the tumor. These results therefore corroborate previous reports of the stimulation of de novo angiogenesis in zebrafish embryos by nonglioma cancer cells (18–20).
Figure 3.
Visualization of angiogenesis within zebrafish embryos following transplantation of human glioma cells. A to C, lateral views of live zebrafish embryos after transplantation of U251-RFP cells in fli1:EGFP zebrafish. A, representative zebrafish at 2 dpf showing transplanted human glioma cells (RFP) located separately from the zebrafish endothelium (GFP). B, a representative zebrafish at 5 dpf showing the developing zebrafish endothelium, with portions of the vasculature approaching the U251-RFP tumor cell masses (arrowheads). Scale bar for A and B, 50 μm. C, an image at higher magnification showing a single distinct GFP-expressing zebrafish blood vessel growing into a U251-RFP tumor cell mass. Scale bar, 10 μm in C.
Exposure to temozolomide does not appreciably affect embryonic development
To investigate the effects of temozolomide on injected human cancer cells, we first established the effects of temozolomide on zebrafish embryonic development. We assessed the survival and morphology of embryos treated with temozolomide alone compared with control embryos exposed to vehicle only (DMSO; neither group was exposed to IR). Embryos in the experimental group were exposed to temozolomide for 12 hours at a final concentration of 100 μmol/L in embryo medium, and, following treatment, the embryos were observed throughout the first 7 dpf of life for survival and morphology. We found that the survival of temozolomide-treated embryos was not significantly different from control embryos. Specifically, the survival of embryos treated with temozolomide (n = 250) at 24, 48, 120, and 168 hours after treatment were 93.0 ± 4.6%, 90.1 ± 3.9%, 88.2 ± 4.9%, and 86.3 ± 5.1%, respectively. The survival of control embryos (n = 260) during the identical time periods were 94.2 ± 6.1%, 91.3 ± 4.7%, 89.1 ± 3.9%, and 87.3 ± 5.2. There were also no appreciable morphologic effects when the control group was compared with the temozolomide group (representative treated or control embryos at the 2 and 5 dpf stages are shown in Supplementary Fig. S3A–B).
Ionizing radiation decreases the survival of human glioma cells growing within zebrafish embryos
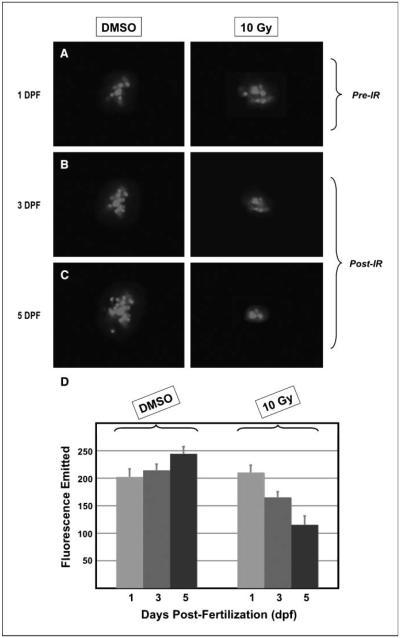
We have previously established parameters for testing the effects of IR on zebrafish embryos (15). This allowed us to determine that the ideal stage to test the relative effects of IR on transplanted tumor cells would be at 1 dpf. At that age, most of the embryos tolerate doses of radiation that are in the therapeutic range for human cancer cells without detectable normal tissue toxicity. Embryos containing U251:RFP tumors were therefore either mock–irradiated or irradiated with 10 Gy at 1 dpf. Embryonic transparency permitted tracking tumor growth via serial microscopy and the ability to measure the fluorescence emitted by the RFP-expressing human cells within the tumor. Images of representative control (DMSO) and irradiated (10 Gy) embryos are shown in Fig. 4A to C, with embryos imaged immediately before IR at 1 dpf (Pre-IR, Fig. 4A) and then after IR at 3 and 5 dpf (Post-IR, Fig. 4B and C). The fluorescence emitted by each group of tumors growing within the embryos was measured and quantitated (Fig. 4D). We found that within mock-irradiated control embryos, the emitted fluorescence increased over time, in accordance with continuing proliferation of the human glioma cells and the visibly increased size of the tumor mass. In contrast, the emitted fluorescence of the tumors in the irradiated group (10 Gy) decreased over this period, which was in accordance with the visibly reduced size of the irradiated tumors, suggesting that the IR led to the death of at least a proportion of the injected U251:RFP cells (Fig. 4D). These results together indicate that exposure to IR inhibited the growth and survival of human glioma cells growing within zebrafish embryos. Furthermore, the inhibitory effects of IR on the human cancer cells could be detected visually as well as measured by the fluorescence emitted by surviving cells.
