Abstract
Context
A family history of colorectal cancer in a first-degree relative increases the risk of developing colorectal cancer. However, the influence of family history on cancer recurrence and survival among patients with established disease remains uncertain.
Objective
To examine the association of family history of colorectal cancer with cancer recurrence and survival of patients with colon cancer.
Design, Setting, and Participants
Prospective observational study of 1,087 patients with stage III colon cancer enrolled in a randomized adjuvant chemotherapy trial (CALGB 89803) between April 1999 and May 2001. Patients provided data on family history at baseline and were followed up until March 2007 for disease recurrence and death (median follow-up 5.6 years). In a subset of patients, we assessed microsatellite instability (MSI) and expression of the mismatch repair (MMR) proteins, MLH1 and MSH2, in tumor specimens.
Main Outcome Measure
Disease-free survival, recurrence-free survival, and overall survival according to the presence or absence of a family history of colorectal cancer.
Results
Among 1,087 eligible patients, 195 (17.9%) reported a family history of colorectal cancer in a first-degree relative. Cancer recurrence or death occurred in 57/195 patients (29%; 95% confidence interval [CI], 23%-36%) with a family history of colorectal cancer and 343/892 patients (38%; 95% CI, 35%-42%) without a family history. Compared to patients without a family history, the adjusted hazard ratios (HR) among those with ≥1 affected first-degree relatives were 0.72 (95% CI, 0.54-0.96) for disease-free survival (DFS), 0.74 (95% CI, 0.55-0.99) for recurrence-free survival (RFS), and 0.75 (95% CI, 0.54-1.05) for overall survival (OS). This reduction in risk of cancer recurrence or death associated with a family history became stronger with an increasing number of affected first-degree relatives. Compared to participants without a family history of colorectal cancer, those with 1 affected relative had a multivariate HR of 0.77 (95% CI, 0.57-1.04) for DFS. For participants with ≥2 affected relatives, we observed a greater reduction in risk (multivariate HR for DFS, 0.49; 95% CI, 0.23-1.04; p for trend with increasing number of affected relatives=0.01). Of note, the improved disease-free survival associated with a family history was independent of tumoral MSI or MMR status.
Conclusion
Among patients with stage III colon cancer receiving adjuvant chemotherapy, a family history of colorectal cancer is associated with a significant reduction in cancer recurrence and death.
Introduction
Approximately 16-20% of patients with colorectal cancer have a first-degree relative with colorectal cancer.1 Beyond rare but highly penetrant hereditary colorectal cancer syndromes, numerous studies have demonstrated that a history of colorectal cancer in a first-degree relative increases the risk of developing the disease by approximately two-fold.2-5 However, the influence of family history on cancer recurrence and survival among patients with established colon cancer remains uncertain. A study of the Utah Geneology Database and Cancer Registry observed that family history of colon cancer had little impact on colorectal cancer patient survival;6 in contrast, an analysis of a Japanese tumor registry demonstrated an improved prognosis.7 Interpretation of these conflicting data is limited by a lack of detailed information regarding tumor stage and treatment in either of these studies.
We therefore prospectively examined the influence of family history of colorectal cancer on survival of patients with stage III colon cancer who participated in a large clinical trial of adjuvant chemotherapy sponsored by the National Cancer Institute (NCI). Because detailed information about family history of colorectal cancer was assessed at study entry, we were able to prospectively analyze the influence of family history while adjusting for other predictors of cancer recurrence and survival. In addition, among a subset of subjects with archived tumor specimens, we were able to assess whether the association between family history and survival was independent of tumoral microsatellite instability (MSI) or expression of mismatch repair (MMR) proteins.
Patients and Methods
Study Population
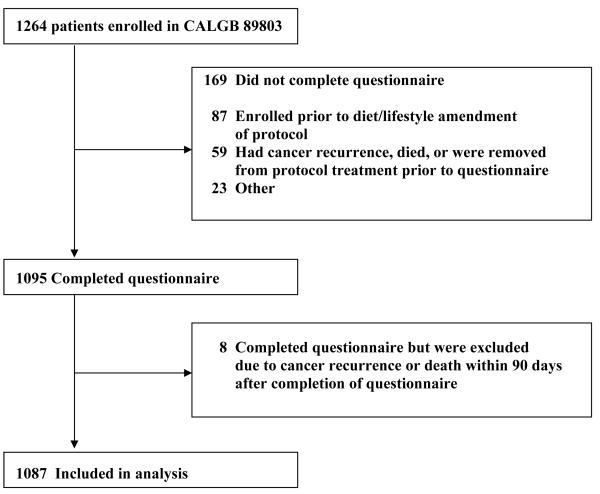
Patients in this study were participants in the NCI-sponsored Cancer and Leukemia Group B (CALGB) adjuvant therapy trial for stage III colon cancer (CALGB 89803). This study was a randomized trial comparing therapy with weekly fluorouracil and leucovorin (FU/LV) to therapy with weekly irinotecan, fluorouracil, and leucovorin (IFL).8 Between April 1999 and May 2001, 1,264 patients were enrolled in the treatment trial. As part of the original randomized clinical trial protocol, CALGB 89803, we included a plan to study diet, lifestyle, and family history on patient survival. A validated self-administered questionnaire assessing diet and lifestyle habits as well as family history of colorectal cancer was administered to patients midway through their adjuvant therapy (4 months after surgical resection).9, 10 The protocol amendment to survey family history was activated after the first 87 patients were enrolled; therefore, only the subsequent 1177 patients were offered the diet and lifestyle companion study. An additional 59 patients experienced cancer recurrence, death, or removal from the protocol before receiving the questionnaire. Thus, 1,118 patients were eligible to complete the questionnaire, of whom 1,095 patients (98%) completed the survey. Consistent with prior analyses, we excluded patients who experienced cancer recurrence or death within 90 days of completing the questionnaire to avoid potential bias in risk factor assessment related to underlying illness,9 thus leaving 1,087 patients eligible for analysis. Figure 1 illustrates adherence with completion of the questionnaire and derivation of the final sample size.
