Abstract
Biological therapies directed at proinflammatory cytokines have irrevocably changed the landscape of treatment of rheumatoid arthritis (RA) and other autoimmune diseases. With the advances in our knowledge in cytokine signaling, the question emerges whether targeting intracellular signaling might also be a safe and efficacious strategy. Janus kinases or Jaks are critical for a large family of cytokines and the first Jak inhibitor has been approved by the FDA for the treatment of myelofibrosis. Late phase clinical trials have been completed for another Jakinib in RA. It is therefore timely to consider this new category of drugs and reflect on their potential roles, present and future, in the treatment of RA and related disorders.
Role of Type I/II cytokines in RA and related diseases
Cytokines are critical for host defense and immunoregulation, but also major players in the immunopathogenesis of autoimmune diseases. Practically, rheumatologists can adduce the success of recombinant cytokine receptors and monoclonal antibodies against cytokines as evidence for the immunopathological role of these factors 1 What the practicing physician may be less cognizant of is the complexity of cytokines and their diversity of their structure.
Based on structure, several major families of cytokines can be recognized. Two major classes are the so-called Type I and Type II cytokine receptors. Type I receptors bind several interleukins (ILs), colony stimulating factors and hormones such erythropoietin, prolactin and growth hormone. Type II receptors bind interferons and IL-10 related cytokines.
Genome wide association scans (GWAS) have identified a plethora of Single-Nucleotide Polymorphisms (SNPs) conferring genetic susceptibility in autoimmune diseases such as rheumatoid arthritis (RA), 2 psoriasis, 3 inflammatory bowel disease (IBD) 4 and ankylosing spondylitis 5. Polymorphisms of genes encoding type I cytokine receptors and their signaling elements are now firmly linked to various autoimmune diseases. For instance, IL-23R, IL12B, JAK2, and STAT3 polymorphisms are associated with IBD and psoriasis and IBD. STAT4 polymorphisms are associated with RA, systemic lupus erythematosus and Sjogren’s syndrome. Other evidence of culpability of type I/II cytokines in autoimmunity comes from their detection in the context of disease. Rheumatoid arthritis, for instance, is associated with overproduction of IL-6, IL-12, IL-15, IL-23, granulocyte-macrophage colony stimulating factor (GM-CSF) and interferons. 2
Signaling via Type I/II Cytokine Receptors
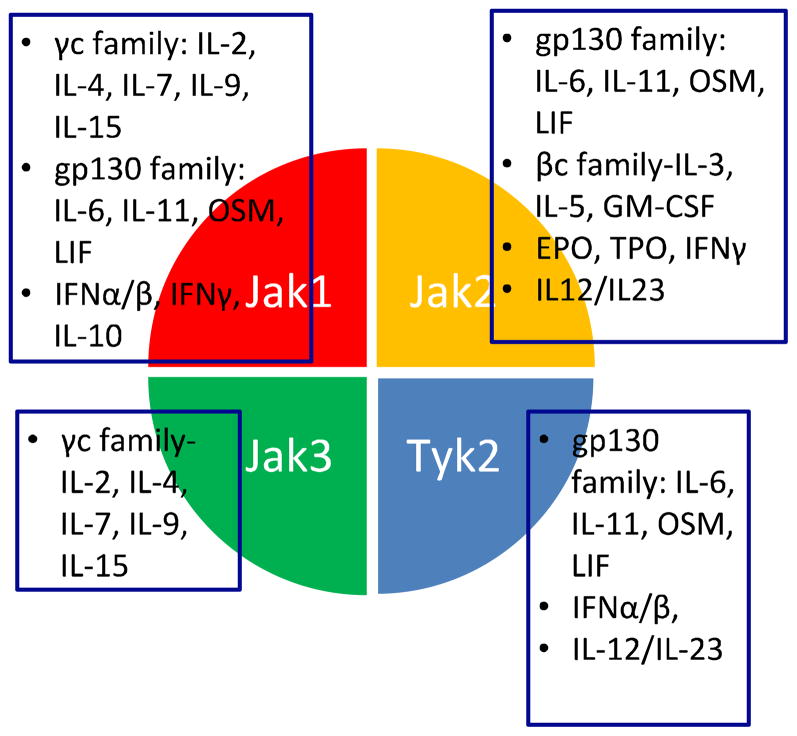
In contrast to other receptors, whose intracellular domains encode kinase or other enzymatically active domains, these receptors lack such elements. Instead, the cytoplasmic domain of Type I and II cytokine receptors bind to members of a specific kinase family, known as the Janus kinases (Jaks) which include Tyk2, Jak1, Jak2 and Jak3 (Figure 1). 6 Cytokine receptors are paired with different Jaks, which are activated upon cytokine binding (Figure 2). Because Jaks are phosphotranferases, they catalyze the transfer of phosphate from ATP to various substrates such as cytokine receptors. This modification allows the recruitment of various signaling molecules including members of the signal transducer and activator of transcription (STAT) family of DNA binding proteins. 7 STATs are another important Jak substrate. Phosphorylation of STATs promotes their nuclear accumulation and regulation of gene expression.
