Abstract
Osteopontin (OPN) is a pro-inflammatory cytokine that can be secreted from many cells including activated macrophages and T-lymphocytes. Elevated levels of osteopontin in the plasma, cerebrospinal fluid or brain of individuals with neurodegenerative diseases such as multiple sclerosis (MS), Parkinson’s and Alzheimer’s disease and more recently in HIV-associated neurocognitive disorder has been reported. However, except for the case of MS, little is known regarding the molecular mechanisms by which OPN may exacerbate disease. Alternatively, OPN through its ability to promote cell survival may in some contexts function in the brain in a protective capacity. OPN has several protein motifs that allow it to engage with several different signaling pathways involved in immunity and inflammation. A better understanding of the cellular pathways that are regulated by OPN in cells of the central nervous system is required to uncover its putative role in neuronal homeostasis.
Keywords: Neurodegeneration, Integrin, CD44, HIV-associated neurocognitive disorder, Macrophage, Microglia
While we know of no infectious agent that can induce the development of multiple sclerosis (MS), Parkinson’s or Alzheimer’s disease, it is well established that HIV infection of the central nervous system (CNS) can lead to the development of neurological dysfunction. The neuropathogenic insults mediated by HIV infection can result in neuronal degeneration, which, in the absence of treatment, can progress to frank neuronal loss. Osteopontin (OPN) is a multifunctional, pro-inflammatory cytokine, which has been linked to cancer, cell-mediated immunity, inflammation, and neurodegenerative diseases including MS [1,2], Alzheimer’s [3–5], and Parkinson’s [6,7] diseases, frontotemporal dementia [8] and more recently, shown to be increased in the cerebrospinal fluid of individuals infected with HIV-1 [9]. Only in the case of multiple sclerosis is there some insight regarding the biological pathways and molecular mechanisms to explain how OPN may contribute to the neurodegenerative process in this disease [2]. There is an urgent need to better understand the regulation of OPN expression, how it modulates HIV replication and to identify and study the critical signaling pathways that are modulated by OPN. In this review, we will explore the putative links between OPN, immunity, inflammation, and neurodegeneration in HIV infection. This line of investigation will likely prove to have farreaching implications regarding translational therapeutic interventions, as current knowledge strongly suggests that common pathological processes, particularly with regard to inflammation, exist amongst several different neurodegenerative diseases.
Before the availability of antiretrovirals, HIV-associated dementia was an AIDS-defining illness seen in 20–30% of infected individuals and was associated with a severe decline in CD4+ T-lymphocytes [10]. Clinical features include short-term memory loss, mental slowing, reading and comprehension difficulties, apathy, gait disturbance, tremor and impairment of fine motor dexterity [10]. Neuropathological features of HIV-associated dementia include HIV encephalitis, reactive astrocytosis, myelin pallor, microglial nodules, an increase in activated macrophages and resident microglia, and multi-nucleated giant cells [10,11]. While HIV-associated dementia has declined significantly with the introduction of combination antiretroviral therapy, milder yet insidious forms of cognitive impairment that affect activities of daily living remain prevalent at a relatively high frequency [12,13]. The term HIV-associated neurocognitive disorders (HAND) is now used to more accurately reflect the spectrum of disease now seen in the clinic [14]. There is general consensus that the inopportune release from activated HIV-infected macrophages of cytokines and factors that are toxic to cells in the CNS leads to injury, death and dysfunction of neurons as well as the aberrant function of glia [15].
A major focus of the field is to identify a set of biomarkers that can be used not only to predict which individuals may be at risk for HIV-associated neurocognitive impairment, but also for use as a clinical marker to diagnose disease or serve as a reference marker to determine the efficacy of therapeutic agents. Animal models for HIV provide an excellent platform for studying how viral infection alters host cell gene expression in the CNS. Microarray analyses using the simian immunodeficiency virus (SIV) rhesus macaque as a model for HIV CNS disease identified OPN as a significantly upregulated RNA message in the brains of infected monkeys with SIV encephalitis (SIVE) [16]. Further studies showed that OPN levels were elevated in the plasma of SIV-infected macaques with SIVE and that an increased level of monocytes expressing a receptor for OPN, CD44v6 could predict which animals would develop CNS disease [17]. In vitro studies using an artificial blood-brain-barrier model suggested that OPN-mediated recruitment of monocytes to the brain and the ability of OPN to promote monocyte survival helped to fuel CNS disease in the macaque model [18].