Figure 4.
Exposure to IR reduces the size and fluorescence emitted by human glioma cells growing in zebrafish embryos. Embryos containing U251:RFP tumors were exposed to DMSO (as in Fig. 5) or irradiated with 10 Gy and imaged on sequential days. A to C, images taken under fluorescence of representative zebrafish embryos after transplantation of U251:RFP cells, and imaged immediately before exposure to DMSO (left column) or 10 Gy irradiation (right column) at 1 dpf (A), 3 dpf (B), or 5 dpf (C). The tumor masses in the embryos treated with DMSO have visibly grown in size at both 3 and 5 dpf, while the tumor masses in the irradiated embryo show regression in size at both time points. D, the fluorescence emitted by the tumor masses of either treatment group was measured at all three time points, and the mean levels of emitted fluorescence are plotted in the histograms in arbitrary fluorescence units (bars, SE). These data were derived from three replicate experiments encompassing the following numbers of embryos: n = 22 for the “DMSO” group and n = 18 for the “10 Gy” group.
Irradiation in the presence of temozolomide results in the greatest inhibition of human glioma cells growing within zebrafish embryos
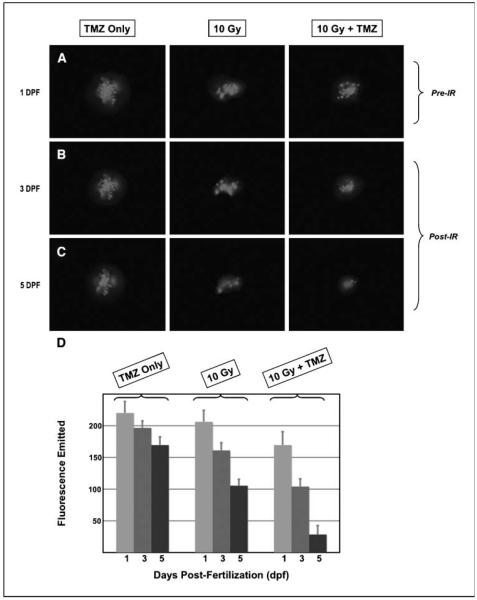
We next tested whether temozolomide might contribute to the effects of IR on the human glioma cells growing within embryos. In these experiments, embryos containing U251:RFP tumors were irradiated at 1 dpf in the presence or absence of temozolomide. The embryos were serially imaged and the size of each tumor was measured. The effects of temozolomide combined with IR were also evident in the measured fluorescence emitted by the tumors growing within the embryos. Images of representative embryos treated either with temozolomide only (TMZ only), with only IR (10 Gy), or with IR in the presence of temozolomide (10 Gy + TMZ) are shown in Fig. 5A to C. The fluorescence emitted by each tumor mass was measured immediately before irradiation as well as on subsequent days. The embryos were imaged immediately before IR (Pre-IR) and then at 3 and 5 dpf; that is, 2 and 4 days after IR (Post-IR). The fluorescence emitted by each tumor mass was quantitated at each of these times (Fig. 5D). As in previous experiments, the IR resulted in serial decreases in the amount of emitted fluorescence (compare fluorescence at days 3 and 5 with day 1 in embryos irradiated with 10 Gy; middle portion of the histogram in Fig. 5D). Temozolomide alone had only a minor effect on the emitted fluorescence of the tumor mass (left portion of the histogram). In contrast, combined temozolomide followed by IR resulted in the greatest decreases in the fluorescence emitted by the human tumor cells (right portions of Fig. 5A–C, and the histogram in Fig. 5D). Of note, only the combined IR and temozolomide resulted in complete eradication of tumor masses in some of the embryos (21% of the embryos).
Figure 5.
Combined temozolomide and IR results in the greatest inhibition of tumor growth and fluorescence emitted from human malignant glioma cells grown in zebrafish embryos. Embryos containing U251:RFP tumors were exposed to temozolomide alone (TMZ Only), irradiated with 10 Gy, or temozolomide followed by 10 Gy IR (10 Gy and 10 Gy + TMZ, respectively) and imaged on sequential days. A to C, images taken under fluorescence of representative zebrafish embryos at 1 dpf (A), 3 dpf (B), or 5 dpf (C). D, the fluorescence emitted by the tumor masses of each treatment group was measured at all three time points. Columns, mean levels of emitted fluorescence in arbitrary fluorescence units; bars, SE. These data were derived from three replicate experiments encompassing the following numbers of embryos: n = 16 for the “TMZ only” group, n = 18 for the “10 Gy” group, and n = 19 for the “10 Gy + TMZ” group.
