Abstract
Current dogma is that meaningful recovery of function after spinal cord injury (SCI) likely will require a combination of therapeutic interventions comprised of regenerative/neuroprotective transplants, addition of neurotrophic factors, elimination of inhibitory molecules, functional sensorimotor training, and/or stimulation of paralyzed muscles or spinal circuits. We routinely use: (1) peripheral nerve grafts to support and direct axonal regeneration across an incomplete cervical or a complete thoracic transection injury, (2) matrix modulation with chondroitinase (ChABC) to facilitate axonal extension beyond the distal graft-spinal cord interface and (3) exercise, such as forced wheel walking, bicycling or step training on a treadmill. We (and others) have demonstrated an increase in spinal cord levels of endogenous neurotrophic factors with exercise, which may be useful in facilitating elongation and/or synaptic activity of regenerating axons and plasticity of spinal neurons below the level of injury
Keywords: rehabilitation, neurotrophic factors, neurotransplantation, cFos
Introduction
Spinal cord injury (SCI) initiates a progressive state of degeneration, with structural, biochemical and physiological changes occurring over weeks to months post injury. Secondary responses to the initial trauma include inflammation, excitotoxicity, apoptosis of neurons and glia, axon retraction, glial scarring, demyelination and exposure of myelin-associated inhibitory molecules, changes in electrophysiological properties of neurons, and aberrant sprouting/plasticity of spared nerve fibers.1–4 When viewed on a whole, clinicians and researchers are faced with multiple changes ranging from molecular to gross anatomical disturbances that need to be addressed, while having the knowledge that no single treatment approach to date has been effective in promoting recovery of volitional movement. Different therapeutic strategies have in the past focused on a single aspect of the injury response, be it neuroprotection, neuroregeneration or neurorehabilitation. In contrast, many recent studies indicate that a greater effect may be realized when a combination of interventions is implemented. In this truncated review we describe the need to balance the effort to promote recovery through use of neural tissue transplantation with the attempt to diminish growth inhibitory factors such as chondroitin sulfate proteoglycans that prevent regeneration of axons, while incorporating exercise as a treatment strategy to help shape cell and molecular plasticity distal to an injury site. With ongoing research we will establish whether exercise has beneficial effects on the regenerative efforts of neurons directly injured by trauma to the spinal cord.
Results
Spinal cord injury and transplantation models
One approach used to promote the regeneration and integration of injured axons across a spinal cord lesion is neural tissue transplantation, where the intent is to provide a substrate that will support and guide axons towards a specified target. Much has been written about the sources of these tissues, with strong evidence of axonal growth into and through transplants of embryonic neural tissue, Schwann cells, segments of peripheral nerve, genetically modified fibroblasts and stem cells.5–9 Other reports suggest that biomaterial scaffolds effectively maintain continuity between injured surfaces of the spinal cord to facilitate axon growth.10–12 It is important to recognize that most of these transplant approaches have been applied to multiple forms of SCI, including hemisection and complete transection aspirations, penetrating knife cuts and contusion injuries. Survival and integration of the transplant in a variety of injury situations suggests that an injury model can be chosen to address a specific set of questions rather than having the need to mimic a human injury condition. This provides for greater variability of research design in which to collect a broad range of information about transplant survival, support of axonal growth, integration with host tissues, the possibility of immunorejection etc.
There has been considerable success with the use of peripheral nerve grafts (PNGs), following the groundbreaking demonstration by Aguayo and colleagues of axon regeneration by adult mammalian neurons.13–14 There are several advantages to the use of PNGs for promoting axon regeneration and these are highlighted in a recent review.15 Briefly, it is possible to direct growing axons towards a specific target, which in our case is the intermediate gray of spinal cord distal to an injury; the target site can be treated to make it either more attractive or less inhibitory for the extension of axons beyond the distal end of the PNG; the PNG contains a pure population of regenerating axons that provides a valuable resource for the assessment of molecular signaling during regrowth; axons coursing through the PNG and the path of axonal outgrowth from the PNG can be mapped out by application of tracer compounds directly to the PNG; behavioral recovery due to regenerated axons can be evaluated by cutting the PNG and determining subsequently if there is a loss of functional activity. Several of these features are highlighted in Figure 1A. The basic techniques of creating a lesion cavity, of transplanting a PNG to form a bridge across the lesion, and intraspinal microinjection of matrix-modifying enzymes are available (see Ref. 16).
Figure 1.