In contrast to neurons, macrophages and microglia can be productively infected with HIV and hence these cells play a central role in the pathogenesis of cognitive impairment in HIV infection. Therefore, we focused on identifying host factors that are modulated by the virus in this cell type using an in vitro model of primary human monocyte-derived macrophages infected with a green-fluorescent-tagged HIV [19,20]. Using PCR-subtractive hybridization we identified OPN as a protein that was significantly upregulated in HIV-infected monocyte-derived macrophages [9]. Knockdown studies in human macrophages revealed that HIV replication is impaired 50%, suggesting that OPN makes a significant positive contribution to the ability of HIV to replicate in macrophages [9]. Using a surrogate cell culture model, OPN was shown to enhance HIV replication through a pathway involving the activation of NF-κB. Moreover, the effect of OPN required an intact NF-κB binding site within the HIV-1 promoter [9].
These in vitro findings were corroborated with ex vivo studies using tissues from the multicenter Northeast AIDS Dementia Cohort (NEAD). HIV-infected individuals with advanced disease underwent extensive neurocognitive testing and sampling of body fluids in a longitudinal fashion [21–23]. While no difference in the level of the protein in plasma was found, mean OPN levels were significantly elevated in the cerebrospinal fluid (CSF) of HIV-infected persons compared to controls [9]. This is in contrast to an earlier study that used a different cohort of patients that found differences in OPN levels in the plasma amongst individuals with differing degrees of impairment, but not in CSF [18]. This difference between the two studies might be explained by the use in the former study of an in-house OPN ELISA assay developed using monoclonal antibodies specifically to the OPN isoform that was increased in HIV-infected macrophages. Commercially available OPN ELISAs do not distinguish between the three different isoforms or cleaved forms of OPN that can potentially exist in a biological sample [24]. Western blot analyses of brain tissue from the occipital lobe of HIV-infected individuals confirmed that OPN expression was significantly increased in those with moderate to severe cognitive impairment compared to normal controls [9].
Interestingly, HIV-infected individuals in the NEAD cohort without cognitive impairment had mean OPN levels in the CSF that were significantly higher than normal controls, suggesting a chronic and sustained release of OPN by cells in the CNS [9]. In HIV-infected individuals with the most severe cognitive impairment, mean OPN levels in the CSF were 2-fold higher than that seen in samples from patients with relapsing-remitting MS [9]. It is likely that although HIV-infected individuals were on antiviral therapy, damage to the CNS continued to occur, leading to the chronic release of inflammatory agents that then worked in a feedback loop leading to further neuronal degeneration. Also to be considered in the HIV NEAD cohort, is the impact of past and/or current use of drugs of abuse on the expression of inflammatory mediators and CNS damage. Residual replication of HIV in the CNS may be another contributing factor to chronic inflammation as antiviral regimens differ in their ability to effectively penetrate the blood-brain-barrier [25].