Figure 1.
Derivation of Cohort Size
Patients were eligible for the treatment trial (and thus this companion study) if they had undergone a complete surgical resection of the primary tumor within 56 days of study entry and had regional lymph node metastases but no evidence of distant metastases (stage III colon cancer). Patients were required to have a baseline Eastern Cooperative Oncology Group performance status of 0-2 (ambulatory) and have adequate bone marrow, renal, and hepatic function. Race or ethnicity was self-reported and recorded in the hospital database at each participating center. Classifications included White, Hispanic, Black, Oriental, Native Hawaiian, Native American, Indian, Filipino, Other, and Unknown. These data along with other demographic information were reported by each participating center to the CALGB Statistical Center. All patients provided written informed consent, approved by the institutional review board of each participating institution.
Family History Assessment
Participants were asked, “Have any of the following relatives (father, mother, one sibling, additional sibling) had colon or rectal cancer?” with an option for yes or no for each relative. Patients were instructed to include any deceased relative and not count half-siblings. Additionally, for each affected relative, patients provided information about the decade of relative’s age at first diagnosis (<age 50, age 50-59, age 60-69, age 70+, age unknown). No questions were asked about family size, and no attempt was made to validate reports of cancer in family members.
Measurement of Microsatellite Instability
Among patients with available tumor specimens, DNA was extracted from paraffin-embedded tumor and non-tumor tissue. Polymerase chain reaction analysis was conducted using 10 DNA microsatellite markers (BAT25, BAT26, BAT40, BAT34C4, D5S346, D17S250, ACTC, D18S55, D10S197, and MYCL) as described previously.11 Only cases with ≥5 evaluable microsatellite markers were included. Those showing instability in at least 40% of the loci tested were classified as having high-frequency MSI (MSI-H). Cases with no evaluable markers showing instability were classed as microsatellite stable (MSS), and the remainder was classed as MSI-low (MSI-L).12
Immunostaining for the mismatch repair (MMR) proteins MLH1 and MSH2 was undertaken as described previously.11 If either MLH1 or MSH2 demonstrated lack of staining, the tumor was considered to be MMR deficient. Tumors possessing staining for both MLH1 and MSH2 were considered to be MMR intact.12
Among the 1087 patients included in this analysis, 125 were classified as having MSI-H tumors, 614 had MSI-L or MSS tumors, and 348 did not have blocks available for genotyping analysis. Furthermore, 87 patients were classified as having tumors with deficient staining for the MMR proteins, 580 had tumors with intact MMR staining, and 420 did not have blocks available for immunohistochemical analysis. In multivariate analyses, MSI status and staining for DNA MMR proteins were coded using an indicator variable to reflect missing data.
Study End Points
For this study, the primary end point was disease-free survival (DFS), defined as time from study enrollment to tumor recurrence, occurrence of a new primary colon cancer, or death as a result of any cause. In addition, recurrence-free survival (RFS) was defined as the time from study enrollment to tumor recurrence, death with evidence of recurrence, or occurrence of a new primary colon tumor. For RFS, patients who died without known tumor recurrence were censored at the last documented evaluation by the treatment provider. Finally, overall survival (OS) was defined as the time from study enrollment to death as a result of any cause.
Statistical Methods
All three endpoints (DFS, RFS, and OS) were examined using Kaplan-Meier curves and the log-rank test.13 Cox proportional hazards regression was used to determine the simultaneous impact of potential confounders.14 The proportionality of hazards assumption for the effect of family history was tested by examining it as a time-dependent covariate in the Cox model. The time-dependent family history covariate was not statistically significant (p=0.95), indicating that the assumption of proportional hazards was appropriate. Covariates with missing variables were coded with indicator variables in adjusted models. We tested for linear trend by entering the number of relatives (0, 1, 2 or more) affected with colorectal cancer as a continuous variable into the multivariate model. Tests of interactions between family history of colorectal cancer and potentially modifying covariates including treatment were assessed by entering the cross product of family history and the covariate of interest. Statistical significance was considered at the 0.05 level. We used SAS version 9.1 (SAS Inc., Cary, North Carolina) for all statistical analyses. Patient registration, clinical data collection, and statistical analyses were conducted by the CALGB Statistical Center, and all analyses were based on the study database frozen on March 2, 2007. Median follow-up time was calculated among surviving patients from the time of enrollment to the time at which the study database was frozen. Using the Clark’s C15, the completeness of follow-up for this study was 83.25%. Applying Wu’s modification16 to adjust for unreported deaths, a more realistic assessment of the completeness of follow-up was 85.0%.
Results
Results for the clinical trial have been previously reported. No significant differences were found between the study treatment arms in either OS or DFS.8
Baseline characteristics for the 1,087 patients for whom data on family history were captured are presented in Table 1. Among these 1,087 participants, 195 (17.9%) reported a family history of colorectal cancer in ≥1 first-degree relative. Compared with patients without a family history, those with a family history were less likely to have presented with clinical bowel obstruction (p=0.02). Other potentially prognostic patient and tumor characteristics did not differ significantly according to family history.
