Abstract
Rationale
The impact of diabetes mellitus on bone marrow (BM) structure is incompletely understood.
Objective
Investigate the effect of type-2 diabetes mellitus (T2DM) on BM microvascular and hematopoietic cell composition in patients without vascular complications.
Methods and Results
Bone samples were obtained from T2DM patients and nondiabetic controls (C) during hip replacement surgery and from T2DM patients undergoing amputation for critical limb ischemia. BM composition was assessed by histomorphometry, immunostaining, and flow cytometry. Expressional studies were performed on CD34pos immunosorted BM progenitor cells (PCs). Diabetes mellitus causes a reduction of hematopoietic tissue, fat deposition, and microvascular rarefaction, especially when associated with critical limb ischemia. Immunohistochemistry documented increased apoptosis and reduced abundance of CD34pos-PCs in diabetic groups. Likewise, flow cytometry showed scarcity of BM PCs in T2DM and T2DM+critical limb ischemia compared with C, but similar levels of mature hematopoietic cells. Activation of apoptosis in CD34pos-PCs was associated with upregulation and nuclear localization of the proapoptotic factor FOXO3a and induction of FOXO3a targets, p21 and p27kip1. Moreover, microRNA-155, which regulates cell survival through inhibition of FOXO3a, was downregulated in diabetic CD34pos-PCs and inversely correlated with FOXO3a levels. The effect of diabetes mellitus on anatomic and molecular end points was confirmed when considering background covariates. Furthermore, exposure of healthy CD34pos-PCs to high glucose reproduced the transcriptional changes induced by diabetes mellitus, with this effect being reversed by forced expression of microRNA-155.
Conclusions
We provide new anatomic and molecular evidence for the damaging effect of diabetes mellitus on human BM, comprising microvascular rarefaction and shortage of PCs attributable to activation of proapoptotic pathway.
Keywords: bone marrow, diabetes mellitus type 2, macroangiopathy, microangiopathy, stem cells
An imbalance between circulating proinflammatory and proangiogenic cells leading to counterproductive cell recruitment to sites of endothelial injury contributes to vascular complications in patients with diabetes mellitus.1–6 Studies in animal models suggest that the altered spectrum of circulating cells is consequent to a deregulated control of cell mobilization from bone marrow (BM).7–10 In line, clinical data show that the BM of diabetic patients has an impaired capacity to release hematopoietic stem cells (HSCs) after stimulation with granulocyte colony-stimulating factor,11 a defect for which the term diabetic stem cell (SC) mobilopathy was recently proposed.12 Moreover, diabetes mellitus might impinge on the integrity of SCs/progenitor cells (PCs) by altering the marrow microenvironment, which consists of stromal, endosteal, and microvascular cells.10,13,14
Besides providing oxygen and nutrients, the marrow microvasculature plays a key role in the regulation of hematopoiesis.15,16 Furthermore, vascular sinusoids constitute a dedicated interface for cell exchanges with the peripheral circulation. In a mouse model, we showed that type 1 diabetes mellitus causes microvascular rarefaction, resulting in critical hypoperfusion, depletion of SCs at the level of the endosteal niche, and altered transendothelial cell trafficking.17 It would be of paramount importance to understand whether similar microvascular pathology occurs in BM of patients with diabetes mellitus. Current knowledge is limited to flow cytometry analysis of aspirates, which showed reduction of CD34pos-PCs in BM of diabetic patients.5 However, this sampling procedure is not suited to define the anatomic structure of the marrow and may provide inaccurate SC counts if the marrow is remodeled. Moreover, the molecular mechanisms underlying diabetes mellitus-induced SC depletion are incompletely understood. We showed that experimental diabetes mellitus causes an elevation in reactive oxygen species, infringes on DNA integrity, and induces apoptosis of Sca-1posc-Kitpos cells.17 In addition, signaling mechanisms that maintain the self-renewal capacity and prevent senescence of SCs, like the polycomb group gene Bmi-1,18 are downregulated in BM cells from diabetic mice.13
Recent evidence indicates that microRNAs (miRs) regulate the maturation of different hematopoietic lineages.19–21 In particular, a restricted subset of miRs expressed in CD34pos HSCs is hierarchically organized in a circuitry that controls proliferation, viability, and differentiation.21,22 Among HSC-associated miRs, miR-221 reportedly regulates terminal stages of erythropoiesis via repression of c-Kit, whereas miR-155 acts upstream of miR-221 holding HSCs at an early stem-progenitor stage through inhibition of differentiation-associated molecules, like CCAAT/enhancer-binding protein-β, cAMP response element-binding protein, JUN and FOS.22,23 In addition, miR-155 inhibits the forkhead transcription factor FOXO3a.24 In hematopoietic cells with incurred DNA damage, FOXO3a induces cell cycle arrest and apoptosis, via transcriptional regulation of the cyclin-dependent kinase inhibitor p27Kip1 and proapoptotic Bcl-2 family member Bim.25–27 By inhibiting FOXO3a, miR-155 exerts prosurvival effects in HSCs.