As a member of the innate immune system, macrophages in peripheral tissues or the brain play a central, first-line of defense role against intracellular pathogens that target this cell type. Interestingly, a role for osteopontin in immunity was discovered through efforts to identify the gene responsible for resistance to Rickettsia [26]. OPN knockout mice challenged with other intracellular pathogens including Mycobacterium tuberculosis, Listeria monocytogenes, Flavivirus, Rotavirus, and herpes simplex virus type 1 are also more susceptible to disease and death [27–30]. A clue to how OPN stimulates cell-mediated immunity came from the early literature describing a factor denoted Early T-lymphocyte Activation-1 (ETA-1), a secreted protein found in activated T-cells, that was later found to be identical to OPN that stimulates the release of IL-12 from macrophages and dendritic cells, thus driving the development and activation of T-helper-1, T-cells [31–33]. OPN can act as a chemoattractant for T-cells and cells of the monocyte lineage [34] and a non-secreted form of OPN has been shown to stimulate through a TLR9 pathway, the release of interferon alpha from dendritic cells [35]. A recent study suggests that OPN, again through its regulation of IL-12 release from antigen presenting cells, can alter the development of CD8+ T-cell memory cells in influenza infection [36]. Identifying specific cytokine pathways in macrophages and microglia that are modulated by OPN and impact their function in neurodegenerative processes will allow greater understanding regarding the mode of action of OPN in the brain.
A possible role for OPN in neurodegenerative disease was first reported in MS. Gene and protein expression analyses in experimental autoimmune encephalitis (EAE) and on MS plaques showed that OPN was significantly upregulated [37,38]. Similar findings have been reported for plasma [39,40] and more recently increased levels of OPN were found in the CSF of MS patients [41]. In the EAE animal model of MS, OPN knockout mice were protected from development of disease [37]. Hur et al., showed that OPN, through its ability to activate the transcription factor NF-κB and stimulate Fox3a, prevented apoptosis of myelin-reactive T-cells thus implicating OPN in the exacerbation of MS [2]. Recently, it was reported that OPN is significantly increased in the CSF of individuals with human African trypanosomiasis suffering from meningeal encephalomyelitis caused by parasites that have crossed the blood-brain-barrier and invaded the brain [42].
The motif-rich structure of OPN gives us clues to its ability to function in several different cellular pathways. Osteopontin is a 314 amino acid multifunctional protein that was discovered in bone, transformed cell lines, macrophages and T-cells [43,44]. In bone it was identified as a highly phosphorylated secreted phosphoprotein-1 (SPP-1, gene name) involved in bone mineralization. OPN can also be modified by glycosylation, and together with bone sialoprotein, dentin matrix protein I, dentin phosphosialoprotein, matrix extracellular phosphoprotein, is a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family of proteins [45]. At the carboxyl terminus is a putative CD44/heparin binding domain. This domain and a putative calcium-binding sequence were identified using recombinant OPN proteins and tested for interactions using biochemical assays. OPN has an Arg-Gly-Asp (RGD) integrin-binding domain followed by a second domain that has also been shown to bind to several different subsets of integrins [43]. Post-translational modifications to OPN, particularly phosphorylation has been shown to play a role in its function [46]. Indeed, few studies have used molecular approaches to analyze deletion mutants of OPN to determine which domains of OPN are required for its specific functions. Interestingly, cleavage of OPN by thrombin results in two proteins that are functionally active. The N-terminal half that can bind to integrins was shown to stimulate IL-12 release, while interaction of OPN with CD44 through its C-terminal end was required for blocking IL-10 production [33]. Moreover, phosphorylation of OPN was required for its ability to stimulate IL-12 release [33]. These results suggest that specific OPN motifs can signal through distinct signaling pathways. When the protein is intact, perhaps interaction with yet to be identified modulatory proteins inhibit or direct signaling through specific pathways.
Monocytes released from the bone marrow enter tissues after a few days where sensing cues from the local microenvironment, differentiate into mature macrophages specialized for their required functions in the lung, liver, kidney, gut, lymph nodes, at mucosal surfaces and in brain. Studies from mice have revealed that microglia in the brain are formed during ontogeny and that local proliferation of microglia at this site can occur [47]. Interestingly, in the bone, monocytes differentiate into multinucleated giant cells called osteoclasts through CD44-mediated fusion induced by OPN [48]. The recognition that cells regulating bone homeostasis differentiate from monocytes and retain many attributes of these cells has led to the emerging field of osteoimmunology [49–51]. In addition, findings suggest that cytokine release from T-cells, monocytes and B-cells plays an important role in regulating the bone resorption and formation functions of osteoclasts, osteoblasts and of mesenchymal stem cells in the bone marrow niche [48,50,52]. While age and antiretroviral drug use are factors in bone mineral density loss, HIV infection is an independent risk factor for the development of osteopenia and osteoporosis [50,53]. Indeed, rats expressing the HIV envelope transgene show significant decreases in bone mineral density compared to normal controls [54]. While associations between the turnover of factors important in bone homeostasis such as osteoprotegerin and receptor activator of NF-κB ligand (RANKL) in HIV-infected patients have been examined [53], little is known about any potential relationship between OPN and the risk for osteopenia or osteoporosis in these individuals [55,56].