Table 1.
Baseline Characteristics by Family History of Colorectal Cancer.
| Family History of Colorectal Cancer | |||
|---|---|---|---|
| No | Yes# | p-value* | |
| No. of patients | 892 | 195 | |
| Age, median (range), years | 60 (24-85) | 63 (21-85) | 0.24† |
| Male (%) | 488 (55) | 119 (61) | 0.11 |
| Race (%) | |||
| White | 789 (89) | 171 (88) | 0.83 |
| Black | 61 (7) | 14 (7) | |
| Other | 39 (4) | 10 (5) | |
| Performance Status± (%) | |||
| 0 | 658 (75) | 139 (73) | 0.52 |
| 1-2 | 214 (25) | 51 (27) | |
| Body mass-index, median (range), kg/m2 |
27.3 (15.6-51.7) | 26.8 (17.5-49.3) | 0.32† |
| Treatment Arm (%) | |||
| FU/LV | 446 (50) | 102 (52) | 0.58 |
| IFL | 446 50) | 93 (48) | |
| Tumor Location (%) | |||
| Right colon | 486 (56) | 115 (61) | 0.26 |
| Left colon | 385 (44) | 75 (39) | |
| Post-op CEA (%) | |||
| ≤ 5 | 765 (92) | 168 (94) | 0.28 |
| >5 | 69 (8) | 10 (6) | |
| Invasion through bowel wall† (%) | |||
| T1-2 | 117 (13) | 25 (13) | 0.99 |
| T3-4 | 753 (87) | 165 (87) | |
| Positive lymph nodes (%) | |||
| 1-3 | 553 (63) | 124 (65) | 0.17 |
| ≥ 4 | 321 (37) | 66 (35) | |
| Tumor differentiation (%) | |||
| Well | 52 (6) | 9 (5) | 0.70 |
| Moderate | 616 (71) | 133 (70) | |
| Poor | 203 (23) | 49 (26) | |
| Clinical Bowel Obstruction (%) | 212 (24) | 31 (16) | 0.02 |
| Clinical Bowel Perforation (%) | 37 (4) | 11 (6) | 0.34 |
| Smoking Status (%) | |||
| Current | 80 (9) | 27 (14) | 0.13 |
| Past | 393 (45) | 85 (44) | |
| Never | 408 (46) | 83 (43) | |
| Median household income in 1999φ, median (range) |
$40,542 ($20,480-$122,956) |
$41,367 ($17,963-$97,918) |
0.26† |
| Physical Activity, median (range), MET h/wk |
4.9 (0-125.2) | 4.5 (0-147.4) | 0.48 |
| Dietary Intake [median (range)] | |||
| Red meat, servings/wk | 3.2 (0-22.6) | 3.2 (0-15.1) | 0.80† |
| Processed meats, servings/wk | 2.4 (0-33.0) | 2.6 (0-16.0) | 0.15† |
| Refined grains, servings/d | 3.2 (0-21.6) | 2.9 (0.5-16.3) | 0.38† |
| Dessert, servings/d | 1.2 (0-10.8) | 1.1 (0-19.3) | 0.84† |
| Total fat, g/d | 73.6 (0-127.6) | 75.0 (26.8-117.2) | 0.50† |
Note: Age, body-mass index, performances status, and postoperative CEA were based on patient status at initiation of chemotherapy (entry into the treatment trial).
Abbreviations: CEA, carcinoembryonic antigen; PS, performance status; FU, 5-fluorouracil; LV, leucovorin; IFL, irinotecan, 5-FU, and leucovorin; MET, metabolic equivalent tasks.
By χ2test unless otherwise noted.
By Wilcoxon rank sum.
A performance status of 0 indicates the patient was fully active; PS 1, restricted in physically strenuous activity but ambulatory and able to carry out light work; PS 2, ambulatory and capable of all self-care but unable to carry out any work activities, up and about more than 50% of waking hours.
A grade of T1 or T2 indicates the level of invasion was through the bowel wall not beyond the muscle layer; T3 or T4, the level of invasion was through the bowel wall beyond the muscle layer.
Median household income was determined based on the median household income in the patient’s zip code area according to the US National Census in 2000.
Percentages may not add to 100 due to rounding.
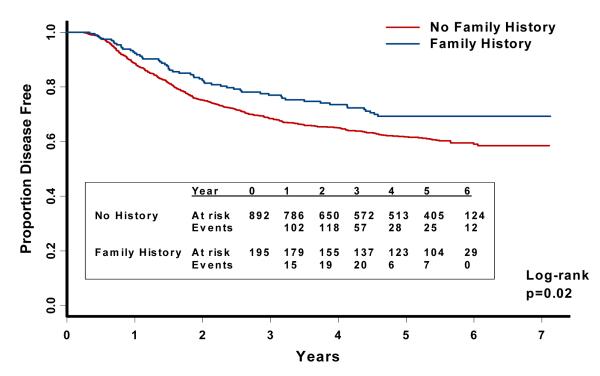
The median follow-up time from enrollment was 5.6 years (10th and 90th percentiles were 3.3 and 6.4 years, respectively). The predefined, primary endpoint of this analysis was DFS (time to cancer recurrence or death as a result of any cause). A family history of colorectal cancer was associated with a significant reduction in the risk of cancer recurrence or mortality (Figure 2). This relationship remained largely unchanged after adjusting for other predictors of cancer recurrence (Table 2). Compared with patients without a family history, those with a family history had a multivariate hazard ratio (HR) of 0.72 (95% CI, 0.54-0.96) for cancer recurrence or death. Cancer recurrence or death occurred in 57/195 patients (29%; 95% CI, 23%-36%) with a family history of colorectal cancer compared with 343/892 patients (38%; 95% CI, 35%-42%) without a family history.