Figure 1.
Usage of different Jaks by various cytokines
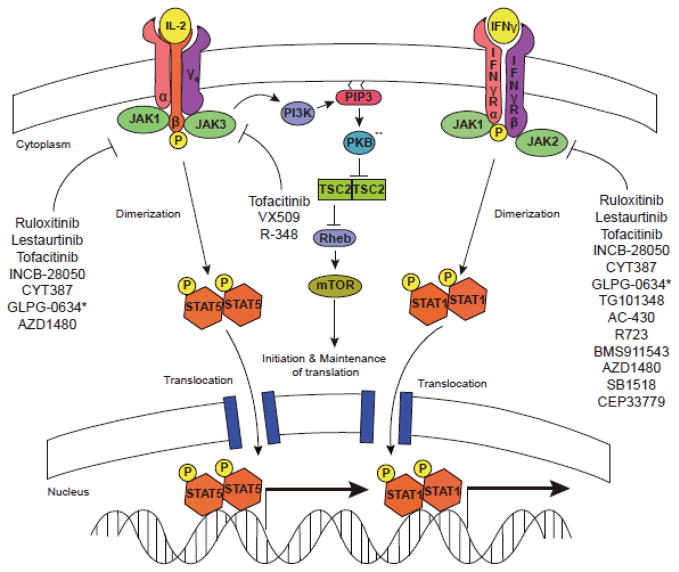
Figure 2. Jakinibs block multiple aspects of cytokine signaling.
Cytokine binding to its cognate receptor leads to phosphorylation of the intracellular domain of the tyrosine kinase receptor by specific Jaks. STATs are then recruited, bind to the receptor and become phosphorylated by Jaks. This results in STAT dimerization, translocation, and regulation of gene transcription. Cytokines also activate the PKB (Akt) and mTOR. Though not carefully studied, it is highly likely that blocking proximal cytokine signals will disrupt all downstream pathways. ** Also referred to as AKT.
Elegant work from mutagenized cell lines and later, knockout mice support the critical and specific role Jaks signaling by Type I/II cytokines and not other pathways. 8 In vivo evidence of the non-redundant functions in humans emerged from primary immunodeficiency patients. 9
It is important both conceptually and practically to bear in mind that receptors for cytokines like TNF, IL-1 and IL-17 are structurally distinct from Type I/II cytokine receptors; these cytokines are not dependent upon Jaks for signaling. 10–12
Targeting kinases
Work over the past twenty-five years has established that protein phosphorylation is a fundamentally important mode of intracellular signal transduction. 13 Thanks to the completion of the human genome, we now know the identity of all these players: there are over 500 kinases in the human kinome, which can be divided into eight families. The Jaks belong to the tyrosine protein kinase family of which there are 90 members. Structurally, the catalytic domains of all these kinases are highly conserved. Consequently, one might imagine that generating therapeutically useful kinase inhibitors would be an enormous challenge. However, it is now clear that kinases are actually very good targets and chemists have become skilled in generating reasonably selective inhibitors. So far, 13 inhibitors have entered clinical use and are approved by the FDA. Clearly, the overall strategy of targeting kinases is no longer theoretical.