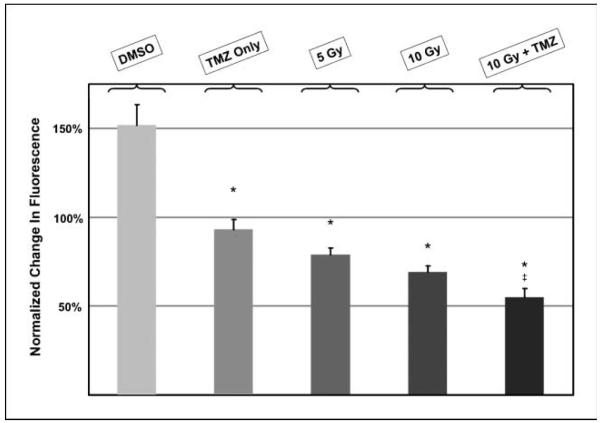
These results encouraged us to expand the treatment groups. We repeated the experiments, with the additional control of embryos with U251:RFP tumors exposed only to DMSO, as well as other embryos with tumors that were exposed to irradiation with a lower dose of irradiation. The fluorescence emitted by tumors growing within control embryos exposed to only DMSO continued to increase so that by day 5 after fertilization (5 dpf), the fluorescence was ~50% greater than at the start of the experiment (first column in the histogram shown in Fig. 6). The tumors in embryos exposed to temozolomide in contrast alone did not grow, and so by the end of the experiment showed significantly less fluorescence than control tumors. Tumors that had been irradiated with either 5 or 10 Gy were visibly smaller and showed significantly less fluorescence as well (as indicated by the third and fourth columns, compared with the first column, in the histogram shown in Fig. 6). Finally, of all the treatments, the greatest degree of tumor regression, and consequently the greatest decrease in emitted fluorescence, was noted in embryos exposed to temozolomide followed by IR (compare last two columns in the histogram in Fig. 6).
Figure 6.
Comparison of the effects of treatments on the cumulative changes in fluorescence emitted by human malignant glioma cells in zebrafish embryos. The cumulative changes in the mean fluorescence emitted by U251-RFP tumor masses in each treatment group between the beginning of the experiment at 24 hpf and the conclusion at 120 hpf. The treatment groups included embryos treated with DMSO or temozolomide alone (TMZ Only), 5 Gy and 10 Gy IR, and temozolomide combined with 10 Gy IR (10 Gy + TMZ). Columns, mean percentage changes in each treatment group; bars, SE. The values were determined from three replicate experiments involving a total of 93 embryos. *, P < 0.001 compared with embryos treated with DMSO only. ‡, P < 0.05 compared with embryos treated with 10 Gy.
In summary, these results together indicate that that irradiation plus temozolomide leads to a significantly greater degree of cell death in the human glioma cells growing as tumor masses in zebrafish embryos compared with those treated with either temozolomide or irradiation alone. In addition, the transparency (i.e., “optical clarity”) of the zebrafish allows the response of the human cancer cells to treatment can be detected visually as well as via measurement of emitted fluorescence.
Discussion
The zebrafish has been established as a vertebrate model system useful for studying human diseases (27, 28). The considerable physiologic and genetic homology of zebrafish to higher-level vertebrates implies that discoveries would have a high probability of relevance to mammals including humans. The rapid development of zebrafish embryos, which allows visualization of major organs in the first few days of life, greatly facilitates experimental throughput. The logistics of care tend to be economic and relatively simple. The permeability and aqueous environment of the embryos render studies of the effects of small molecules practical. Finally, the small size of embryos renders the zebrafish amenable to microplate-based studies, and therefore potentially suitable for high-throughput screening for discovering novel molecules with useful translational properties.
Extending the power of this vertebrate model system, recent reports have shown the feasibility of transplanting cancer cells into zebrafish embryos to study tumor proliferation and growth mechanisms (18–20). In addition to the advantages discussed in the preceding paragraph, the zebrafish embryo lacks antitumor immunity and so will not reject transplanted human tumor cells, in part because the T-cell receptor a gene is not expressed in extrathymic sites before 9 dpf (29, 30). The suitability of tumor xenografts in zebrafish embryos as a system that usefully models the behavior of mammalian tumors and tumor interactions within the host microenvironment has been further highlighted by evidence that tumor xenografts induce angiogenesis in the zebrafish embryonic host (18, 20).