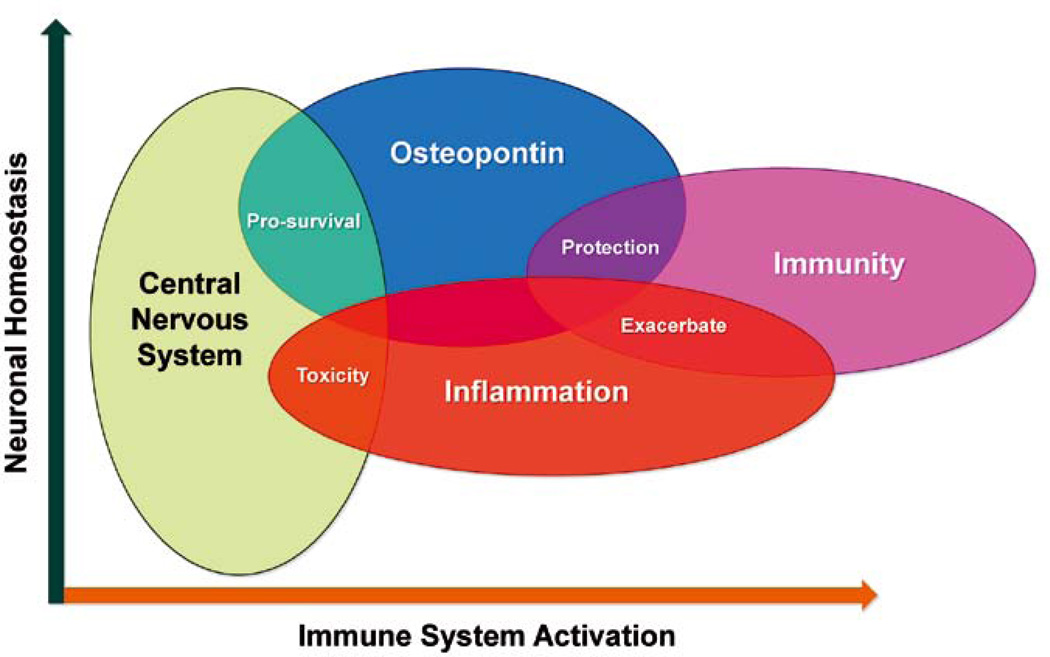
OPN is a good example of an early-response signaling factor that acts at the neuroimmune axis (Figure 1). Early sentinels, macrophages become activated responding to the infiltration of intracellular pathogens like HIV into the CNS or to neuronal damage and increase their expression of OPN, a danger signal that in turn leads to the expression of inflammatory cytokines that drive protective adaptive immune responses. As we have learned with HIV, it is a pathogen that has evolved to hijack aspects of the host immune response [57]. OPN via activation of NF-κB signaling, results in the inadvertent upregulation of HIV replication thus exacerbating virus replication [9]. Through signaling via its receptors, various integrins and CD44, OPN can activate pro-survival pathways [34], elaborate the release of cytokines from macrophages, T-cells and perhaps from other cells in the CNS. Indeed, a subset of neurons can express OPN and its receptors [3,6,7,58–61]. A recent study found a significant reduction of proliferating oligodendrocytes in the brains of OPN−/− neonatal mice after hypoxic-ischemic brain injury, suggesting that OPN plays a role in cell survival and repair [62]. Sustained chronic activation of macrophages perhaps both in the periphery and the CNS, release of OPN and other inflammatory molecules can lead to toxicity and neuronal degeneration as associations with MS suggests. Studies have shown in the context of the developing skull or after mechanical stress post calvarial suture that abundant OPN expressing osteoblasts are recruited to the site of growth or injury respectively, and are involved in repair [63–65]. Whether OPN from the latter source contributes to the CSF pool of the cytokine is not currently known.