Jakinibs in 2012
The critical function of Jaks in cytokine signaling has made them targets for industry to consider. At present there are a number of such inhibitors in clinical use or being tested in clinical trials.
Ruxolitinib and Baracitinib
The discovery that gain-of-function JAK2 mutations underlie the myeloproliferative disorders including polycythemia vera (PV), essential thrombocythemia (ET) and myelofibrosis (MF) was a great breakthrough in understanding the pathophysiology of these disorders. 14 The identification of these mutations also provided a rationale for purposefully targeting this enzyme. Ruxolitinib is a Jak1/2 inhibitor that is now approved by the FDA approved for the treatment of intermediate- and high risk MF. 15–17 Ruxolitinib reduces splenomegaly and systemic symptoms and also improves overall survival.
However, ruxolitinib has also been studied in RA where preliminary results were promising in terms of efficacy and safety in a phase IIa trial. 18 Ruxolitinib has also been used as a topical formulation in psoriasis with promising results. 19 Like ruxolitinib, baracitinib (formerly designated INCB028050) is also a Jak1/Jak2 inhibitor which showed efficacy in a highly active RA patient group resistant to disease modifying drugs and biologics with superior results in higher doses up to 4 or 8 mg once daily within 2 weeks with dose dependent side effects to include decrease of hemoglobin and neutrophil count and increase of LDL and creatinine with good overall tolerability. 20
Tofacitinib
Tofacitinib (former CP-690,550) was actually the first Jak inhibitor to be tested in the clinic. Tofacitinib inhibits Jak3 and Jak1 and to a lesser extent Jak2. It has little effect on Tyk2. 21 Across the kinome, it has selectivity remarkably sparing other kinases showing its high specificity compared to others. 22
Because of prominent role of Type I/II cytokines in driving autoimmunity and the effect of tofacitinib on these cytokines, this drug has been tested in a range of settings from RA, IBD, and psoriasis to renal transplantation rejection and dry eyes. 23–28 Phase III trials have shown efficacy for tofacitinib in RA patients who have failed DMARDS, both as monotherapy24 and in combination with methotrexate 25. These findings are consistent with prior phase II trials. 27,29 Of interest, tofacitinib was not inferior to standard of care therapy, namely, adalimumab in combination with methotrexate 30 There is evidence that structural damage was also averted31; however, further investigation will be needed to substantiate this. Of note, tofacitinib was efficacious in patients who failed multiple biologics. 24 For all these reasons, tofacitinib has recently been recommended for approval in the US for moderate to severe RA in patients who failed other DMARDs and/or biologics. If approved, tofacitinib would be the first Jakinib approved for RA.
Other Jakinibs
The picture is made complicated in that VX-509, a reportedly specific Jak3 inhibitor, was also efficacious in a Phase IIa study in RA. 32 Moreover, a reportedly selective Jak1 inhibitor, GLPG0634, also met its primary endpoint in a Phase IIa RA trial with no anemia and no lipid abnormalities observed. 33 CEP-33779, a selective Jak2 inhibitor, showed efficacy in two preclinical arthritis models 34. Thus, the relative contribution of the different Jaks in disease pathogenesis and the utility of selective blockade remains to be determined. At present, there are no selective Tyk2 inhibitors in clinical trials.
Mechanism of Action of First-Generation Jakinibs
An increasing body of evidence implicates specific cytokines and cell subsets as drivers of pathogenesis in different autoimmune diseases. Many of these key cytokines use the Jak/STAT pathway to exert their effects rendering them amenable to therapeutic blockade with Jakinibs. Given the apparent pathogenic role of a variety of cytokines like IL-6, IL-12, IL-23, interferons and GM-CSF in RA, psoriasis, IBD, AS and other autoimmune diseases, the ability of Jakinibs to block such cytokines is likely a major aspect of their mechanism of action.