High-grade gliomas are rapidly progressive brain tumors that portend a uniformly grim outcome in diagnosed patients, both in adults and in the young (9). Effective and safe new treatments are needed. Model systems that facilitate investigations of the efficacy and safety of anticancer agents on human glioma cells would aid the development of such novel treatment strategies and paradigms. Although previous efforts have described the usefulness of zebrafish as a model system for studying the developmental effects of radiation therapy (15, 31, 32), in this work, we have extended the usefulness of zebrafish for studying the antitumor efficacy and normal tissue safety of conventional anticancer treatments. The permeability of the embryo and the aqueous environment allows the testing of the effects of combined radiation and chemotherapy on transplanted human tumor cells. The transparency of the zebrafish facilitates detection and serial monitoring of the resultant tumor masses, and even of individual cancer cells, before and after treatment. Because of the resolution that this system permits, the effects of treatment are readily detectable within days following treatment. These features of this model system in turn should therefore allow increased experimental throughout.
One can envision further applications and potential implications of the model system we have described. This system may be useful in testing the efficacy of combining temozolomide with radiation therapy or other systemic agents for human solid malignancies, while simultaneously assessing for potential effects on normal tissue function or development. This system allows testing potential bystander effects that may influence the radiosensitivity of cancer cells (33). Recent biological-based targeting agents have been proposed for treating gliomas (34); the effects of these novel agents on development and efficacy against human cancer cells could be efficiently evaluated in the zebrafish embryonic system we describe. Such pilot investigations could include the testing of the efficacy and safety of sequencing or combining specific novel agents with standard anticancer treatment. This system therefore serves as a potential bridge to more resource- and time-intensive studies such as in mice or other higher order mammals, and ultimately to clinical trials.
Supplementary Material
Acknowledgments
Grant support: Office of Research and Development Medical Research Service; Department of Veterans Affairs (Advanced Career Research Award); NIH grants CA107956, P01CA075138, and 1R21NS061737; and Radiation Biology training grant C5T32CA009677 (G.A. Geiger).
We thank the other members of the Kao Laboratory for expert assistance, especially Melissa Dowling, Frances Lee, Stephanie Yee, and Kaitrin Baloue; Dr. Ann Kennedy for support for G.A. Geiger; Drs. Steve Hahn and Constantinos Koumenis, whose support helped make this research possible; and Dr. Michael Pack (Division of Gastroenterology, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA) for the generous gifts of materials and fli1:EGFP zebrafish.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarti A, Dicker A, Mehta M. The contribution of epidermal growth factor receptor (EGFR) signaling pathway to radioresistance in human gliomas: a review of preclinical and correlative clinical data. Int J Radiat Oncol Biol Phys. 2004;58:927–31. doi: 10.1016/j.ijrobp.2003.09.092. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura JL, Karlsson A, Arvold ND, et al. PKB/Akt mediates radiosensitization by the signaling inhibitor LY294002 in human malignant gliomas. J Neurooncol. 2005;71:215–22. doi: 10.1007/s11060-004-1718-y. [DOI] [PubMed] [Google Scholar]
- 4.Athanassiou H, Synodinou M, Maragoudakis E, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23:2372–7. doi: 10.1200/JCO.2005.00.331. [DOI] [PubMed] [Google Scholar]
- 5.Brandes AA, Vastola F, Basso U, et al. A prospective study on glioblastoma in the elderly. Cancer. 2003;97:657–62. doi: 10.1002/cncr.11097. [DOI] [PubMed] [Google Scholar]
- 6.Chinot OL, Barrie M, Frauger E, et al. Phase II study of temozolomide without radiotherapy in newly diagnosed glioblastoma multiforme in an elderly populations. Cancer. 2004;100:2208–14. doi: 10.1002/cncr.20224. [DOI] [PubMed] [Google Scholar]
- 7.Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24:2563–9. doi: 10.1200/JCO.2005.04.5963. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 9.Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5:144–51. doi: 10.1634/theoncologist.5-2-144. [DOI] [PubMed] [Google Scholar]