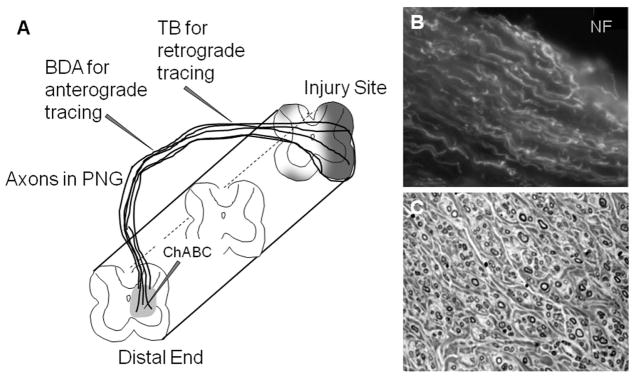
Axon regeneration into peripheral nerve grafts. (A) Diagrammatic representation of a PNG bridging a spinal cord lesion with a demonstration of axonal growth into and through the distal end of the graft where ChABC was delivered to degrade inhibitory matrix molecules. This illustration shows the flexibility of the PNG in that grafts of a specified length can be created, the distal end can be apposed to a specific region or level of the spinal cord and the graft is external to the spinal canal which facilitates isolation for electrophysiological testing and microinjection of tracers into the graft. (B) Regenerating axons immunolabeled with antibody to neurofilament are oriented in a longitudinal array within the PNG. (C) Evaluation of successful grafting includes quantitation of the number of myelinated axons observed in a transverse section through the PNG. Here myelinated axons of various diameters are seen surrounded by fibrous-like connective tissue.
Over the last 25 years we and others have used PNGs to define the regenerative effort of supraspinal and propriospinal neurons after a variety of spinal cord injuries. From this body of work we know that acute and chronically injured neurons exhibit the capacity for regeneration when provided with an appropriate substrate and that these newly growing axons processes will extend to the distal end of the PNG.17,18 Both descending motor axons and ascending sensory axons exhibit a pattern of linear growth within the graft (Fig. 1B) and axons of small to large caliber were well myelinated (Fig. 1C). We have observed similar results when grafting into spinal cord injured adult rats or adult cats.19, 20 After treatment with Chondroitinase to degrade chondroitin sulfate proteoglycans of the glial scar it is observed that approximately 10–15% of the axons in the PNG extend beyond the distal graft to integrate with adjacent spinal neurons17. These axons do not extend very far from the PNG (<1 mm) but many form synaptic contacts with spinal neurons and the observed increase in cFos protein in spinal cord neurons after electrical stimulation of the PNG is indicative of the formation of functional synapses between regenerated axons and their target. Functional recovery due to synaptic connections made by axons growing into and out of the grafts is directly tested by evaluating kinematic data, EMG activity and recording of spinal cord field potentials after electrical stimulation of the PNG.20–25
On the positive side it is encouraging that there is a robust regenerative response after a traumatic spinal cord injury. After a cervical level injury we have traced axonal growth into PNGs by all major descending spinal pathways except the corticospinal tract.26 After a lower thoracic injury significant regeneration by thoracic propriospinal neurons was observed but few supraspinal neurons responded to an injury at this distant level by regenerating their axon.27 Ascending sensory fibers exhibit a strong regenerative effort without regard to the level of injury as dorsal root ganglion neurons (both small and large neurons) and thoracic propriospinal neurons have been identified as sources of axons regenerating into a PNG that was apposed to the caudal surface of the injury site. In adult rats the mean number of myelinated axons within PNGs is about 1200 regardless of whether they arise from descending or ascending spinal pathways. In adult cats we have observed more than 2400 myelinated axons in our grafts, which likely reflects the larger diameter of the nerve used for grafting and the ability to have room for more axonal ingrowth. Despite demonstrating anatomical and behavioral recovery by axons extending across a lesion, the overall benefit of Chondroitinase treatment is not very compelling as the majority of axons remain in the transplant (no matter what the tissue source) with only a small number reaching target areas and there is no convincing evidence of restoration of volitional control of movement. It is our mission to develop better strategies to encourage outgrowth by a greater percentage of those axons that have regenerated into a PNG, with the rationale that greater axonal integration with spinal cord neurons will result in greater functional reorganization.
Exercise promotes neural plasticity
The capacity for CNS reorganization28, 29 is a well known property that may underlie many examples of functional recovery.30 SCI leads to rapid changes in motoneuron (MN) activity patterns because of the interruption of descending supraspinal and propriospinal pathways. Removal of descending presynaptic inhibition of group Ia axons may contribute to the decrease in reflex threshold and loss of habituation by MNs.31–33 Changes in reflex circuitry properties likely underlie the progressive development of a hyper-reflexive state that eventually leads to spasticity. After SCI, the H-reflex exhibits lower negative modulation with increasing stimulation frequency as compared to intact animals; whereas in exercised animals there is a restoration of frequency dependent depression as a measure of H-reflex excitability34 and prevention of atrophy of hind limb muscles.35 Thus a component of the attenuating effects of SCI on motor systems with passive exercise may reside in the spinal cord and may include the afferent connections to MNs or the properties of the MNs themselves. In collaboration with Phil Gardiner36, rats with a complete lower thoracic transection injury that received cycling exercise demonstrated retention of MN resting membrane potential and spike trigger level compared to unexercised animals, consistent with influences on ion conductance’s at or near the initial segment. This study supports therapeutic roles for exercise as a means to slow the deleterious responses of MNs to SCI and the ability of exercise to support structural and physiological plasticity in components of neuromuscular junctions associated with motoneurons located below the level of injury.