Figure 2.
Disease-free survival according to family history of colorectal cancer.
Table 2.
Unadjusted and multivariable adjusted hazard ratios for recurrence-free survival, disease-free survival, and overall survival, according to presence of family member with colorectal cancer
| Family Member with Colon or Rectal Cancer |
||
|---|---|---|
| No | Yes | |
|
|
||
| Cancer recurrence or death from any cause (Disease-Free Survival) |
||
| No. of events | 343 | 57 |
| No. at risk | 892 | 195 |
| Unadjusted HR (95% CI) | Referent | 0.71 (0.54-0.94) |
| Adjusted HR (95% CI)* | Referent | 0.72 (0.54-0.96) |
| Cancer recurrence (Recurrence-Free Survival) |
||
| No. of events | 316 | 52 |
| No. at risk | 892 | 195 |
| Unadjusted HR (95% CI) | Referent | 0.71 (0.53-0.95) |
| Adjusted HR (95% CI)* | Referent | 0.74 (0.55-0.99) |
| Overall Mortality | ||
| No. of events | 247 | 43 |
| No. at risk | 892 | 195 |
| Unadjusted HR (95% CI) | Referent | 0.75 (0.54-1.03) |
| Adjusted HR (95% CI)* | Referent | 0.75 (0.54-1.05) |
Multivariate HRs and 95% CIs are adjusted for age (years), gender, race, performance status (0 v 1-2), depth of invasion (T1-2 v T3-4), number of positive lymph nodes (1-3 v 4 or more), presence of clinical perforation at the time of surgery, presence of bowel obstruction at the time of surgery, postoperative CEA (<5, ≥ 5), grade of tumor differentiation (undifferentiated or poorly differentiated v well or moderately differentiated), and treatment arm.
To isolate the influence of family history on cancer recurrences, we used the endpoint of RFS in secondary analyses. Compared with patients without a family history of colorectal cancer, those with a family history had a multivariate HR of 0.74 (95% CI, 0.55-0.99) for cancer recurrence (Table 2). Cancer recurrence occurred in 52/195 patients (27%; 95% CI, 21%-33%) with a family history of colorectal cancer and 316/892 patients (35%; 95% CI, 32%-39%) without a family history. Moreover, adjusted HR for overall mortality among patients with a family history compared to patients without a family history was 0.75 (95% CI, 0.54-1.05). Death occurred in 43/195 patients (22%; 95% CI, 17%-28%) with a family history of colorectal cancer and 247/892 patients (28%; 95% CI, 25%-31%) without a family history.
The apparent benefit associated with family history was stronger with an increasing number of affected family members (Figure 3, Table 3). Although the vast majority of patients with a family history reported one affected relative, there was a significant trend for improvement in DFS with an increasing number of family members. Compared with participants without a family history of colorectal cancer, those with 1 affected relative had a multivariate HR of 0.77 (95% CI, 0.57-1.04) for cancer recurrence or death. For participants with ≥2 affected relatives, we observed a greater reduction in risk (multivariate HR, 0.49; 95% CI, 0.23-1.04; p for trend with increasing number of affected relatives=0.01). Cancer recurrence or death occurred in 50/165 patients (30%; 95% CI 24%-38%) with 1 affected relative, 7/30 patients (23%; 95% CI 12%-41%) with ≥2 affected relatives, and 343/892 patients (38%; 95% CI, 35%-42%) without a family history. We observed similar trends for cancer recurrence (RFS; p for trend=0.03) and overall mortality (OS; p for trend=0.09) (Table 3).
Figure 3.
Disease-free survival according to number of family members with history of colorectal cancer.
Table 3.
Unadjusted and multivariate adjusted hazard ratios for disease-free survival, and overall survival, according to number of family members with colorectal cancer
| Number of Family Members with Colorectal Cancer |
||||
|---|---|---|---|---|
| 0 | 1 | 2 or more | p-trend | |
|
|
||||
| Cancer recurrence or death from any cause (Disease-Free Survival) |
||||
| No. of events | 343 | 50 | 7 | |
| No. at risk | 892 | 165 | 30 | |
| Unadjusted HR (95% CI) | Referent | 0.75 (0.56-1.01) | 0.51 (0.24-1.09) | |
| Adjusted HR (95% CI)* | Referent | 0.77 (0.57-1.04) | 0.49 (0.23-1.04) | 0.01 |
| Cancer recurrence (Recurrence-Free Survival) |
||||
| No. of events | 316 | 46 | 6 | |
| No. at risk | 892 | 165 | 30 | |
| Unadjusted HR (95% CI) | Referent | 0.75 (0.55-1.02) | 0.49 (0.22-1.09) | |
| Adjusted HR (95% CI)* | Referent | 0.79(0.58-1.08) | 0.49 (0.22-1.11) | 0.03 |
| Overall Mortality | ||||
| No. of events | 247 | 36 | 7 | |
| No. at risk | 892 | 165 | 30 | |
| Unadjusted HR (95% CI) | Referent | 0.75 (0.53-1.06) | 0.74 (0.35-1.58) | |
| Adjusted HR (95% CI)* | Referent | 0.77 (0.54-1.10) | 0.65 (0.30-1.40) | 0.09 |
Multivariate HRs and 95% CIs are adjusted for age (years), gender, race, performance status (0 v 1-2), depth of invasion (T1-2 v T3-4), number of positive lymph nodes (1-3 v 4 or more), presence of clinical perforation at the time of surgery, presence of bowel obstruction at the time of surgery, postoperative CEA (<5, ≥ 5), grade of tumor differentiation (undifferentiated or poorly differentiated v well or moderately differentiated), and treatment arm.