Figure 1.
Osteopontin (OPN) as an inflammatory mediator at the neuroimmune axis. OPN secreted from activated macrophages and microglia, and possibly other cells in the CNS can, through the release of specific cytokines such as IL-12, stimulate cell-mediated immune responses of the adaptive arm of the immune system. Inadvertently, OPN via activation of signaling pathways that induce NF-κB, can upregulate HIV-1 replication, thus exacerbating virus replication. OPN can also modulate the expression of other inflammatory cytokines. When OPN release is chronic and sustained, toxicity can result that can lead to the dysfunction and degeneration of neurons and perhaps of glia as well. Alternatively, in models of hypoxic ischemia, OPN can promote cell survival by inhibition of apoptotic pathways.
Current anti-HIV therapies that are effective in suppressing viral load do not completely reverse inflammation in the CNS [66, 67]. Identifying the critical signaling pathways that are regulated by OPN in macrophages and microglia and whether there are any direct effects of OPN on neurons is necessary for a greater understanding of its putative role in neuronal degeneration in HIV infection and for the development of strategies to counter its action.
Acknowledgements
This work was supported by a grant from the Margaret Q. Landenberger Foundation and R21MH095646 from the National Institutes of Mental Health.
Footnotes
The author declares no conflict of interests.
References
- 1.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: Attenuated experimental autoimmune enchephalomyelitis in eta-1/osteopontin-deficient mice. J. Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 2.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 2006;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 3.Wung JK, Perry G, Kowalski A, Harris PL, Bishop GM, Trivedi MA, et al. Increased expression of the remodeling- and tumorigenic-associated factor osteopontin in pyramidal neurons of the Alzheimer’s disease brain. Curr. Alzheimer Res. 2007;4:67–72. doi: 10.2174/156720507779939869. [DOI] [PubMed] [Google Scholar]
- 4.Wirths O, Breyhan H, Marcello A, Cotel MC, Brück W, Bayer TA. Inflammatory changes are tightly associated with neurodegeneration in the brain and spinal cord of the APP/PS1KI mouse model of Alzheimer’s disease. Neurobiol. Aging. 2010;31:747–757. doi: 10.1016/j.neurobiolaging.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Comi C, Carecchio M, Chiocchetti A, Nicola S, Galimberti D, Fenoglio C, et al. Osteopontin is increased in the cerebrospinal fluid of patients with Alzheimer’s disease and its levels correlate with cognitive decline. J. Alzheimers Dis. 2010;19:1143–1148. doi: 10.3233/JAD-2010-1309. [DOI] [PubMed] [Google Scholar]
- 6.Maetzler W, Berg D, Schalamberidze N, Melms A, Schott K, Mueller JC, et al. Osteopontin is elevated in Parkinson’s disease and its absence leads to reduced neurodegeneration in the MPTP model. Neurobiol. Dis. 2007;25:473–482. doi: 10.1016/j.nbd.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Iczkiewicz J, Jackson MJ, Smith LA, Rose S, Jenner P. Osteopontin expression in substantia nigra in MPTP-treated primates and in Parkinson’s disease. Brain Res. 2006;1118:239–250. doi: 10.1016/j.brainres.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Mattson N, Rüetschi U, Pijnenburg YA, Blankenstein MA, Podust VN, Li S, et al. Novel cerebrospinal fluid biomarkers of axonal degeneration in frontotemporal dementia. Mol. Med. Report. 2008;1:757–761. doi: 10.3892/mmr_00000025. [DOI] [PubMed] [Google Scholar]