The lumbosacral spinal cord apparently can learn several tasks where specific supraspinal and sensory information associated with movement influence and shape the activity of spinal networks. Even after complete transection injuries, cats can be trained to stand and to step on a treadmill.37 They are capable of adjusting the movement to change in speed or weight support and relearn a task after an interruption or change in training type.38–41 Sensory feedback is critical to coordinate limb function in spinalized cats during treadmill training suggesting that spinal pathways can integrate dynamic sensory inputs provided by repetitive activation to shape motor output.42, 43 We have been applying training procedures to SCI rats44, 45 (Fig. 2) and preventing afferent feedback to drive spinal networks during exercise through either T12–S2 dorsal rhizotomy or pyridoxine toxicity46. Either of these approaches prevented rate dependent reflex modulation and there was no exercise related increase in brain derived neurotrophic factor (BDNF) mRNA (Fig. 3) indicating that input arising from large diameter proprioceptors is required to realize the beneficial effects of exercise after SCI.
Figure 2.
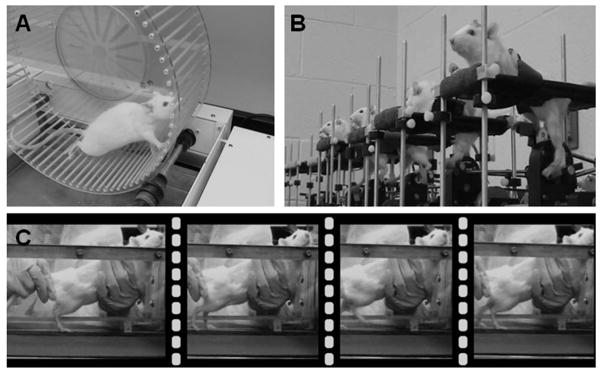
Exercise devices used for spinal cord injured rats. (A) A motorized exercise wheel (Lafayette Instruments, Lafayette, IN) is useful for rats that have a unilateral injury so that they can maintain a slow walking speed using 3 limbs initially, until the impaired limb regains some measure of function. Changes in speed and duration of exercise are routinely implemented to encourage rats to perform at a higher level. (B) Motorized cycles (custom-made) for spinalized rats provide rhythmic sensory input to the lumbar spinal cord as they move the hind limbs through a complete range of motion. As little as 15 minutes of cycling per day is sufficient to increase intraspinal neurotrophic factor levels. (C) Step training of spinalized rats on a motorized treadmill provides loading of hind limb muscles which does not occur with cycling. Similar to treadmill training of cats (see Rossignol and Martinez, this issue), spinalized rats require assistance with balance and perineal stimulation to initiate locomotion.
Figure 3.
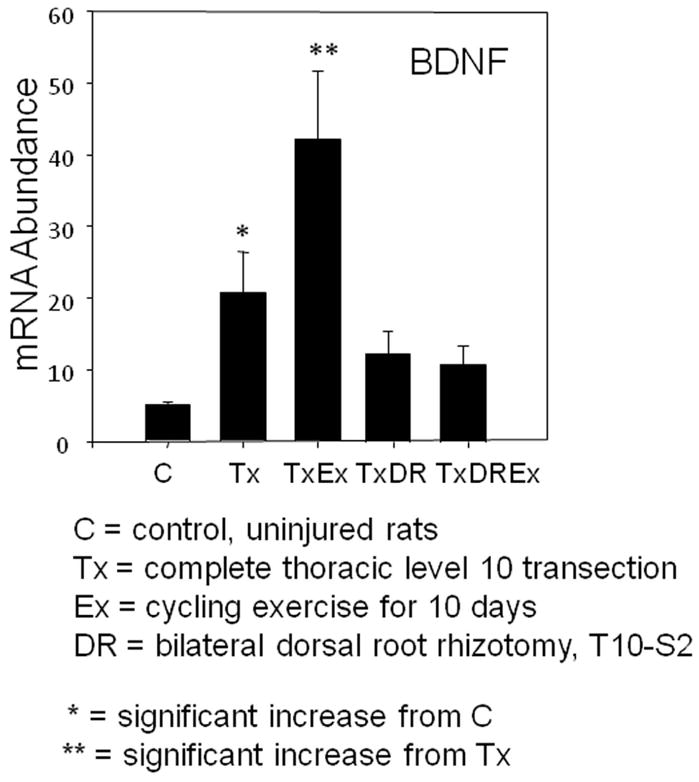
Deafferentation abolishes exercise dependent increase in neurotrophic factor mRNA. Rats with a complete thoracic transection injury show significant increase in levels of BDNF mRNA after cycling exercise. When dorsal root axons caudal to the injury are removed bilaterally there is no benefit from exercise of these rats. This indicates the necessity of sensory information being transmitted to the spinal cord during exercise.