We also examined whether the effect of family history varied according to the age at which the first-degree relative was diagnosed with colorectal cancer. Among patients whose relative was diagnosed with colorectal cancer at an age <50 years, the adjusted HR for cancer recurrence or mortality (DFS) was 0.68 (95% CI, 0.36-1.29), compared with patients without a family history of colorectal cancer. For patients with a family member diagnosed with colorectal cancer at age ≥50 years, the adjusted HR for cancer recurrence or mortality was 0.73 (95% CI, 0.53-1.00). Cancer recurrence or death occurred in 10/36 patients (28%; 95% CI, 16%-44%) and 46/155 patients (30%; 95% CI, 23%-37%) with a history of colorectal cancer diagnosed in a family member at an age <50 years and ≥50 years, respectively.
We considered the possibility that patients with a family history of colorectal cancer might have a different prognosis related to earlier detection of their cancers. Although adjusting for depth of invasion (T stage) and nodal status (N stage) and the presence of clinical obstruction or perforation would minimize such biases, we further addressed this concern by repeating our analyses after excluding patients with earlier stage T1 and T2 tumors. However, restricting the analysis to patients with T3 and T4 tumors did not materially alter our results. Compared with patients without a family history, those with one or more affected first-degree relatives experienced a multivariate HR for cancer recurrence or death of 0.69 (95% CI, 0.51-0.94). Among patients with T3-4 tumors, cancer recurrence or death occurred in 49/165 patients (30%; 95% CI, 23%-37%) with a family history of colorectal cancer and 310/753 patients (41%; 95% CI, 38%-45%) without a family history. We also repeated our analyses after excluding patients with a cancer detected in less than four positive lymph nodes (N1). Among patients with N2 disease (≥ 4 positive lymph nodes), our results remained largely unchanged: the adjusted HR for cancer recurrence or death among patients with a family history of colorectal cancer was 0.76 (95% CI, 0.49-1.17). Cancer recurrence or death occurred in 26/66 patients (39%; 95% CI, 29%-51%) with a family history of colorectal cancer and 156/321 patients (49%; 95% CI, 43%-54%) without a family history.
We also assessed the association between family history and disease-free survival across strata of other potential predictors of patient outcome (Figure 4). The effect of family history on the risk of cancer recurrence or mortality was not significantly modified by baseline performance status, number of positive lymph nodes, or treatment arm. In contrast, the effect of family history appeared to differ according to patient age and gender. Among patients <50 years, a family history of colorectal cancer was associated with an adjusted HR for cancer recurrence or death of 1.19 (95% CI, 0.61-2.31); among patients ≥50 years, the adjusted HR for family history was 0.66 (95% CI, 0.48-0.91). For patients <50 years, cancer recurrence or death occurred in 14/40 patients (35%; 95% CI, 22%-50%) and 61/172 patients (35%; 95% CI, 29%-43%) with and without a family history, respectively. For patients ≥50 years, cancer recurrence or death occurred in 43/155 patients (28%; 95% CI, 21%-35%) and 282/720 patients (39%; 95% CI, 36%-43%) with and without a family history, respectively. Among male patients, a family history of colorectal cancer was associated with an adjusted HR for cancer recurrence or death of 0.63 (95% CI, 0.44-0.91); among female patients, the adjusted HR for family history was 1.03 (95% CI, 0.64-1.63). For male patients, cancer recurrence or death occurred in 35/119 patients (29%; 95% CI, 22%-38%) and 202/488 patients (41%; 95% CI, 37%-46%) with and without a family history, respectively. For female patients, cancer recurrence or death occurred in 22/76 patients (29%; 95% CI, 20%-40%) and 141/404 patients (35%; 95% CI, 30%-40%) with and without a family history, respectively. Nonetheless, tests for interaction between patient age and the presence of a family history and patient gender and the presence of a family history did not reach statistical significance (P=.18 and .19, respectively). In addition, the effect of family history appeared stronger when the primary tumor was located in the right colon (cecum to splenic flexure) relative to the left colon (splenic flexure to the rectosigmoid junction). Among patients with tumors located in the right colon, the adjusted HR for DFS in patients with a family history was 0.61 (95% CI, 0.41-0.90), compared with those without a family history of colorectal cancer. Among patients with tumors located in the left colon, the adjusted HR for DFS was 1.01 (95% CI, 0.65-1.56). For patients with right-sided tumors, cancer recurrence or death occurred in 32/115 patients (28%; 95% CI, 20%-37%) and 195/486 patients (40%; 95% CI, 36%-45%) with and without a family history, respectively. For patient with left-sided tumors, cancer recurrence or death occurred in 25/75 patients (33%; 95% CI, 24%-45%) and 138/385 patients (36%; 95% CI, 31%-41%) with and without a family history, respectively. Nonetheless, a test of interaction between the site of the primary tumor and the presence of a family history did not reach statistical significance (p=0.16).
Figure 4.