- 9.Brown A, Islam T, Adams R, Nerle S, Kamara M, Eger C, et al. Osteopontin enhances HIV replication and is increased in the brain and cerebrospinal fluid of HIV-infected individuals. J. Neurovirol. 2011;17:382–392. doi: 10.1007/s13365-011-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann. Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 11.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann. Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 12.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, et al. HIV-associated neurocognitive disorder before and during the era of combination antiretroviral therapy: differences in rates, nature and predictors. J. Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol. Dis. 2010;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdo TH, Wood MR, Fox HS. Osteopontin prevents monocyte recirculation and apoptosis. J. Leukoc. Biol. 2007;81:1504–1511. doi: 10.1189/jlb.1106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcondes MC, Lanigan CM, Burdo TH, Watry DD, Fox HS. Increased expression of monocyte CD44v6 correlates with the development of encephalitis in rhesus macaques infected with simian immunodeficiency virus. J. Infect. Dis. 2008;197:1567–1576. doi: 10.1086/588002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdo TH, Ellis RJ, Fox HS. Osteopontin is increased in HIV-associated dementia. J. Infect. Dis. 2008;198:715–722. doi: 10.1086/590504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown A, Gartner S, Kawano T, Benoit N, Cheng-Mayer C. HLA-A2 down-regulation on primary human macrophages infected with an M-tropic EGFP-tagged HIV-1 reporter virus. J. Leukoc. Biol. 2005;78:675–685. doi: 10.1189/jlb.0505237. [DOI] [PubMed] [Google Scholar]
- 20.Brown A, Zhang H, Lopez P, Pardo CA, Gartner S. In vitro modeling of the HIV-macrophage reservoir. J. Leukoc. Biol. 2006;80:1127–1135. doi: 10.1189/jlb.0206126. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler-Heitbrock HWL, Fingerle G, Ströbel M, Schraut W, Stelter F, Schütt C, et al. The novel subset of CD14+ CD16+ blood monocytes exhibits features of tissue macrophages. Eur. J. Immunol. 1993;23:2053–2058. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 22.Marder K, Albert SM, McDermott MP, McArthur JC, Schifitto G, Selnes OA, et al. Inter-rater reliability of a clinical staging of HIV-associated cognitive impairment. Neurology. 2003;60:1467–1473. doi: 10.1212/01.wnl.0000064172.46685.82. [DOI] [PubMed] [Google Scholar]
- 23.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J. Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 24.Anborgh PH, Wilson SM, Tuck AB, Winquist E, Schmidt N, Hart R, et al. New dual monoclonal ELISA for measuring plasma osteopontin as a biomarker associated with survival in prostate cancer: clinical validation and comparison of multiple ELISAs. Clin. Chem. 2009;55:895–903. doi: 10.1373/clinchem.2008.117465. [DOI] [PubMed] [Google Scholar]
- 25.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lampe MA, Patarca R, Iregui MV, Cantor H. Polyclonal B cell ativation by the Eta-1 cytokine and the development of systemic autoimmune disease. J. Immunol. 1991;147:2902–2906. [PubMed] [Google Scholar]
- 27.Saito Y, Kon S, Fujiwara Y, Nakayama Y, Kurotaki D, Fukuda N, et al. Osteopontin small interfering RNA protects mice from fulminant hepatitis. Hum. Gene Ther. 2007;18:1205–1214. doi: 10.1089/hum.2007.069. [DOI] [PubMed] [Google Scholar]
- 28.Rollo EE, Hempson SJ, Bansal A, Tsao E, Habib J, Rittling SR, et al. The cytokine osteopontin modulates the severity of rotavirus diarrhea. J. Virol. 2005;79:3509–3516. doi: 10.1128/JVI.79.6.3509-3516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patarca R, Saavedra RA, Cantor H. Molecular and cellular basis of genetic resistance to bacterial infection: the role of the early T-lymphocyte activation-1/osteopontin gene. Crit. Rev. Immunol. 1993;13:225–246. [PubMed] [Google Scholar]