Change in neurotrophic factor levels with exercise
Neurotrophic factors (NTFs) are a class of polypeptide growth factors that promote neuron survival, modulate neuronal function, and enhance plasticity. For example, BDNF47–50 and neurotrophin-3 (NT3)51, 52 have been shown to enhance survival of damaged neurons and to contribute to recovery of function following injury. With regard to SCI, exogenous NTFs rescue neurons after injury53, 54 and in combination with a neural tissue transplant promote the regenerative effort of acute and chronically injured neurons.6, 49, 55–57 Our studies with PNGs have demonstrated that exogenous delivery of several NTFs, such as BDNF, glial cell line-derived neurotrophic factor (GDNF), or NT3, significantly increases the number of chronically injured neurons that regenerate an axon into the PNG.17, 49, 58
There is also evidence that neurotrophic factors play a role in maintaining and promoting function at the peripheral neuromuscular junction (NMJ). Indeed, recent studies demonstrate that NMJs in hindlimb muscles innervated by MNs caudal to spinal lesions are not stable but extensively disassembled resulting in substantial loss of nerve-muscle connectivity as early as 2 weeks after SCI,59 where vulnerable NMJs lose synaptic organization by either excessive sprouting of their nerve terminals or failing to maintain muscle acetylcholine receptors (AChRs). On the other hand, cycling exercise that increased neuromuscular activity in muscles caudal to a complete transection suppressed sprouting and enhanced receptor maintenance, thereby stabilizing synaptic connectivity in hind limb muscles.
Exercise and subsequent activation of neural networks influences transcription of neurotrophic factors both centrally and peripherally in skeletal muscle. In the developing CNS, activity stabilizes synapses60 and disuse leads to synaptic elimination (i.e. pruning).61, 62 In intact adult rats treadmill training increases BDNF and NT3 expression (protein and mRNA) in both spinal motoneurons and skeletal muscle.63–65 BDNF and NT3 are localized by immunohistochemistry to the ventral horn and NT3 staining also is dense in the substantia gelatinosa. Elimination of hindlimb EMG activity by complete thoracic and sacral cord transections and bilateral lumbosacral dorsal rhizotomy (i.e. spinal isolation)66 results in greatly diminished activity and reduced transcription of neurotrophins. Levels of neurotrophins (BDNF, NT3) and the downstream effector (synapsin I) were significantly diminished in the lumbar cord. BDNF and NT3 expression also has been shown to be down-regulated after moderate spinal cord contusion and increased with treadmill training67, cycle training68 or voluntary locomotion in freely moving running wheels.69
We examined the effects of non-robotic, step training on a treadmill or motorized cycling on the expression of neurotrophic factors in the spinal cord45. Each of these exercise paradigms led to comparable increases in BDNF, NT3, NT4, and GDNF protein levels in the lumbar enlargement (L4–L6) as well as in upper lumbar (L2–3) and thoracic (T12–L1) regions just caudal to the lesion site. Interestingly there also was a significant increase in levels of NT3 in the thoracic spinal cord proximal to the level of complete transection injury. The increase in spinal NTFs and recovery of H-reflex frequency-dependent depression was positively correlated and required the afferent feedback provided by exercise46 suggesting that normalization of reflexes in the injured spinal cord is effectively triggered by afferent activity and local increase in NTFs. Motor recovery after SCI has been linked directly to the increase in BDNF levels within the lumbar spinal cord70 and a recent review of BDNF treatments after SCI71 suggests that exercise is the “safest intervention” to achieve beneficial effects without some of the undesired effects often seen with exogenous delivery of BDNF over a prolonged period.
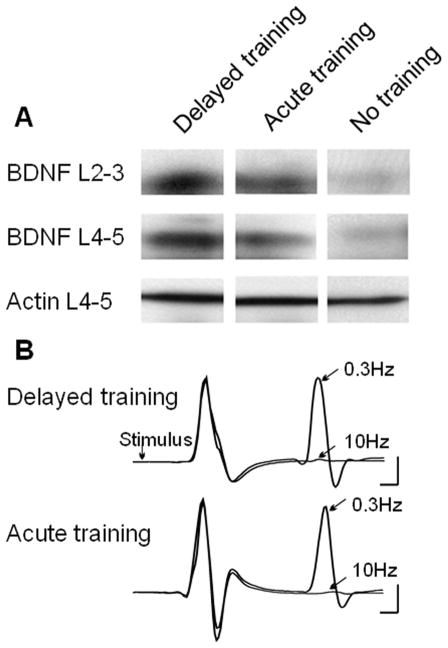
One issue that we and others have faced over the years is whether the effect of exercise is due to the latest bout or whether it is a cumulative effect over the entire period of exercise. Also it was not clear how a delay in exercise might affect levels of NTFs in the spinal cord and recovery of MN reflex activity. To address this we prepared three groups of adult rats with complete lower thoracic transection injuries. Animals in Group 1 began exercising 5 days after injury for 5 consecutive days. Animals in Group 2 were subjected to only 1 bout of exercise (on the last day of exercise for Group 1 animals). Animals in Group 3 were subjected to 5 days of exercise beginning 4 weeks after the transection injury. Western blot analysis of protein from the lumbar enlargement of these animals indicated that the effect of exercise on NTF production was indeed cumulative, as there was a significant increase in BDNF with 5 days of exercise (Group 1) over animals in Group 2 that received only a single bout of exercise (data not shown). Perhaps of greater importance was the observation of comparable levels of BDNF in animals of Group 1 and Group 3 indicating the potential for exercise to increase NTFs with acute or delayed application (Fig. 4A). In these same groups we determined that there was no significant difference in frequency dependent depression of the H-reflex when training was provided acutely or after a 4 week delay (Fig. 4B). Whether this is related to the expression of NTFs with training is not known but these results indicate a capacity for considerable neuroplasticity remains long after the initial injury to the spinal cord.