Stratified Analysis of Disease-Free Survival (Comparison of patients with a family history of colorectal cancer to those without a family history)
We also examined whether family history modified the effect of adjuvant chemotherapy assignment on disease-free survival. Among patients with a family history of colorectal cancer, those randomized to receive irinotecan, 5-fluorouracil, and leucovorin (IFL) had an adjusted HR for cancer recurrence of death of 1.07 (95% CI, 0.60-1.92) when compared to those who received 5-fluorouracil and leucovorin (FU/LV). Similarly, among patients without a family history of colorectal cancer, the adjusted HR of death or recurrence for patients treated with IFL was 1.02 (95% CI, 0.83-1.27) compared to those who received FU/LV. For patients with a family history of colorectal cancer, cancer recurrence or death occurred in 27/93 patients (29%; 95% CI 21%-39%) randomized to receive IFL and 30/102 patients (29%; 95% CI 21%-39%) randomized to receive FU/LV. For patients without a family history of colorectal cancer, cancer recurrence or death occurred in 177/446 patients (40%; 95% CI 35%-44%) randomized to receive IFL and 166/446 patients (37%; 95% CI 33%-42%) randomized to receive FU/LV.
Finally, we considered the possibility that the association between family history and improvement in disease-free survival might be related to microsatellite instability (MSI) status. Information regarding MSI status, as determined by genotyping, was available for 739 patients; information regarding immunostaining for the DNA mismatch repair (MMR) proteins MLH1 and MSH2 was available for 667 patients. The prevalence of MSI-High (MSI-H) tumors was 24% (30/125, 95% CI 17%-32%) and 15% (95/614, 13%-19%) among patients with and without a family history of colorectal cancer, respectively. The prevalence of tumors with deficient staining for MMR proteins was 21% (24/115, 14%-29%) and 11% (63/552, 95% CI 9%-14%) among patients with and without a family history of colorectal cancer, respectively. Results remained largely unchanged after adjustment for MSI status: the adjusted HR for DFS was 0.73 (95% CI, 0.55-0.97) among patients with a family history of colorectal cancer compared with those without a family history. Similarly, the adjusted HR for DFS was 0.73 (95% CI, 0.55-0.97) among patients with a family history of colorectal cancer compared with those without a family history after adjusting for MMR status. Furthermore, the effect of family history on DFS did not appear to be modified by either MSI or MMR status (p for interaction=.51 and .45, respectively).
Discussion
In a cohort of patients with stage III colon cancer treated with surgery and adjuvant chemotherapy, a history of colorectal cancer in a first-degree relative was associated with a significant reduction in cancer recurrence and mortality. Moreover, the apparent benefit associated with family history increased significantly with an increasing number of affected first-degree relatives, and the effect of family history appeared to be independent of tumoral microsatellite instability or DNA mismatch repair status
Numerous studies have demonstrated that family history of colorectal cancer increases the risk of developing colorectal cancer.2-5 However, few studies have examined the influence of family history of colorectal cancer on subsequent outcomes in patients with established cancer. Our findings are consistent with reports from a large registry of Japanese patients, which demonstrated improved five-year survival among patients with colorectal cancer with a family history of the disease.7 In contrast, Slattery and Kerber found no overall effect of family history on survival of patients with colon cancer.6 However, the latter study was based on patients identified through a Utah cancer registry and had limited information on treatment, follow-up care, disease stage, and other prognostic factors.
Our study has several strengths because it is based on patients enrolled in an NCI-sponsored clinical trial. First, all patients had lymph node-positive cancer, reducing the impact of heterogeneity by disease stage. Second, treatment and follow-up care were standardized, and the date and nature of recurrence were recorded prospectively. Finally, extensive and detailed information on other prognostic factors was routinely collected.
Several limitations of this study deserve comment. First, because we relied on self-reported family history, misclassification of family history status may be possible. However, prior studies have demonstrated such data to be reliable.17 Moreover, because the data on family history were collected at study baseline before cancer recurrence, any errors in recall would have attenuated rather than exaggerated a true association with patient outcome.
Additionally, we did not collect information regarding number of siblings, and the likelihood of having a family history of the disease may vary according to the number of siblings at risk for the disease. However, it is unlikely that family size independently affects survival. Moreover, less detailed information of kindred size is likely non-differential and would only bias our results toward finding no association between family history and survival.
Our analysis sought to assess the influence of family history beyond the rare, well-characterized hereditary colorectal cancer syndromes of familial adenomatous polyposis (FAP) or hereditary nonpolyposis colorectal cancer (HNPCC). Beyond obtaining data on colorectal cancer in first-degree family members, our trial did not specifically elicit information about the presence of familial adenomatous polyposis (FAP) or hereditary nonpolyposis colorectal cancer (HNPCC); nonetheless, fewer than 5% of colorectal cancer cases are attributable to these syndromes. Numerous studies demonstrate that a common (i.e., non-syndromic) family history of colorectal cancer significantly increases the risk of developing colorectal cancer, and the current analysis suggests that the presence of a common (i.e., non-syndromic) family history is significantly associated with an improved cancer survival. Although we cannot exclude the possibility that patients with multiple affected relatives in our trial may have included some patients with FAP or HNPCC, the vast majority of patients reporting a family history of colorectal cancer in this study were likely to have an undefined or sporadic familial predisposition towards developing colorectal cancer. Moreover, even among patients with only one affected relative, we observed a 25% reduction in cancer-specific and overall mortality.