The present study examines the damaging action of diabetes mellitus on human BM using femoral bone specimens collected from orthopedic surgery. To determine whether macrovascular disease can further disrupt BM integrity, we also studied patients with type 2 diabetes mellitus (T2DM) undergoing amputation for critical limb ischemia (CLI). Furthermore, we investigated the influence of diabetes mellitus and high glucose (HG) on miR expression in CD34pos immunosorted BM PCs and the ability of miR-155 to reverse FOXO3a upregulation in HG-challenged CD34pos cells.
Methods
A Supplemental Methods section is available in the Online Data Supplement.
Patients and Study Protocol
The study complied with the principles stated in the Declaration of Helsinki and was covered by institutional ethical approval (protocol number 20/2010). Eligible subjects were screened from a consecutive series referring to MultiMedica Hospital for hip replacement surgery (nondiabetic controls and T2DM patients) or limb amputation (T2DM patients with CLI) from August 2010 to November 2012. T2DM and CLI were defined according to the American Diabetes Association and the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II), respectively.28 Exclusion criteria were acute infection, immune diseases, current or past hematologic disorders or malignancy, drug-induced diabetes mellitus, unstable angina, recent (within 6 months) myocardial infarction or stroke, liver failure, dialysis because of renal failure, pregnancy, and lack of consent to participate in the study. Online Table I illustrates main clinical data of the 82 subjects who entered the study.
Immunohistochemistry, flow cytometry, and molecular biology analyses were performed on femoral head leftovers from orthopedic surgery and proximal part of amputated femurs. In addition, a 30 mL peripheral blood (PB) sample was obtained by venipuncture the day before interventional procedures.
Results
Characteristics of the Study Population
As shown in Online Table I, groups were similar with regard to age and sex distribution. T2DM patients showed a higher body mass index than controls and T2DM+CLI, whereas the T2DM+CLI group had the highest percentage of smokers and a longer duration of diabetes mellitus. Hypertension was more frequent in the 2 diabetic groups than in controls, and cardiovascular complications were common in T2DM+CLI patients. Insulin was the preferred antidiabetic medication in T2DM+CLI, whereas T2DM patients were mainly treated with oral antidiabetic drugs including glitazones. Between 40% and 52% of diabetic patients were taking statins. Fasting glucose was higher in the 2 diabetic groups, especially in T2DM+CLI.
Diabetes Mellitus Alters BM Composition
Histomorphometry demonstrates a remarkable remodeling of BM from diabetic patients, consisting of decreased hematopoietic tissue, fat deposition, and bone rarefaction (Figure 1A and 1B). The effect of diabetes mellitus was significant, incremental in T2DM+CLI patients and independent of background factors (Online Table III). Multiple regression analysis showed that the dependent variables, hematopoietic and fat fractions, can be predicted by grouping factor, fasting glucose and duration of diabetes mellitus, with no effect of other independent variables except for body mass index, which in association with duration of diabetes mellitus predicts the abundance of fat in BM (Online Table V).
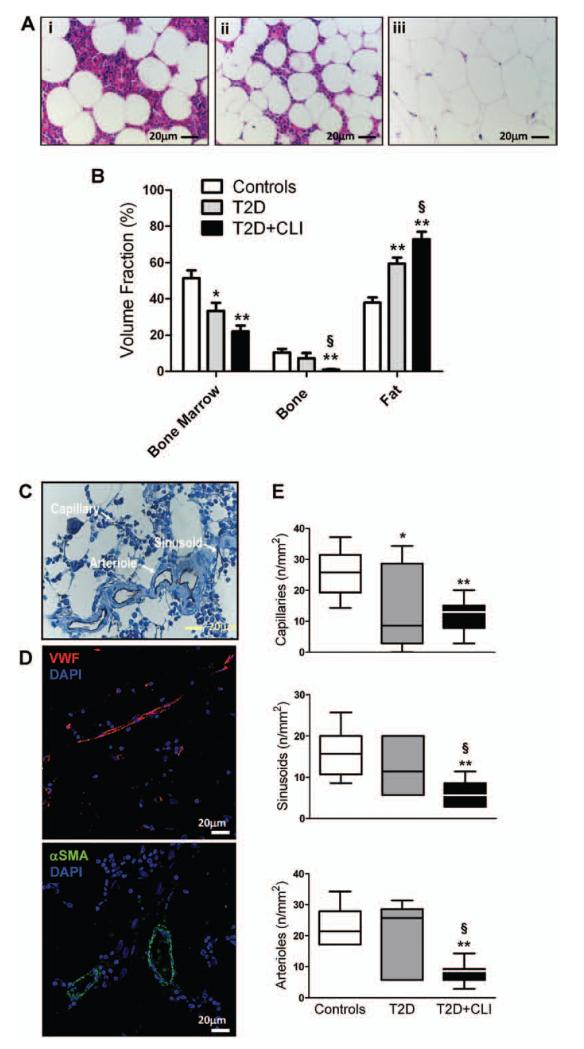
Figure 1. Diabetes mellitus induces bone marrow (BM) remodeling and vascular rarefaction.