Figure 4.
Neurotrophin expression with acute or delayed exercise after SCI. (A) Western blot data demonstrates the presence of comparable levels of BDNF in the lumbar spinal cord when cycling exercise was initiated 5 days after injury (acute) or 28 days after injury (delayed). (B) Frequency dependent depression of the H reflex was restored in spinalized rats that received acute or delayed cycling exercise. Together these observations demonstrate the potential for exercise dependent plasticity in the injured spinal cord at short and long intervals after SCI.
Cell and molecular changes with exercise
Downstream effectors of BDNF including TrkB, synapsin I, and CREB also increase with exercise and are conversely diminished by botulinum induced paralysis.64 The impact on synapsin is particularly interesting because it is postulated to play an important role in neurotransmitter release and maintenance of synaptic contacts.72, 73 In a series of studies we found that exercise after SCI led to a significant increase in the distribution of immunolabeled synapsin around neurons in the intermediate gray and ventral horn of the lumbar spinal cord (data not shown). Whether this is indicative of an increase in synaptic sites per neuron or an increase in the density of synaptic vesicles per synapse is unknown, but the results clearly demonstrate an effect of exercise. Correlating with this is the observation of an increase in the number of neurons expressing cFos in dorsal horn and intermediate gray regions of the lumbar spinal cord following hind limb exercise of spinalized rats (Fig. 5). An increase in cFos is used as an indication of increased neuronal activity resulting from synaptic activity74, 75, in this case during exercise. It is not clear if long term consequences may arise from this transient increase in neuron activity but it is tempting to put forward the notion that neurons in a heightened state of activity may be an attractive target for regenerating axons entering the area from a nerve graft. Continuing the theme of cellular plasticity after exercise we identified an increase in the number of interneurons expressing phosphorylated-S6 (P-S6) and an increase in dendritic branching and content of P-S6 by motoneurons after cycling of SCI rats.76 The absence of P-S6 expression in exercised rats concurrently treated with rapamycin is an indication of the role of mTOR in this example of exercise-dependent plasticity.
Figure 5.
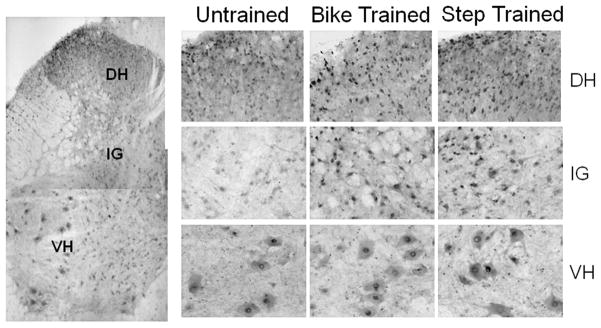
Immunolabeling for cFos after SCI and exercise. With bike or step training there is an increase in the number of neurons in the L4 dorsal horn (DH) and intermediate gray (IG) compared to neurons in untrained animals. The base level of cFos in motoneurons of the ventral horn (VH) does not appear to be changed with exercise. The expression of cFos is used as an indicator of neuron activity following hind limb exercise of spinalized rats.
In other experiments we examined the expression of microRNAs (miRs) in spinal cord tissue caudal to a transection injury with or without hindlimb exercise. Significant changes in response to cycling exercise were identified in genes associated with apoptosis and cell growth signaling pathways.77 Upregulation of miR21 was associated with decrease in expression of mRNA for PTEN and PDCD4, which are upstream of caspase expression. Also, miR15b was downregulated in association with upregulation of Bcl2 mRNA (another signaling molecule upstream of caspase expression) and cycling exercise led to a decrease in protein levels of caspase 7. We also measured changes in miRs that affect expression of PTEN, AKT, and mTOR and S6 mRNA, which have been recently implicated in axon regeneration after optic nerve or spinal cord injury.78, 79 The increased expression of miR21 after exercise was associated with a decrease in PTEN message and protein and an increase in AKT message and protein. A decrease in miR199a-3p with exercise was associated with an increase in mTOR and S6 message and protein. Thus it is tempting to suggest that modulation of the PTEN/AKT/mTOR pathway is one mechanism by which exercise might influence axon growth by neurons affected by SCI.
Summary
Several thousand axons regenerate into PNGs yet only 10–15% of these appear to exit the graft when inhibitory proteoglycans in the spinal cord are digested prior to apposition of the distal graft end.17, 19 We have identified functional changes attributable to this relatively modest axonal outgrowth but it is important to know if the number of axons extending beyond the graft can be increased and if such an increase would result in more dramatic functional recovery. Our previous work58 demonstrated that short term delivery of exogenous NTFs enhanced the regenerative effort of chronically injured neurons and several recent studies demonstrate that creation of neurotrophic gradients effectively increase axonal regeneration beyond a transplant of neural precursor cells.80, 81 As a non-invasive treatment strategy, hind limb exercise of spinalized rats significantly increases the level of NTFs in spinal cord tissue both proximal and distal to an injury site45, increases the expression of cFos in dorsal horn and intermediate gray neurons, promotes expression of molecules involved in regulation of protein synthesis and restores reflex activity of motoneurons (Table 1). These results advocate that this intervention may be useful for increasing the growth response of injured neurons, both in acute and chronic injury situations. As a first step towards enhancing the possibility of reconnection of axons across an injury it is important to determine if we can increase the number of axons available at the distal end of the PNG to form new contacts once they re-enter the spinal cord. An increase in the number of outgrowing axons could result in more synaptic contacts being made with spinal cord neurons, possibly leading to an enhanced behavioral and/or functional outcome. A key feature of this work will be to test whether repetitive stimulation of spinal cord circuitry will be beneficial in attracting axons to areas of the spinal cord affected by exercise. In effect, exercise may be useful in creating suitable target areas within the spinal cord according to some of the plasticity observed so far. There remain several fundamental areas of exercise dependent plasticity to explore and results will influence our views of how to move forward with combination treatment strategies for functional recovery after SCI.