We cannot completely exclude the possibility that patients with a family history may experience an improved prognosis due to earlier detection of malignancy. However, the effect of family history persisted after adjusting for other patient and disease characteristics associated with cancer recurrence or survival. Additionally, because patients were entered into a clinical trial that was restricted to stage III cancer, pathologic stage, administration of adjuvant therapy, and follow-up care were reasonably uniform among all participants. Moreover, the benefit associated with family history remained largely unchanged across the number of positive lymph nodes as well as baseline performance status. Although the effect of family history appeared to be modified by patient age, sex, and tumor location, tests for interaction were not significant, possibly because of limited statistical power. Further investigation to explore the relationship between family history and these factors is required.
Finally, because our study was based on a well-defined cohort enrolled in a clinical trial, our results may not be generalizable to a larger population of patients with colon cancer. However, the rate of family history in this cohort is similar to the general population of patients with colon cancer, and there is no evidence that family history appreciably varies according to participation in a clinical trial.
Studies suggest the importance of genetic contributions to the development of familial colorectal cancers.18-20 However, the relationship between family history and outcome is likely to be complex and may be influenced by a confluence of genetic and environmental factors. We considered whether shared environmental or lifestyle factors might contribute to our findings but found no significant difference in smoking, median household income, body-mass index, diet, and physical activity patterns between patients with and without a family history of colorectal cancer (Table 1).
Beyond rare, well-characterized hereditary colorectal cancer syndromes (e.g. FAP or HNPCC), our data support the hypothesis that a relatively common though less penetrant genetic predisposition may not only influence colorectal cancer risk but also patient survival. This finding may reflect a distinct underlying molecular and pathogenic mechanism in cancers that develop in the setting of a common (i.e., sporadic) family history. For example, data suggest that family history of colorectal cancer is associated with tumors with a higher frequency of microsatellite instability (MSI-H),21, 22 which may be associated with improved prognosis compared to microsatellite stable tumors.23-27 Consistent with the observation that MSI-H tumors are more common in the right colon,12, 28 the beneficial effect of family history in the current study appeared greater among patients with right-sided colon cancer. However, our results remained largely unchanged after adjustment for MSI or MMR status, and we found no significant interaction between family history and MSI or MMR. This suggests that the association between a sporadic (i.e., non-syndromic) family history and reduction in risk of cancer recurrence or death may be independent of MSI or MMR. Nonetheless, family history may influence cancer prognosis through other pathways. Further studies are needed to more fully elucidate potential mechanisms by which a common family history may influence the outcome for patients with colorectal cancer.
Acknowledgements
Cancer and Leukemia Group B (CALGB) 89803 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, M.D., Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601) as well as support from Pharmacia & Upjohn Company, now Pfizer Oncology. Dr. Chan is supported in part by a T32 grant from the National Cancer Institute (T32 CA009001) and CA118553.
Role of the Sponsor: The sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Clinical Centers: The following institutions participated in this study:
Baptist Cancer Institute CCOP, Memphis, TN–Lee S. Schwartzberg, M.D., supported by CA71323
Christiana Care Health Services, Inc. CCOP, Wilmington, DE–Stephen Grubbs, M.D.., supported by CA45418
University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, M.D., supported by CA47559
University of Chicago, Chicago, IL –Gini Fleming, M.D., supported by CA41287
Dartmouth Medical School - Norris Cotton Cancer Center, Lebanon, NH–Marc S. Ernstoff, M.D., supported by CA04326
Duke University Medical Center, Durham, NC–Jeffrey Crawford, M.D., supported by CA47577
Dana-Farber Cancer Institute, Boston, MA–Eric P. Winer, M.D., supported by CA32291
Georgetown University Medical Center, Washington, DC, Edward Gelmann, M.D., supported by CA77597
Cancer Centers of the Carolinas, Greenville, SC–Jeffrey K. Giguere, M.D, supported by CA29165
University of Illinois MBCCOP, Chicago, IL–Lawrence E. Feldman, M.D., supported by CA74811
University of Iowa, Iowa City, IA–Gerald Clamon, MD, supported by CA47642
North Shore - Long Island Jewish Medical Center, Manhasset, NY–Daniel R Budman, M.D., supported by CA35279
University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, M.D., supported by CA31983
University of Massachusetts Medical School, Worcester, MA–William V. Walsh, M.D., supported by CA37135
Massachusetts General Hospital, Boston, MA–Michael L. Grossbard, M.D., supported by CA12449
Mount Sinai Medical Center, Miami, FL–Rogerio Lilenbaum, MD, supported by CA45564
University of Minnesota, Minneapolis, MN–Bruce A Peterson, M.D., supported by CA16450
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C Perry, M.D., supported by CA12046
Mount Sinai School of Medicine, New York, NY–Lewis R. Silverman, M.D., supported by CA04457
Memorial Sloan-Kettering Cancer Center, New York, NY, Clifford Hudis, MD, supported by CA77651
University of Nebraska Medical Center, Omaha, NE–Anne Kessinger, M.D., supported by CA77298
Long Island Jewish Medical Center, Lake Success, NY–Marc Citron, M.D., supported by CA11028
The Ohio State University Medical Center, Columbus, OH–Clara D Bloomfield, M.D., supported by CA77658
Rhode Island Hospital, Providence, RI–William Sikov, M.D., supported by CA08025
Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, M.D., supported by CA02599
Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC–James N. Atkins, M.D., supported by CA45808
Southern Nevada Cancer Research Foundation CCOP, Las Vegas, NV–John Ellerton, M.D., supported by CA35421
Syracuse Hematology-Oncology Assoc. CCOP, Syracuse, NY–Jeffrey Kirshner, M.D., supported by CA45389
University of Tennessee Memphis, Memphis, TN–Harvey B. Niell, M.D., supported by CA47555
University of California at San Diego, San Diego, CA–Joanne Mortimer, M.D., supported by CA11789
University of California at San Francisco, San Francisco, CA–Alan P. Venook, M.D., supported by CA60138
Vermont Cancer Center, Burlington, VT–Hyman B. Muss, M.D., supported by CA77406
Wake Forest University School of Medicine, Winston-Salem, NC–David D Hurd, M.D., supported by CA03927
Walter Reed Army Medical Center, Washington, DC–Thomas Reid, M.D., supported by CA26806
Washington University School of Medicine, St. Louis, MO–Nancy Bartlett, MD, supported by CA77440
Weill Medical College of Cornell University, New York, NY–Scott Wadler, M.D., supported by CA07968
Footnotes
Financial Disclosures: None reported.