A and B, Histomorphometric analysis shows replacement of marrow with fat and bone rarefaction. A, Representative microphotograph of hematoxylin and eosin-stained BM sections: (i) Control, (ii) type-2 diabetes mellitus (T2DM) patient, and (iii) T2DM patient with critical limb ischemia (CLI). B, Bar graph showing average data of marrow fractions. C, Representative microphotograph of human BM showing CD31-positive vascular structures. Arrows indicate the type of vessel. D, Confocal microscopy photographs of human BM showing vascular structures stained with the endothelial marker von Willebrand factor (VWF) and the vascular smooth muscle marker α-smooth muscle actin (αSMA). Nuclei are stained blue by 4′,6-diamidino-2-phenylindole (DAPI). E, Bar graph showing average data of microvascular density. *P<0.05 and **P<0.01 versus Controls, §P<0.05 versus T2DM. Controls, n=10; T2DM, n=7; T2DM+CLI, n=10.
Diabetes Mellitus and CLI Additively Reduce Vascular Density in Human BM
We next assessed the marrow microvasculature by immunohistochemistry. Capillaries were recognized as CD31-positive structures whose lumen-size does not exceed the diameter of an erythrocyte, whereas venous sinusoids were identified as larger irregular structures containing several erythrocytes (Figure 1C). Furthermore, microvessels and arterioles were identified by confocal and fluorescence microscopy using the endothelial marker von Willebrand factor and the vascular smooth muscle cell marker α-smooth muscle actin (Figure 1D).
Analysis of microvascular density identified a significant difference among groups for all sets of vascular structures (P=0.005; Figure 2E). Pairwise comparison indicates a large decrease of capillary density in BM of T2DM patients (P<0.05 versus controls) and a further reduction of all 3 vascular fractions in T2DM+CLI patients (P<0.01). The effect of diabetes mellitus on BM microvascular density was confirmed when considering background covariates (Online Table III). In multiple regression analysis, the dependent microvascular variables were predicted by grouping factor, duration of diabetes mellitus, and fasting glucose, in linear combination with hypertension as far as capillaries and sinusoids are concerned (Online Table V). Furthermore, we found that vascular density is directly correlated with the hematopoietic fraction and inversely correlated with fat abundance in BM (P<0.01 for both comparisons).
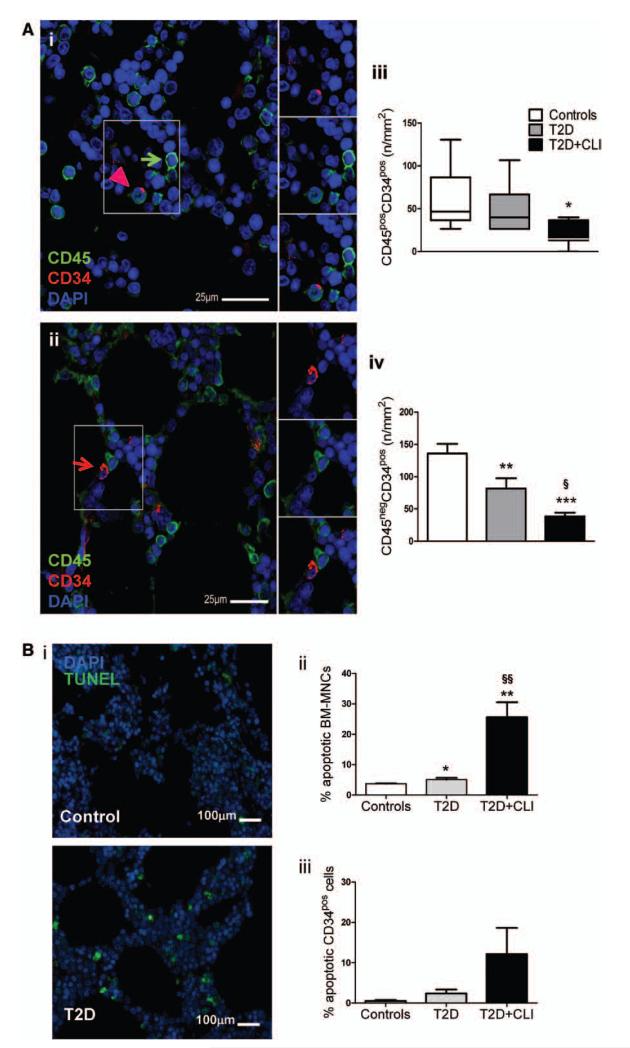
Figure 2. Reduced abundance of CD34pos cells in bone marrow (BM) of type-2 diabetes mellitus (T2DM) patients.