- 30.Nau GJ, Liaw L, Chupp GL, Berman JS, Hogan BL, Young RA. Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect. Immun. 1999;67:4223–4230. doi: 10.1128/iai.67.8.4223-4230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin/Eta-1. Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 32.Patarca R, Freeman GJ, Singh RP, Wei FY, Durfee T, Blattner F, et al. Structural and functional studies of the early T-lymphocyte activation 1 (Eta-1) gene. J. Exp. Med. 1989;170:145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jannson M, Zawaideh S, et al. Eta-1 (Osteopontin): An early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 34.Wang KX, Denhardt DT. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat. Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morimoto J, Sato K, Nakayama Y, Kimura C, Kajino K, Matsui Y, et al. Osteopontin modulates the generation of memory CD8+ T cells during influenza virus infection. J. Immunol. 2011;187:5671–5683. doi: 10.4049/jimmunol.1101825. [DOI] [PubMed] [Google Scholar]
- 37.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 38.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 39.Vogt MH, Floris S, Killestein J, Knol DL, Smits M, Barkhof F, et al. Osteopontin levels and increased disease activity in relapsing remitting multiple sclerosis patients. J. Neuroimmunol. 2004;155:155–160. doi: 10.1016/j.jneuroim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Comabella M, Pericot I, Goertsches R, Nos C, Castillo M, Blas Navarro J, et al. Plasma osteopontin levels in multiple sclerosis. J. Neuroimmunol. 2005;158:231–239. doi: 10.1016/j.jneuroim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Braitch M, Nunan R, Niepel G, Edwards LJ, Constantinescu CS. Increased osteopontin levels in the cerebrospinal fluid of patients with multiple sclerosis. Arch. Neurol. 2008;65:633–635. doi: 10.1001/archneur.65.5.633. [DOI] [PubMed] [Google Scholar]
- 42.Tiberti N, Hainard A, Lejon V, Robin X, Ngoyi DM, Turck N, et al. Discovery and verification of osteopontin and Beta-2-microglobulin as promising markers for staging human African trypanosomiasis. Mol. Cell. Proteomics. 2010;9:2783–2795. doi: 10.1074/mcp.M110.001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sodek J, Ganss B, McKeee MD. Osteopontin. Crit. Rev. Oral Biol. Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 44.Denhardt DT, Guo X. Osteopontin: A protein with diverse functions. FASEB J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- 45.Bellahcène A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-liked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat. Rev. Cancer. 2008;8:212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J. Cell. Biochem. 2007;102:912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- 47.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki K, Takeyama S, Sakai Y, Yamada S, Shinoda H. Current topics in pharmacological research on bone metabolism: inhibitory effects of bisphosphonates on the differentiation and activity of osteoclasts. J. Pharmacol. Sci. 2006;100:189–194. doi: 10.1254/jphs.fmj05004x2. [DOI] [PubMed] [Google Scholar]
- 49.Ofotokun I, Weitzmann MN. HIV and bone metabolism. Discovery Med. 2011;11:385–393. [PMC free article] [PubMed] [Google Scholar]