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
References
- 1.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003 Mar 6;348(10):919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994 Dec 22;331(25):1669–1674. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 3.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001 Oct;96(10):2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 4.Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer: a review of the epidemiology. Epidemiol Rev. 1993;15(2):499–545. doi: 10.1093/oxfordjournals.epirev.a036132. [DOI] [PubMed] [Google Scholar]
- 5.Slattery ML, Kerber RA. Family history of cancer and colon cancer risk: the Utah Population Database. J Natl Cancer Inst. 1994 Nov 2;86(21):1618–1626. doi: 10.1093/jnci/86.21.1618. [DOI] [PubMed] [Google Scholar]
- 6.Slattery ML, Kerber RA. The impact of family history of colon cancer on survival after diagnosis with colon cancer. Int J Epidemiol. 1995 Oct;24(5):888–896. doi: 10.1093/ije/24.5.888. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and pathological analyses of patients with a family history of colorectal cancer. Registry Committee, Japanese Research Society for Cancer of the Colon and Rectum. Jpn J Clin Oncol. 1993 Dec;23(6):342–349. [PubMed] [Google Scholar]
- 8.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007 Aug 10;25(23):3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006 Aug 1;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. Jama. 2007 Aug 15;298(7):754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 11.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002 Feb 15;20(4):1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 12.Bertagnolli MM, Compton CC, Niedzwiecki D, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, 5-fluorouracil and leucovorin in stage III colon cancer. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings. 2006;Vol 24(No. 18S) doi: 10.1200/JCO.2008.18.2071. abstract 10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Therneau T, Grambsch P. Modeling Survival Data. Springer; New York, NY: 2000. [Google Scholar]
- 14.Jones MP, Crowley J. A general class of nonparametric tests for survival analysis. Biometrics. 1989 Mar;45(1):157–170. [PubMed] [Google Scholar]
- 15.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002 Apr 13;359(9314):1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Takkenberg JJ, Grunkemeier GL. Measuring follow-up completeness. Ann Thorac Surg. 2008 Apr;85(4):1155–1157. doi: 10.1016/j.athoracsur.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Kerber RA, Slattery ML. Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol. 1997 Aug 1;146(3):244–248. doi: 10.1093/oxfordjournals.aje.a009259. [DOI] [PubMed] [Google Scholar]
- 18.Cannon-Albright LA, Skolnick MH, Bishop DT, Lee RG, Burt RW. Common inheritance of susceptibility to colonic adenomatous polyps and associated colorectal cancers. N Engl J Med. 1988 Sep 1;319(9):533–537. doi: 10.1056/NEJM198809013190902. [DOI] [PubMed] [Google Scholar]
- 19.Cannon-Albright LA, Thomas TC, Bishop DT, Skolnick MH, Burt RW. Characteristics of familial colon cancer in a large population data base. Cancer. 1989 Nov 1;64(9):1971–1975. doi: 10.1002/1097-0142(19891101)64:9<1971::aid-cncr2820640935>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007 Oct 15;21(20):2525–2538. doi: 10.1101/gad.1593107. [DOI] [PubMed] [Google Scholar]
- 21.Ogino S, Kirkner G, Cantor M. Family History of Sporadic Colorectal Cancer Modifies Risks for Specific Molecular Pathology in Tumors: Data from a Prospective Cohort of 86,220 Women. Modern Pathology. 2005;18:114A. al. e. [Google Scholar]
- 22.Ricciardiello L, Goel A, Mantovani V, et al. Frequent loss of hMLH1 by promoter hypermethylation leads to microsatellite instability in adenomatous polyps of patients with a single first-degree member affected by colon cancer. Cancer Res. 2003 Feb 15;63(4):787–792. [PubMed] [Google Scholar]
- 23.Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000 Jan 13;342(2):69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 24.Parc Y, Gueroult S, Mourra N, et al. Prognostic significance of microsatellite instability determined by immunohistochemical staining of MSH2 and MLH1 in sporadic T3N0M0 colon cancer. Gut. 2004 Mar;53(3):371–375. doi: 10.1136/gut.2003.019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001 Sep;10(9):917–923. [PubMed] [Google Scholar]
- 26.Benatti P, Gafa R, Barana D, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005 Dec 1;11(23):8332–8340. doi: 10.1158/1078-0432.CCR-05-1030. [DOI] [PubMed] [Google Scholar]
- 27.Sinicrope FA, Rego RL, Halling KC, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006 Sep;131(3):729–737. doi: 10.1053/j.gastro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Sinicrope FA, Rego RL, Foster N, et al. Microsatellite instability accounts for tumor site-related differences in clinicopathologic variables and prognosis in human colon cancers. Am J Gastroenterol. 2006 Dec;101(12):2818–2825. doi: 10.1111/j.1572-0241.2006.00845.x. [DOI] [PubMed] [Google Scholar]