A, Representative confocal microscopy photographs of human BM showing the presence of CD45pos (green arrow), CD45posCD34pos (pink arrowhead) (i), and CD45negCD34pos cells (red arrow) (ii). Nuclei are stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Bar graphs showing the average density of CD45posCD34pos cells (iii, median and 5%–95% distribution) and CD34posCD45neg cells (iv, mean±SEM). B, Increased abundance of apoptotic mononuclear cells and CD34pos cells in BM from diabetic patients. Representative microphotographs of fluorescent terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) positive cells (i) and bar graphs showing average data (ii and iii). *P<0.05, **P<0.01 and ***P<0.001 versus controls, §P<0.05 and §§P<0.01 versus T2DM. Controls, n=10; T2DM, n=7; T2D+critical limb ischemia (CLI), n=8.
Altogether, these data indicate that diabetes mellitus causes microangiopathy in human BM, with vascular rarefaction being aggravated by CLI. Among associated risk factors, hypertension interacts with diabetes mellitus in influencing capillary and sinusoid density.
Diabetes Mellitus Reduces the Abundance of Hematopoietic Progenitors in BM
BM-derived CD34pos cells are a well-characterized population that have been used for hematopoietic reconstitution and more recently for the treatment of myocardial and peripheral ischemia.29 We investigated the abundance of CD45posCD34pos and CD45negCD34pos cells in BM by confocal microscopy (Figure 2Ai and 2Aii). ANOVA detected a difference among groups with regard to the CD45posCD34pos fraction (P=0.008) and pairwise comparison showed a large reduction of this cell population in T2DM+CLI compared with controls (P<0.05) (Figure 2Aiii). Likewise, CD45negCD34pos cells were different among groups (P=0.001) because of a large reduction in T2DM (P=0.008 versus controls) and T2DM+CLI patients (P=0.0001 versus controls) (Figure 2Aiv). The difference among groups was confirmed after consideration of background factors by ANCOVA (Online Table III). Grouping factor was a strong predictor of the dependent variables, CD45negCD34pos and CD45posCD34pos cells. Furthermore, duration of diabetes mellitus and fasting glucose predicted the abundance of CD45negCD34pos cells (Online Table V). Moreover, in situ detection of DNA fragmentation by terminal deoxynucleotidyl transferase dUTP nick end labeling assay indicates activation of apoptosis in BM-mononuclear cells and CD34pos-PCs of T2DM and T2DM+CLI patients compared with controls (Figure 2B and Online Figure I).
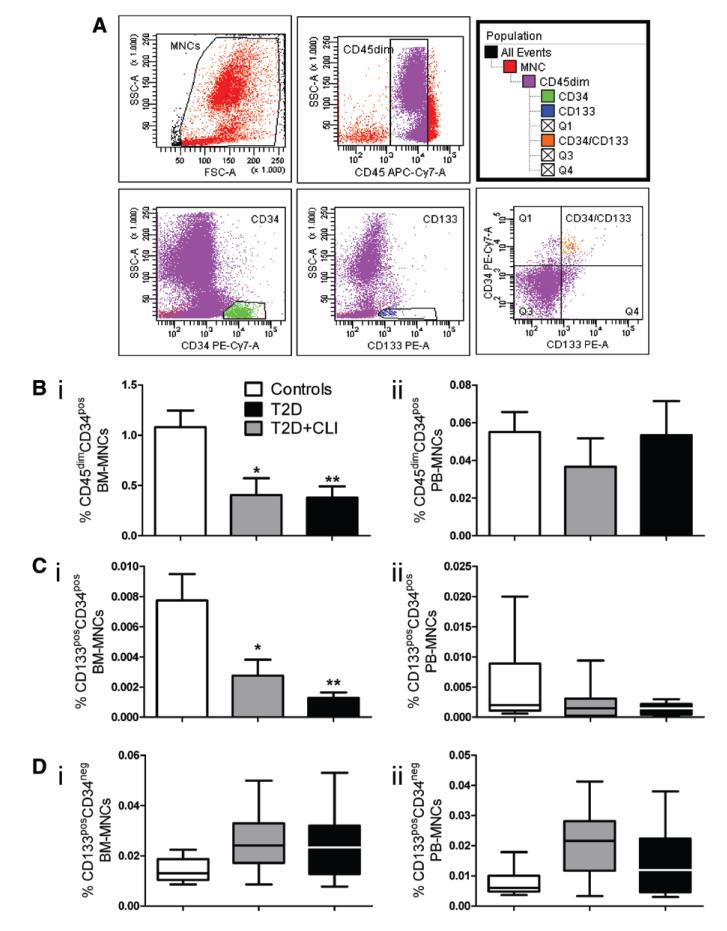
We next used multicolor flow cytometry for quantification of various cell populations in BM and PB (Figure 3A).30 CD45dimCD34pos cells were reduced in BM of T2DM (P=0.02 versus controls) and T2DM+CLI patients (P=0.004), but did not differ between the diabetic groups (Figure 3Bi), with these data being confirmed by ANCOVA (Online Table III). In contrast, PB CD45dimCD34pos cells were similar in control and diabetic patients (Figure 3Bii). The relative abundance of CD45dimCD34pos cells in BM could be predicted independently by grouping factor, duration of diabetes mellitus, and fasting glucose in the multiple regression model (Online Table V).