Mechanistically, tofacitinib blocks common γc cytokines including IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21, all of which are signal through Jak3. In addition, it blocks Jak1, which would result in inhibition of gp130 family including IL-6 and IL-11 as well as type II cytokine receptor family such as IFN-α/β, IFN-γ and IL-10. To a lesser extent the drug blocks Jak2 and therefore blocks βc family such as IL-3, IL-5 and GM-CSF as well as EPO and IFN-γ. 6 Because tofacitinib blocks Jak1 and Jak2, it interferes with the differentiation of IFN-γ producing Th1 cells. It also blocks the generation of pathogenic Th17 cells, which are dependent upon IL-23 21,35. Because tofacitinib blocks IL-4 and IL-21, it might be anticipated that it will interfere with the function of B cells and follicular helper T cells. In addition to blocking the function of lymphocytes (adaptive immunity), tofacitinib also blocks innate immune responses. Specifically, tofacitinib blocks the effects IL-6 and interferons and thereby inhibits chemokine production from synovial fibroblasts. 35,36 In a sepsis model, which is dependent upon IFN-γ, tofacitinib blocked the production of TNF and IL-1. 21 Thus, tofacitinib can interfere with the production and action of TNF. However, TNF signaling per se is not affected; rather, tofacitinib blocks autocrine effects of interferons that mediate TNF effects. 36 In patients with RA patients treated with tofacitinib, serum levels of IL-6 were significantly decreased; presumably, this is due to effects on Type I/II cytokines that induce IL-6. 37 In an RA animal model tofacitinib abrogated osteoclast-mediated arthritic joint structural damage by decreasing RANKL production.38
Because ruxolitinib and baracitinib inhibit Jak1 and Jak2, they block many of the same cytokines as tofacitinib. Deletion of Jak3 impedes lymphocyte development because of its requisite role in cγc cytokine signaling. 39–41 However, gene targeting of Jak1 also results in a SCID phenotype. 42 From this perspective, the expectation would be that these drugs might have very similar mechanisms of action, in terms of the cytokines that are blocked (Figure 3).
Figure 3.
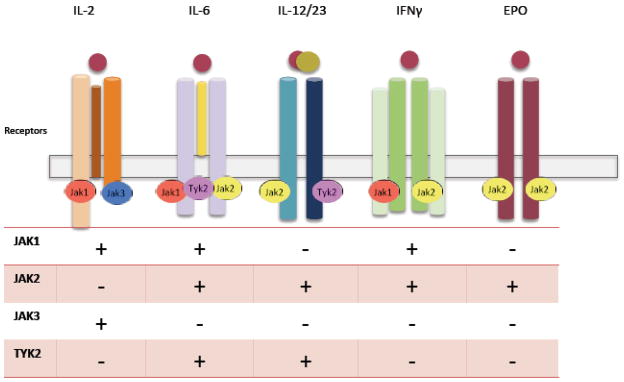
Impact of inhibiting various Jaks on signaling by selected cytokines
Side effects of Jakinibs
An important side effect of Jakinibs is serious bacterial, mycobacterial, fungal and viral infections. In the phase 3 trials of tofacitinib among opportunistic infections, pulmonary tuberculosis (TB) was reported in 3 cases all of which were initially negative upon screening for TB. Increased frequency of non-disseminated Herpes zoster was also reported which may reflect reduction of NK cells by virtue of Jak1 or Jak3 blockade. Whether this accounts for viral infection susceptibility remains to be established. Longer duration adequately powered trials are needed to estimate the risk of common and opportunistic infections. A potential advantage of Jakinibs compared to biologics with respect to infection risk is the relatively short half-life of the former; if infections occur, the drug can be stopped and the immunomodulatory effect is transient.
Jakinibs can cause anemia, thrombocytopenia and neutropenia, likely related to Jak2 inhibition, which is important for erythropoietin signaling and the actions of colony stimulating factors. When used for treatment of myelofibrosis in the setting of thrombocytopenia, the dose of ruxolitinib needs to be adjusted accordingly.