Table 1.
Cell and molecular targets of exercise. A summary of known effects of exercise in the injured spinal cord demonstrates a wide range of action, including local and distant increase in neurotrophic factors, upregulation of cFosexpression in multiple neuron cell types, expression of downstream molecules of the AKT/mTOR pathway that may reflect a strong regenerative potential and retention of muscle size and motoneuron membrane properties and reflex activity. In future experiments, exercise will be employed to attempt to increase the growth of regenerating axons into and then out of a PNG.
| Cell and molecular plasticity with exercise | |||
|---|---|---|---|
| Outcome | Location | Effects | What’s next |
| Increased expression of neurotrophic factors | Below and above injury site | Neuroprotective Reduce axon dieback Rescue injured neurons |
Examine the potential of exercise to promote axon growth into and out of a PNG. |
| Upregulation of cFos expression | Multiple neuronal cell types below injury site | Neuroplasticity Enhanced neuronal activity |
|
| Increased expression of phosphorylated S6 | Interneurons below injury site | Neuroregenerative S6 is a molecule downstream of AKT/mTOR, suggests a strong regenerative potential |
|
| Retention of MN properties, muscle size and modulation of H-reflex | Below injury site | Neurorehabilitative Promote interneuronplasticity Modulate MN excitability Reduce muscle atrophy |
|
Acknowledgments
This work was supported by grants from NIH (NS26380, NS055976, and NS074406 to JDH) and the Craig H. Neilsen Foundation (to MPC).
References
- 1.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 2.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord forms a new intraspinal circuit in adult rats. Nature Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 3.Jones TB, McDaniel EE, Popovich PG. Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Curr Pharm Des. 2005;11:1223–1236. doi: 10.2174/1381612053507468. [DOI] [PubMed] [Google Scholar]
- 4.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. J Neurosci Res. 2002;68:501–510. doi: 10.1002/jnr.10240. [DOI] [PubMed] [Google Scholar]
- 6.Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 7.Murray M, Kim D, Liu Y, Tobias C, Tessler A, Fischer I. Transplantation of genetically modified cells contributes to repair and recovery from spinal injury. Brain Res Brain Res Rev. 2002;40:292–300. doi: 10.1016/s0165-0173(02)00211-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee YS, Lin CY, Robertson RT, Hsiao I, Lin VW. Motor recovery and anatomical evidence of axonal regrowth in spinal cord-repaired adult rats. J Neuropathol Exp Neurol. 2004;63:233–245. doi: 10.1093/jnen/63.3.223-a. [DOI] [PubMed] [Google Scholar]
- 9.Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gros T, Sakamoto JS, Blesch A, Havton LA, Tuszynski MH. Regeneration of long-tract axons through sites of spinal cord injury using template agarose scaffolds. Biomaterials. 2010;31:6719–6729. doi: 10.1016/j.biomaterials.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Zhai P, Chen X, Schreyer DJ, Sun X, Cui F. Bioengineered scaffolds for spinal cord repair. Tissue Eng B. 2011;17:177–194. doi: 10.1089/ten.TEB.2010.0648. [DOI] [PubMed] [Google Scholar]
- 12.Liu T, Houle JD, Xu J, Chan BP, Chan SY. Nanofibrous collagen nerve conduits for spinal cord repair. Tissue Eng A. 2012;18:1057–1066. doi: 10.1089/ten.tea.2011.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–3. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–5. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- 15.Côté MP, Amin AA, Tom VT, Houle JD. Peripheral nerve grafts support regeneration after spinal cord injury. Neurotherapeutics. 2011;8:294–303. doi: 10.1007/s13311-011-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houle JD, Amin A, Cote MP, Lemay M, Miller K, Sandrow H, Santi L, Shumsky J, Tom V. Combining peripheral nerve grafting and matrix modulation to repair the injured rat spinal cord. J Visualized Experiments. 2009;20(33) doi: 10.3791/1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houle JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–90. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houle JD, Tessler A. Repair of chronic spinal cord injury. Exp Neurol. 2003;182:247–260. doi: 10.1016/s0014-4886(03)00029-3. [DOI] [PubMed] [Google Scholar]
- 19.Houle JD, V, Tom J, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cote MP, Hanna A, Lemay MA, Ollivier-Lanvin K, Santi L, Miller K, Monaghan R, Houle JD. Peripheral nerve grafts after cervical spinal cord injury in adult cats. Exp Neurol. 2010;225:173–82. doi: 10.1016/j.expneurol.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salame CG, Dum RP. Central nervous system axonal regeneration into sciatic nerve grafts: physiological properties of the grafts and histologic findings in the neuraxis. Exp Neurol. 1985;90:322–40. doi: 10.1016/0014-4886(85)90022-6. [DOI] [PubMed] [Google Scholar]