- 50.Ofotokun I, Weitzmann MN. HIV: inflammation and bone. Curr. HIV/ AIDS Rep. 2012;9:16–25. doi: 10.1007/s11904-011-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clowes J, Riggs BL, Khosia S. The role of immune system in the pathophysiology of osteoporosis. Immunol. Rev. 2005;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 52.Pirraco RP, Reis RL, Marques AP. Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs. J. Tissue Eng. Regen. Med. 2012 doi: 10.1002/term.535. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Brown TT, Ross AC, Storer N, Labbato D, McComsey GA. Bone turnover, osteoprotegerin/RANKL and inflammation with antiretroviral initiation: tenofovir versus non-tenofovir regimens. Antivir. Ther. 2011;16:1063–1072. doi: 10.3851/IMP1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vikulina T, Fan X, Yamaguchi M, Roser-Page S, Zayzafoon M, Guidot DM, et al. Alterations in the immuno-skeletal interface drive bone distruction in HIV-1 transgenic rats. Proc. Natl. Acad. Sci. USA. 2010;107:13848–13853. doi: 10.1073/pnas.1003020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haskelberg H, Carr A, Emery S. Bone turnover markers in HIV disease. AIDS Rev. 2011;13:240–250. [PubMed] [Google Scholar]
- 56.Walker Harris B, Brown TT. Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. J. Infect. Dis. 2012;205(Suppl. 3):S391–S398. doi: 10.1093/infdis/jis199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coiras M, López-Huertas MR, Sánchez del Cojo M, Mateos E, Alcamí J. Dual role of host cell factors in HIV-1 replication: restriction and enhancement of the viral cycle. AIDS Rev. 2010;12:103–112. [PubMed] [Google Scholar]
- 58.Shin SL, Cha JH, Chun MH, Chung JW, Lee MY. Expression of osteopontin mRNA in the adult rat brain. Neurosci. Lett. 1999;273:73–76. doi: 10.1016/s0304-3940(99)00516-9. [DOI] [PubMed] [Google Scholar]
- 59.Ichikawa H, Itota T, Nishitani Y, Torii Y, Inoue K, Sugimoto T. Osteopontin-immunoreactive primary sensory neurons in the rat spinal and trigeminal nervous systems. Brain Res. 2000;863:276–281. doi: 10.1016/s0006-8993(00)02126-0. [DOI] [PubMed] [Google Scholar]
- 60.Glezer I, Bittencourt JC, Rivest S. Neuronal expression of Cd36, Cd44, and Cd83 antigen transcripts maps to distinct and specific murine brain circuits. J. Comp. Neurol. 2009;517:906–924. doi: 10.1002/cne.22185. [DOI] [PubMed] [Google Scholar]
- 61.Mark MP, Prince CW, Gay S, Austin RL, Butler WT. 44-kDal bone phosphoprotein (osteopontin) antigenicity at ectopic sites in newborn rats: kidney and nervous tissues. Cell Tissue Res. 1988;251:23–30. doi: 10.1007/BF00215443. [DOI] [PubMed] [Google Scholar]
- 62.van Velthoven CT, Heijnen CJ, van Bel F, Kavelaars A. Osteopontin enhances endogenous repair after neonatal hypoxic-ischemic brain injury. Stroke. 2011;42:2294–2301. doi: 10.1161/STROKEAHA.110.608315. [DOI] [PubMed] [Google Scholar]
- 63.Morinobu M, Ishijima M, Rittling SR, Tsuji K, Yamamoto H, Nifuji A, et al. Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. J. Bone Miner. Res. 2003;18:1706–1715. doi: 10.1359/jbmr.2003.18.9.1706. [DOI] [PubMed] [Google Scholar]
- 64.McKee MD, Addison WN, Kaartinen MT. Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton. Cells Tissues Organs. 2005;181:176–188. doi: 10.1159/000091379. [DOI] [PubMed] [Google Scholar]
- 65.Iseki S, Wilkie AO, Heath JK, Ishimaru T, Eto K, Morriss-Kay GM. Fgfr2 and osteopontin domains in the developing skull vault are mutually exclusive and can be altered by locally applied FGF2. Development. 1997;124:3375–3384. doi: 10.1242/dev.124.17.3375. [DOI] [PubMed] [Google Scholar]
- 66.Hazenberg MD, Stuart JW, Otto SA, Borleffs JC, Boucher CA, de Boer RJ, et al. T-cell division in human immunodeficiency virus (HIV-1) infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 67.Connoly N, Riffler S, Rinaldo C. Proinflammatory cytokines in HIV disease - a review and rationale for new therapeutic approaches. AIDS Rev. 2005;7:168–180. [PubMed] [Google Scholar]