Use of jakinibs is associated with hypercholesterolemia. However, this is also consistently observed in RA trials with tocilizumab implying that high LDL, triglycerides and HDL may be mediated by blockade of IL-6 signaling. Standard anti-hyperlipidemic therapy improves the metabolic profile but the overall risk for cardiovascular morbidity will need to be determined in the long term. 43
Small increases in creatinine have been observed with tofacitinib, effects unclear if they are related to the drug’s mechanism of action.
A concern regarding chronic treatment with Jakinibs pertains to the possibility of increased cancer risk. Interferons and NK cells are important in tumor surveillance and the blockade of their action provides the theoretical rationale for development of malignancies mandating increased clinical vigilance. 44 The rate of lymphomas or other lymphoproliferative disorders in phase 3 and long extension studies of tofacitinib in RA was 0.07 per 100 patient-years (95% confidence interval, 0.03 to 0.15) which is comparable with studies of other biologics and the general RA population.25
The future of jakinibs in treating autoimmune disease
Clinical use of Jakinibs
Over the past decade, the biologics have clearly raised the bar with respect to treatment of rheumatic disease. They are highly effective and remarkably safe. However, not all patients respond. Exactly how Jakinibs will fit within the rheumatologist’s armamentarium remains to be seen. It will be of interest to see how a new, highly effective oral agent will be embraced relative to established parenteral drugs. An exciting development is that patients who fail biologics respond to Jakinibs.
Jakinibs in other diseases
Trials in psoriasis, inflammatory bowel disease and transplantation are presently ongoing. In preclinical studies, Jakinibs appear to have efficacy in lupus models. 34,45,46 The possibility of treating patients with SLE is attractive given the prominence of the “interferon signature” in this disease. 47–49 Asthma and allergy is associated with Th2 responses and the action of IL-4. Jak1 and Jak2 are important for IL-4 signaling and the potential utility of tofacitinib and other Jakinibs in these disorders is supported by preclinical data. 50
Selective vs. pan-Jak inhibitors
The kinome is a known entity – so for any new kinase inhibitor (inib) it is a fair question to ask what its selectivity is. Does it inhibit just Jaks or other kinases as well? How specific is it amongst the Jaks? These are important questions for any new drug coming along to understand it’s mechanism of action but also its side effects. The present Jakinibs all block more than one Jak, so all inhibit multiple cytokines. The question going forward is whether more selective Jakinibs will be as effective and potentially safer. While one might assume that more selectivity would be better, this assumption is not always borne out. Just look at the experience with NSAIDS and selective Cox2 inhibitors. Another possible scenario is that multikinase inhibitors might be useful in early phases of disease treatment, when a plethora of inflammatory responses are raging. Later, when disease is more controlled, perhaps a more selective inhibitor might be safe and effective for maintenance therapy.
Lessons learned?
Thanks to the completion of the human genome, there are hundreds of potential therapeutic targets for autoimmune disease. And yet, the cost of generating a new drug typically runs a billion or so dollars. The development of Jakinibs will surely be studied to see if there are lessons that might be gleaned for other classes of new drugs. In contrast to initial views, kinases turn out to be very “druggable” and genetic information unequivocally established the requisite function of Jaks in cytokine signaling. However, knocking out Jak2 in mice resulted in embryonic lethality, so one might have thought that a drug with Jak2 activity would be problematic. It is clear that equating drugs to knockouts is not always useful. If Jaks are good targets, one might imagine that STATs would also be good targets; however, targeting the latter has proven to be extremely difficult.
Conclusions
The development of kinase inhibitors has offered new therapies for diverse clinical entities ranging from malignancy to autoimmunity. Jak inhibitors or Jakinibs initially launched to treat a rare hematologic disorder are now progressing to be used in not only malignancies but common autoimmune disorders as well. The role of Jak inhibitors will hold in the treatment algorithm of diseases ranging from the vasculitides to systemic lupus erythematosus or polymyalgia rheumatica remains to be determined. Where Jakinibs will fit in the spectrum of therapeutic options from DMARDs and steroids to biologics and cyclophosphamide is unknown. However, the excitement is that if approved, Jakinibs will be the first new approved oral therapy for RA in decade.