- 22.Pinzon A, Calancie B, Oudega M, Noga BR. Conduction of impulses by axons regenerated in a Schwann cell graft in the transected adult rat thoracic spinal cord. J Neurosci Res. 2001;64:533–41. doi: 10.1002/jnr.1105. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier P, Réga P, Lammari-Barreault N, Polentes J. Functional reconnections established by central respiratory neurons regenerating axons into a nerve graft bridging the respiratory centers to the cervical spinal cord. J Neurosci Res. 2002;70:65–81. doi: 10.1002/jnr.10379. [DOI] [PubMed] [Google Scholar]
- 24.Nordblom J, Persson JK, Svensson M, Mattsson P. Peripheral nerve grafts in a spinal cord prosthesis result in regeneration and motor evoked potentials following spinal cord resection. Restor Neurol Neurosci. 2009;27:285–95. doi: 10.3233/RNN-2009-0478. [DOI] [PubMed] [Google Scholar]
- 25.Lee YS, Hsiao I, Lin VW. Peripheral nerve grafts and aFGF restore partial hindlimb function in adult paraplegic rats. J Neurotrauma. 2002;19:1203–16. doi: 10.1089/08977150260338001. [DOI] [PubMed] [Google Scholar]
- 26.Sandrow HR, Shumsky JS, Amin A, Houle JD. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin AA, Houle JD. The role of neurotrophic factors and their receptors in ascending and descending axon regeneration through intrapsinal peripheral nerve grafts (PNGs) Society for Neuroscience Abstracts 2010 [Google Scholar]
- 28.Edgerton VR, de Leon RD, Tillakaratne N, Recktenwald MR, Hodgson JA, Roy RR. Use dependent plasticity in spinal stepping and standing. Adv Neurol. 1997;72:233–47. [PubMed] [Google Scholar]
- 29.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J Neurophysiol. 1999;81:85–94. doi: 10.1152/jn.1999.81.1.85. [DOI] [PubMed] [Google Scholar]
- 30.Dobkin BH. Activity-dependent learning contributes to motor recovery. Ann Neurol. 1998;44:158–160. doi: 10.1002/ana.410440204. [DOI] [PubMed] [Google Scholar]
- 31.Gustafsson B, Katz R, Malmsten Effects of chronic partial deafferentation on the electrical properties of lumbar alpha-motoneurones in the cat. Brain Research. 1982;246:23–33. doi: 10.1016/0006-8993(82)90138-x. [DOI] [PubMed] [Google Scholar]
- 32.Hochman S, McCrea DA. Changes of chronic spinalization on ankle extensor motoneurons. I. Composite monosynaptic Ia EPSPs in four motoneuron pools. J Neurophysiol. 1994a;71:1452–1467. doi: 10.1152/jn.1994.71.4.1452. [DOI] [PubMed] [Google Scholar]
- 33.Hochman S, McCrea DA. Effects of chronic spinalization on ankle extensor motoneurons. II. Motoneuron electrical properties. J Neurophysiol. 1994b;71:1468–1479. doi: 10.1152/jn.1994.71.4.1468. [DOI] [PubMed] [Google Scholar]
- 34.Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–131. [PubMed] [Google Scholar]
- 35.Murphy RJL, Dupont-Versteegden EE, Peterson CA, Houle JD. Two experimental strategies to restore muscle mass in adult rats following spinal cord injury. Neurorehab Neural Repair. 1999;13:125–134. [Google Scholar]
- 36.Beaumont E, Houle JD, Peterson CA, Gardiner PF. Fetal spinal cord transplant and passive exercise help to restore motoneuronal properties after spinal cord transection in rats. Muscle & Nerve. 2004;29:234–242. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- 37.Martinez M, Rossignol S. A dual spinal cord lesion paradigm to study spinal locomotor plasticity in the cat. Ann N Y Acad Sci. 2012 doi: 10.1111/j.1749-6632.2012.06823.x. This Volume. [DOI] [PubMed] [Google Scholar]
- 38.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 39.Hodgson J, Roy R, Dobkin B, Edgerton VR. Can the mammalian spinal cord learn a motor task? Med Sci Sports Exer. 1994;26:1491–1497. [PubMed] [Google Scholar]
- 40.Lovely R, Gregor R, Roy R, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- 41.Lovely R, Gregor R, Roy R, Edgerton V. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res. 1990;514:206–218. doi: 10.1016/0006-8993(90)91417-f. [DOI] [PubMed] [Google Scholar]
- 42.Barriere G, Frigon A, Leblond H, Provencher J, Rossignol S. Dual spinal lesion paradigm in the cat: evolution of the kinematic locomotor pattern. J Neurophysiol. 2010;104:1119–1133. doi: 10.1152/jn.00255.2010. [DOI] [PubMed] [Google Scholar]
- 43.Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S. Incomplete spinal cord injury promotes durable functional changes within the spinal locomotor circuitry. J Neurophysiol. 2012;108:124–134. doi: 10.1152/jn.00073.2012. [DOI] [PubMed] [Google Scholar]