Figure 3. Flow cytometry characterization of hematopoietic cells in bone marrow (BM) and peripheral blood (PB) of type-2 diabetes mellitus (T2DM) patients.
A, Gating strategy of multicolor flow cytometry. B to D, Bar graphs showing the abundance of CD45dimCD34pos (Bi and Bii), CD45dimCD133posCD34pos cells (Ci and Cii) and CD45posCD133posCD34neg cells (Di and Dii). *P<0.05, **P<0.01 and ***P<0.001 versus controls. Controls, n=8 to 14; T2DM, n=7 to 9; T2DM+critical limb ischemia (CLI), n=7 to 11.
The surface antigen CD133, a prominin 5 transmembrane glycoprotein 1, has been used to identify primitive hematopoietic and nonhematopoietic PCs31 endowed with proangiogenic and healing activities in animal models32 and patients with acute myocardial infarction.33–35 We found that CD45dimCD133posCD34pos cells are remarkably reduced in BM of T2DM (P=0.02 versus controls) and T2DM+CLI (P=0.01), with no difference between the 2 diabetic groups (P=0.83) (Figure 3Ci and Online Table III). The levels of CD45dimCD133posCD34pos cells were predicted by grouping, duration of diabetes mellitus, and fasting glucose (Online Table V). In contrast, no group difference was found in PB CD45dimCD133posCD34pos cells (P=0.35) (Figure 3Cii). Moreover, there was no difference between diabetic and control subjects with regard to BM and PB CD133posCD34neg cells (Figure 3Di and 3Dii), which are considered very early hematopoietic precursors.36
Differential Effects of Diabetes Mellitus on Endothelial PCs and Lineage Committed Hematopoietic Cells
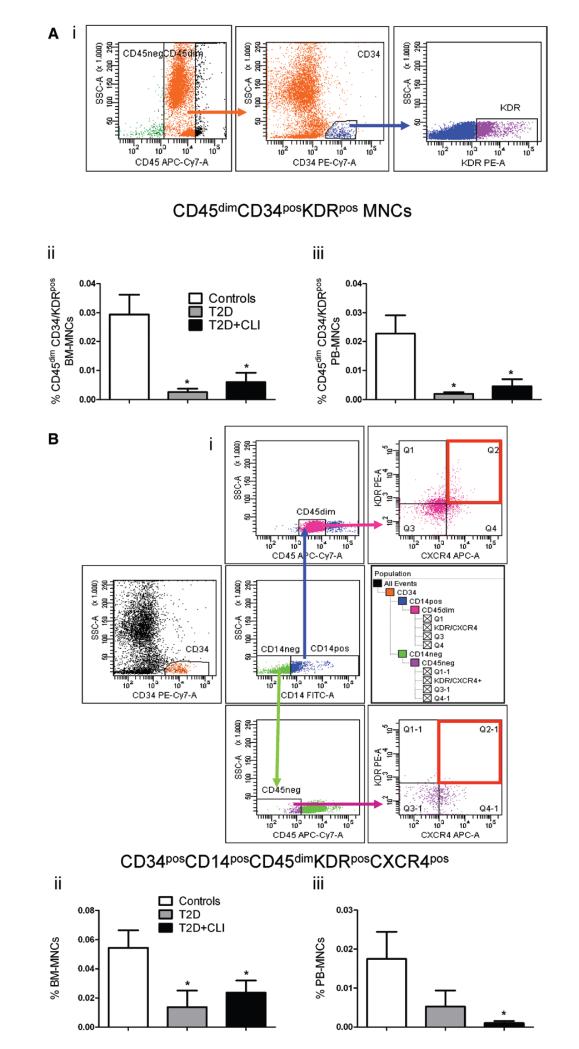
Several BM cell subpopulations, including hematopoietic and nonhematopoietic SCs, have the capacity to differentiate into endothelial cells (ECs) or promote EC growth by paracrine mechanisms. For instance, BM CD34pos kinase insert domain receptor (KDR)pos cells have been postulated to comprise the hemangioblast, that is, a bipotent cell type able to form both ECs and blood cells.37–39 Moreover, CD34posKDRpos cells are reportedly decreased in PB of patients with traditional cardiovascular risk factors including diabetes mellitus.3,40,41 As shown in Figure 4Ai, CD34posKDRpos cells were mainly enriched in the CD45dim mononuclear cell fraction. Moreover, we could confirm a large reduction of BM and PB CD45dimCD34posKDRpos cells in both subgroups of diabetic subjects as compared with controls (P=0.006 for both comparisons), with no difference between T2DM and T2DM+CLI (Figure 4Aii and 4Aiii and Online Table III). The dependent variable, BM CD45dimCD34posKDRpos cells, was predicted by grouping factor, duration of diabetes mellitus, and fasting glucose, whereas PB CD45dimCD34posKDRpos cells were predicted by grouping factor and duration of diabetes mellitus (Online Table V).