References
- 1.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 4.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–25. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 5.Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377(9783):2127–37. doi: 10.1016/S0140-6736(11)60071-8. [DOI] [PubMed] [Google Scholar]
- 6.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 7.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 8.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–14. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36(4):515–28. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21(4):461–5. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 12.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell. 2007;28(5):730–8. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7(9):673–83. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 15.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 17.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–27. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams W, Scherle P, Shi J, Newton R, McKeever E, Fridman J, et al. A randomized placebocontrolled study of INCB018424, a selective Janus kinase1& 2 (JAK1&2) inhibitor in rheumatoid arthritis. Arthritis & rheumatism. 2008;58(9):S431. [Google Scholar]
- 19.Kwatra SG, Dabade TS, Gustafson CJ, Feldman SR. JAK Inhibitors in Psoriasis: A Promising New Treatment Modality. J Drugs Dermatol. 2012;11(8):913–8. [PubMed] [Google Scholar]
- 20.Keystone E, Taylor P, Genovese M, Schlichting D, Beattie S, Gaich C, et al. 12-Week results of a Phase 2B dose-ranging study of LY3009104 (INCB028050), an oral JAJ/JAK2 inhibitor, in combination with traditional DMARDs in patients with rheumatoid arthritis. Annals in Rheumatic Disease. 2012;71(S3) [Google Scholar]
- 21.Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550) J Immunol. 2011;186(7):4234–43. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26(1):127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 23.Liew SH, Nichols KK, Klamerus KJ, Li JZ, Zhang M, Foulks GN. Tofacitinib (CP-690,550), a Janus Kinase Inhibitor for Dry Eye Disease: Results from a Phase 1/2 Trial. Ophthalmology. 2012;119(7):1328–35. doi: 10.1016/j.ophtha.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-Controlled Trial of Tofacitinib Monotherapy in Rheumatoid Arthritis. New England Journal of Medicine. 2012;367(6):495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 25.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or Adalimumab versus Placebo in Rheumatoid Arthritis. New England Journal of Medicine. 2012;367(6):508–19. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 26.Boy MG, Wang C, Wilkinson BE, Chow VF, Clucas AT, Krueger JG, et al. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J Invest Dermatol. 2009;129(9):2299–302. doi: 10.1038/jid.2009.25. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63(8):1150–8. doi: 10.1002/acr.20494. [DOI] [PubMed] [Google Scholar]
- 28.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. The New England journal of medicine. 2012;367(7):616–24. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 29.Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60(7):1895–905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 30.Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64(3):617–29. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 31.van der Heijde D, Tanaka Y, Fleischmann R, Keystone EC, Kremer J, Zerbini CAF, et al. Tofacitinib (CP-690,550), An Oral Janus Kinase Inhibitor, in Combination with Methotrexate Reduced the Progression of Structural Damage in Patients with Rheumatoid Arthritis: a 24-Month Phase 3 Study. Arthritis & rheumatism. 2011:Abstract: 2592. [Google Scholar]
- 32.Fleischmann R, Spencer-Green GT, Fan F, Frankovic B, Luo X, Hoock T, et al. Dose ranging study of VX-509, an oral selective JAK3 inhibitor, as monotherapy in patients with active rheumatoid arthritis (RA) Arthritis and rheumatism. 2011 [Google Scholar]