- 10.Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23:35–61. doi: 10.1016/s0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 11.Barone G, Maurizi P, Tamburrini G, Riccardi R. Role of temozolomide in pediatric brain tumors. Childs Nerv Syst. 2006;22:652–61. doi: 10.1007/s00381-006-0081-z. [DOI] [PubMed] [Google Scholar]
- 12.Burzynski SR. Treatments for astrocytic tumors in children: current and emerging strategies. Paediatr Drugs. 2006;8:167–78. doi: 10.2165/00148581-200608030-00003. [DOI] [PubMed] [Google Scholar]
- 13.Jesuthasan S. Genetics and development. Zebrafish in the spotlight. Science. 2002;297:1484–5. doi: 10.1126/science.1076115. [DOI] [PubMed] [Google Scholar]
- 14.Fishman MC. Genomics. Zebrafish—the canonical vertebrate. Science. 2001;294:1290–1. doi: 10.1126/science.1066652. [DOI] [PubMed] [Google Scholar]
- 15.Geiger GA, Parker SE, Beothy AP, Tucker JA, Mullins MC, Kao GD. Zebrafish as a “biosensor”? Effects of ionizing radiation and amifostine on embryonic viability and development. Cancer Res. 2006;66:8172–81. doi: 10.1158/0008-5472.CAN-06-0466. [DOI] [PubMed] [Google Scholar]
- 16.Bladen CL, Flowers MA, Miyake K, et al. Quantification of ionizing radiation-induced cell death in situ in a vertebrate embryo. Radiat Res. 2007;168:149–57. doi: 10.1667/RR0803.1. [DOI] [PubMed] [Google Scholar]
- 17.Lally BE, Geiger GA, Kridel S, et al. Identification and biological evaluation of a novel and potent small molecule radiation sensitizer via an unbiased screen of a chemical library. Cancer Res. 2007;67:8791–9. doi: 10.1158/0008-5472.CAN-07-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haldi M, Ton C, Seng WL, McGrath P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis. 2006;9:139–51. doi: 10.1007/s10456-006-9040-2. [DOI] [PubMed] [Google Scholar]
- 19.Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233:1560–70. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- 20.Nicoli S, Ribatti D, Cotelli F, Presta M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007;67:2927–31. doi: 10.1158/0008-5472.CAN-06-4268. [DOI] [PubMed] [Google Scholar]
- 21.Westerfield M. A Guide for the laboratory use of zebrafish Danio (Brachydanio) rerio. University of Oregon Press; Eugene (OR): 1995. [Google Scholar]
- 22.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 23.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–18. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 24.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–3. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 25.Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci U S A. 2007;104:17406–11. doi: 10.1073/pnas.0703446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown CK, Khodarev NN, Yu J, et al. Glioblastoma cells block radiation-induced programmed cell death of endothelial cells. FEBS Lett. 2004;565:167–70. doi: 10.1016/j.febslet.2004.03.099. [DOI] [PubMed] [Google Scholar]
- 27.Amatruda JF, Shepard JL, Stern HM, Zon LI. Zebrafish as a cancer model system. Cancer Cell. 2002;1:229–31. doi: 10.1016/s1535-6108(02)00052-1. [DOI] [PubMed] [Google Scholar]
- 28.Stern HM, Zon LI. Cancer genetics and drug discovery in the zebrafish. Nat Rev Cancer. 2003;3:533–9. doi: 10.1038/nrc1126. [DOI] [PubMed] [Google Scholar]
- 29.Danilova N, Hohman VS, Sacher F, Ota T, Willett CE, Steiner LA. T cells and the thymus in developing zebrafish. Dev Comp Immunol. 2004;28:755–67. doi: 10.1016/j.dci.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Willett CE, Zapata AG, Hopkins N, Steiner LA. Expression of zebrafish rag genes during early development identifies the thymus. Dev Biol. 1997;182:331–41. doi: 10.1006/dbio.1996.8446. [DOI] [PubMed] [Google Scholar]
- 31.Bladen CL, Lam WK, Dynan WS, Kozlowski DJ. DNA damage response and Ku80 function in the vertebrate embryo. Nucleic Acids Res. 2005;33:3002–10. doi: 10.1093/nar/gki613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAleer MF, Davidson C, Davidson WR, et al. Novel use of zebrafish as a vertebrate model to screen radiation protectors and sensitizers. Int J Radiat Oncol Biol Phys. 2005;61:10–3. doi: 10.1016/j.ijrobp.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 33.Mothersill C, Smith RW, Agnihotri N, Seymour CB. Characterization of a radiation-induced stress response communicated in vivo between zebrafish. Environ Sci Technol. 2007;41:3382–7. doi: 10.1021/es062978n. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Batt D, Warmuth M. B-Raf kinase inhibitors for cancer treatment. Curr Opin Investig Drugs. 2007;8:452–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.