Figure 4. Flow cytometry characterization of bone marrow (BM) and peripheral blood (PB) endothelial progenitors.
A, Gating strategy of CD45dimCD34posKDRpos mononuclear cells (MNCs) (i), and bar graphs showing the abundance of this population in BM (ii) and PB (iii). B, Gating strategy of CD34posCD14posCD45dimKDRposCXCR4pos cells (i), and bar graphs showing the abundance of this population in BM (ii) and PB (iii). *P<0.05 versus controls. Controls, n=8 to 11; type-2 diabetes mellitus (T2DM), n=6 to 9; T2DM+critical limb ischemia (CLI), n=7 to 10.
We also verified the abundance of proangiogenic PCs defined as CD34posCD14posCD45dimKDRposCXCR4pos mononuclear cells.42,43 This cellular subpopulation was reduced in BM and PB of both diabetic groups, with no difference between T2DM and T2DM+CLI (Figure 4Bi–4Biii). However, this effect was attenuated when considering other covariates (Online Table III). Nonetheless, the reduction of the PB cell fraction was predicted by grouping factor (Online Table V). Furthermore, a recent study showed that BM CD31pos cells are enriched of highly angiogenic cells.44 However, we found no difference among groups regarding this cell population (data not shown).
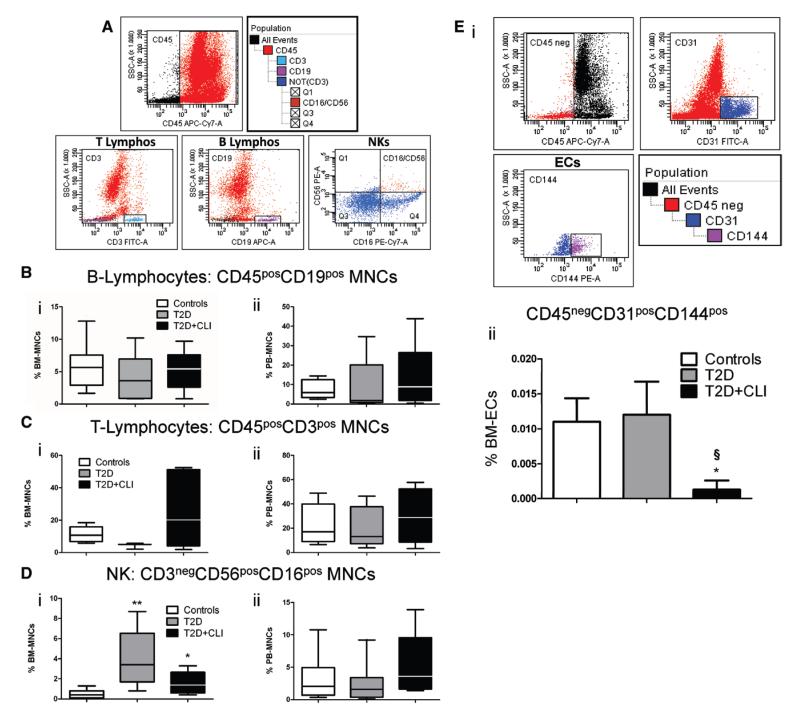
We next verified the influence of diabetes mellitus on lineage committed hematopoietic cells (Figure 5A–5D) and mature ECs (Figure 5E). No difference was found in the BM and PB levels of CD45posCD19pos B-lymphocytes (Figure 5Bi and 5Bii) and CD45posCD3pos T lymphocytes (Figure 5Ci and 5Cii). In contrast, CD3negCD56posCD16pos natural killer (NK) lymphocytes were increased in BM of T2DM patients, with this effect being confirmed after correction for other covariates. In multiple regression analysis, fasting glucose was a predictor of NK lymphocytes (P=0.001). Moreover, PB NK lymphocytes did not differ between diabetic and control subjects (Figure 5Di and 5Dii). Finally, CD45negCD31posCD144pos ECs were reduced in BM of T2DM+CLI (Figure 5E), thus, confirming the in situ data from immunohistochemistry.
Figure 5. Flow cytometry characterization of lineage committed hematopoietic cells and endothelial cells (ECs).