- 33.Vanhoutte FP, Mazur M, Namour F, Van der Aa A, Wigerinck P, Van’t Klooster GAE. Efficacy and safety of GLPG0634, a selective JAK1 inhibitor, after short-term treatment of rheumatoid arthritis; results of a Phase IIa trial. Annals in Rheumatic Disease. 2012;71(S3) [Google Scholar]
- 34.Stump KL, Lu LD, Dobrzanski P, Serdikoff C, Gingrich DE, Dugan BJ, et al. A highly selective, orally active inhibitor of Janus kinase 2, CEP-33779, ablates disease in two mouse models of rheumatoid arthritis. Arthritis Res Ther. 2011;13(2):R68. doi: 10.1186/ar3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeshima K, Yamaoka K, Kubo S, Nakano K, Iwata S, Saito K, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-gamma and interleukin-17 production by human CD4+ T cells. Arthritis Rheum. 2012;64(6):1790–8. doi: 10.1002/art.34329. [DOI] [PubMed] [Google Scholar]
- 36.Rosengren S, Corr M, Firestein GS, Boyle DL. The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Ann Rheum Dis. 2012;71(3):440–7. doi: 10.1136/ard.2011.150284. [DOI] [PubMed] [Google Scholar]
- 37.Yamaoka K, Kubo S, Sonomoto K, Maeshima K, Tanaka Y. A JAK inhibitor, tofacitinib reduces IL-6 and matrix metalloproteinase-3 production in rheumatoid arthritis with suppressed cartilage destruction. Arthritis Research & Therapy. 2012;14(Suppl 1):77. [Google Scholar]
- 38.TP, Jesson MI, Radi ZA, Storer CE, Guzova JA, Bonar SL, et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis and rheumatism. 2012 doi: 10.1002/art.34649. [DOI] [PubMed] [Google Scholar]
- 39.Chen M, Cheng A, Chen YQ, Hymel A, Hanson EP, Kimmel L, et al. The amino terminus of JAK3 is necessary and sufficient for binding to the common gamma chain and confers the ability to transmit interleukin 2-mediated signals. Proc Natl Acad Sci U S A. 1997;94(13):6910–5. doi: 10.1073/pnas.94.13.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Candotti F, Oakes SA, Johnston JA, Giliani S, Schumacher RF, Mella P, et al. Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood. 1997;90(10):3996–4003. [PubMed] [Google Scholar]
- 41.Yoshida K, van den Berg TK, Dijkstra CD. The functional state of follicular dendritic cells in severe combined immunodeficient (SCID) mice: role of the lymphocytes. Eur J Immunol. 1994;24(2):464–8. doi: 10.1002/eji.1830240230. [DOI] [PubMed] [Google Scholar]
- 42.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93(3):373–83. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 43.McInnes IB, Kim H-Y, Lee S, Mandel D, Song Y, Connell CA, et al. Phase 2 study of teh effects of open-label tofactinib (CP-690,550) and double blind atorvastatin on lipids in patients with active rheumatoid arthritis. Ann Rheum Dis. 2011;70(Suppl3):169. [Google Scholar]
- 44.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 45.Kawasaki M, Fujishiro M, Yamaguchi A, Nozawa K, Kaneko H, Takasaki Y, et al. Possible role of the JAK/STAT pathways in the regulation of T cell-interferon related genes in systemic lupus erythematosus. Lupus. 2011;20(12):1231–9. doi: 10.1177/0961203311409963. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Yang N, Zhang L, Huang B, Tan H, Liang Y, et al. Jak/STAT signaling is involved in the inflammatory infiltration of the kidneys in MRL/lpr mice. Lupus. 2010;19(10):1171–80. doi: 10.1177/0961203310367660. [DOI] [PubMed] [Google Scholar]
- 47.Obermoser G, Pascual V. The interferon-alpha signature of systemic lupus erythematosus. Lupus. 2010;19(9):1012–9. doi: 10.1177/0961203310371161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crow MK. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res Ther. 2010;12 (Suppl 1):S5. doi: 10.1186/ar2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacob N, Stohl W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(4):228. doi: 10.1186/ar3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kudlacz E, Conklyn M, Andresen C, Whitney-Pickett C, Changelian P. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. Eur J Pharmacol. 2008;582(1–3):154–61. doi: 10.1016/j.ejphar.2007.12.024. [DOI] [PubMed] [Google Scholar]