- 44.Sandrow-Feinberg HR, Izzi J, Shumsky JS, Zhukareva V, Houle JD. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J Neurotrauma. 2009;26:1–11. doi: 10.1089/neu.2008.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Côté MP, Azzam GA, Lemay MA, Zhukareva V, Houle JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ollivier-Lanvin K, Keeler BE, Siegfried R, Houle JD, Lemay MA. Proprioceptive neuropathy affects normalization of the H-reflex by exercise after spinal cord injury. Exp Neurol. 2010;221:198–205. doi: 10.1016/j.expneurol.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- 48.Friedman B, Kleinfeld D, Ip NY, Verge VM, Moulton R, Boland P, Zlotchenko E, Lindsay RM, Liu L. BDNF and NT4/5 exert neurotrophic influences on injured adult spinal motor neurons. J Neurosci. 1995;15:1044–1056. doi: 10.1523/JNEUROSCI.15-02-01044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye JH, Houle JD. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp Neurol. 1997;143:70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 51.Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- 52.Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giehl KM, Tetzlaff %W. BDNF and NT-3 but not NGF, prevent axotomy-induced death of rat corticospinal neurons in vivo. Eur J Neurosci. 1996;8:1167–1175. doi: 10.1111/j.1460-9568.1996.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 54.Novikova LN, Novikov LN, Kellerth IO. Survival effects of BDNF and NT-3 on axotomized rubrospinal neurons depend on the temporal pattern of neurotrophin administration. Eur J Neurosci. 2000;12:776–780. doi: 10.1046/j.1460-9568.2000.00978.x. [DOI] [PubMed] [Google Scholar]
- 55.Bregman BS, Coumans J-V, Dai HN, Kuhn PL, Lynskey J, McAtee M, Sandhu F. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. In: McKerracher L, Doucet G, Rossignol S, editors. Progress in Brain Research. Elsevier; Montreal: 2002. pp. 258–273. [DOI] [PubMed] [Google Scholar]
- 56.Coumans JV, Lin TT-S, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dolbeare D, Houle JD. Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF) J Neurotrauma. 2003;20:1251–1261. doi: 10.1089/089771503770802916. [DOI] [PubMed] [Google Scholar]
- 59.Burns AS, Jawaid S, Zhong H, Yoshihara H, Bhagat S, Murray M, Roy RR, Tessler A, Son YJ. Paralysis elicited by spinal cord injury evokes selective disassembly of neuromuscular synapses with and without terminal sprouting in ankle flexors of the adult rat. J Comp Neurol. 2007;500:116–33. doi: 10.1002/cne.21143. [DOI] [PubMed] [Google Scholar]
- 60.Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- 61.Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Ann Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- 62.Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 63.Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 64.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 65.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003;987:93–99. doi: 10.1016/s0006-8993(03)03258-x. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Pinilla F, Ying Z, Roy RR, Hodgson J, Edgerton VR. Afferent input modulates neurotrophins and synaptic plasticity in the spinal cord. J Neurophysiol. 2004;92:3423–3432. doi: 10.1152/jn.00432.2004. [DOI] [PubMed] [Google Scholar]
- 67.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 68.Dupont-Versteegden EE, Houle JD, Dennis RA, Zhang J, Knox M, Wagoner G, Peterson CA. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle & Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- 69.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 70.Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weishaupt N, Blesch A, Fouad K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.09.001. In Press. [DOI] [PubMed] [Google Scholar]
- 72.Brock TO, O’Callaghan JP. Quantitative changes in the synaptic vesicle proteins synapsin I and p38 and the astrocyte-specific protein glial fibrillary acidic protein are associated with chemical-induced injury to the rat central nervous system. J Neurosci. 1987;7:931–942. doi: 10.1523/JNEUROSCI.07-04-00931.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang SD, Goldberger ME, Murray M. Plasticity of spinal systems after unilateral lumbosacral dorsal rhizotomy in the adult rat. J Comp Neurol. 1991;304:555–568. doi: 10.1002/cne.903040405. [DOI] [PubMed] [Google Scholar]
- 74.Ahn SN, Guu JJ, Tobin AJ, Edgerton VR, Tillakaratne NJ. Use of c-fos to identify activity-dependent spinal neurons after stepping in intact adult rats. Spinal Cord. 2006;44:547–559. doi: 10.1038/sj.sc.3101862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Ann Rev Neurosci. 1991;14:412–452. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 76.Liu G, Detloff MR, Miller KN, Santi L, Houle JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 2011;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu G, Keeler BE, Zhukareva V, Houle JD. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp Neurol. 2010;226:200–206. doi: 10.1016/j.expneurol.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly J, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu K, Lu Y, Lee JK, Smaara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nature Neuroscience. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Bleasch A, Rosenzweig ES, Havton LA, Zheng B, Connor JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]