A, Gating strategy for identification of B-lymphocytes, T-lymphocytes, and natural killer (NK) cells. B to D, Bar graphs showing the abundance of B-lymphocytes (B), T-lymphocytes (C) and NK cells (D) in bone marrow (BM) (i) and peripheral blood (PB) (ii). E, Gating strategy for identification of BM ECs (i) and bar graph showing average values (ii) *P<0.05 and **P<0.01 versus controls, §P<0.05 versus type-2 diabetes mellitus (T2DM). Controls, n=9 to 16; T2DM, n=7 to 9; T2DM+critical limb ischemia (CLI), n=7 to 8.
Altogether, these data indicate that diabetes mellitus causes a selective reduction of endothelial PCs in BM and PB, without altering the abundance of lineage committed hematopoietic cells with exception of NK lymphocytes which were augmented in BM.
Effect of Diabetes Mellitus on Non-HSCs
This fraction represents a heterogeneous population that comprises CD45negCXCR4posCD34pos SCs, CD34negCD45negCD14negCD90posCD73posCD105pos mesenchymal SCs and cKitpos PCs.45–47 We could not find any difference between diabetic patients and controls with regard to BM CD45negCXCR4posCD34pos SCs and mesenchymal SCs (data not shown). Surprisingly, cKitpos PCs were increased in BM and PB of T2DM patients (P<0.05 versus controls) but not in T2DM+CLI patients (Online Figure II). However, in multiple regression analysis, diabetes mellitus was not a predictor of cKitpos PCs.
Activation of the Proapoptotic FOXO3a Signaling Pathway in BM CD34pos Cells
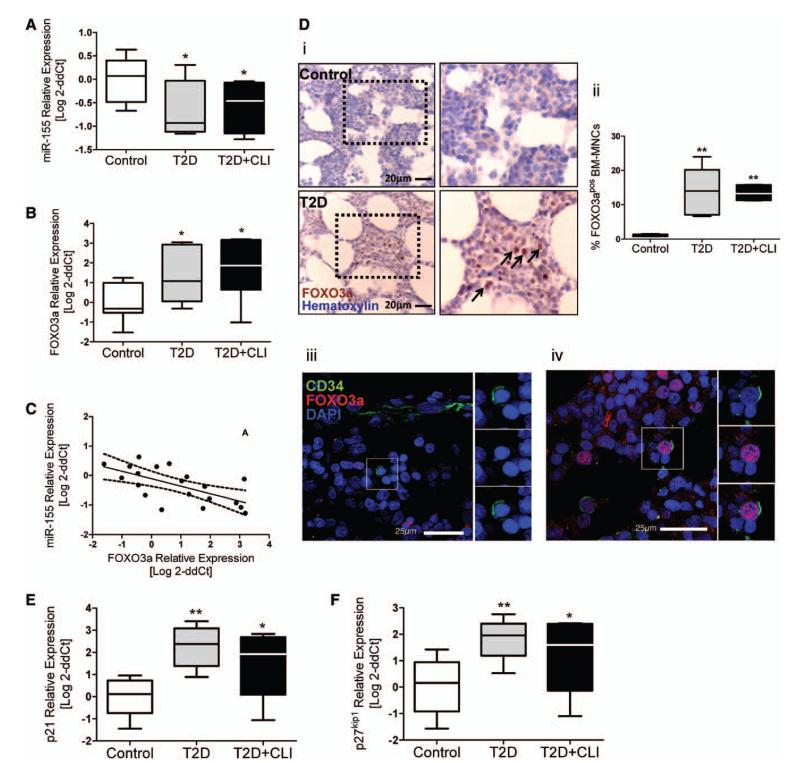
We investigated the expression levels of miR-155 and its validated target FOXO3a24 in CD34pos immunosorted BM PCs. Results indicate a downregulation of miR-155 in CD34pos-PCs from diabetic patients compared with controls (P=0.019) (Figure 6A). In contrast, miR-221 did not differ among groups (data not shown). Moreover, we found FOXO3a mRNA levels to be increased in BM CD34pos-PCs of diabetic patients (P=0.004) (Figure 6B) and inversely correlated to miR-155 levels (R2=−0.41; P=0.002) (Figure 6C). Of note, in situ analysis of FOXO3a expression showed a remarkable increase of the transcription factor in BM cells from both diabetic groups (P<0.0007) (Figure 6Di and 6Dii), with evidence of nuclear translocation which is required for transcriptional activation of target genes (Figure 6Diii and 6Div). In line, we found that the FOXO3a targets p21 and p27kip1 are upregulated in CD34pos cells from diabetic patients (P=0.001 and 0.006, respectively) (Figure 6E and 6F). The effect of diabetes mellitus on miR-115, FOXO3a, and p21 was confirmed when considering background covariates (Online Table VI). P21 and p27kip1 inhibit cell cycle progression by binding to, and inactivating, cyclin-dependent kinase complexes. Analysis of cell cycle by flow cytometry confirmed that CD34pos cells from diabetic BM are stalled at the G1 checkpoint and undergo apoptosis with high frequency (Online Figure III).
Figure 6. Diabetes mellitus-induced expressional changes in bone marrow (BM) CD34pos cells.
A and B, Bar graphs showing mRNA levels of microRNA (miR)-155 (A) and its target FOXO3a (B). Controls, n=10; type-2 diabetes mellitus (T2DM), n=7; T2DM+critical limb ischemia (CLI), n=6. C, Graph showing the inverse correlation between miR-155 and FOXO3a mRNA levels in CD34pos cells. D, Representative microphotographs (i) and bar graph (ii) showing the in situ expression of FOXO3a in BM cells. Confocal microphotographs showing FOXO3a (red) localization in the cytoplasm (iii) and nucleus (iv) of CD34pos cells (green). Nuclei are stained blue with 4′,6-diamidino-2-phenylindole (DAPI). n=5 per group. E and F, Bar graphs showing mRNA levels of CDKN1A/p21 (E) and CDKN1B/p27kip1 (F). Controls, n=10; T2DM, n=7; T2DM+CLI, n=6. *P<0.05 and **P<0.01 versus controls.
We next investigated whether in vitro exposure to HG mimics diabetes mellitus in altering the miR-155/FOXO3a/p21/p27kip1 signaling pathway in CD34pos-PCs. We found that HG decreases cell counts and miR-155 levels and increases FOXO3a (Online Figure IVA–IVC). Moreover, HG increases the levels of p21 and p27kip1 (Online Figure IVD and IVE). The osmotic control mannitol did not alter the expression of miR-155 and related target genes (Online Figure V).
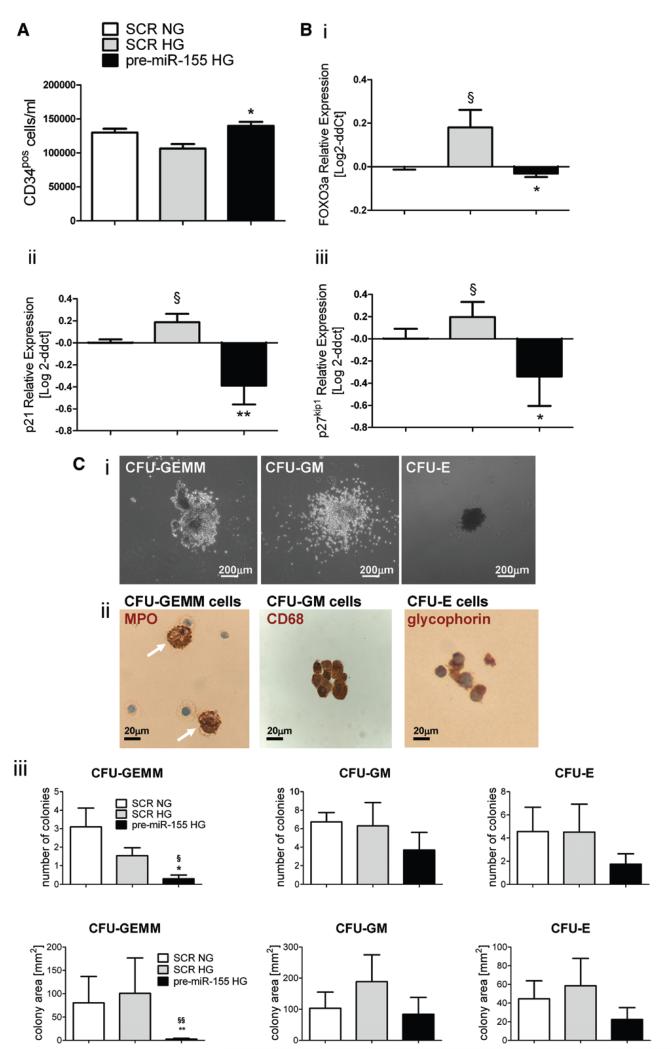
To verify whether miR-155 overexpression can rescue the effect of HG on target genes, we transfected HG-challenged CD34pos cells with pre–miR-155 and confirmed that transfection results in upregulation of related mature miR (Online Figure VI). Of note, forced expression of miR-155 contrasted the diminishing effect of HG on CD34pos cell counts and abrogates the HG-induced upregulation of FOXO3a, p21, and p27kip1 (Figure 7A and 7B). Finally, in inductive colony forming unit assays on HG-challenged CD34pos cells, miR-155-transduced cells generated fewer myeloid and erythroid colonies. Furthermore, the colonies generated by miR-155–transduced cells were smaller than controls (Figure 7C).
Figure 7. MicroRNA (miR)-155 overexpression in bone marrow (BM) CD34pos cells reverts high glucose (HG)-induced expressional changes and reduces the generation of myeloid and erythroid colonies.
A, Bar graphs showing the effect of HG and pre–miR-155 or control scramble (SCR) transfection on CD34pos cell number. *P<0.05 versus SCR HG. Cells were cultured either in normal glucose (NG) (5 mmol/L D-glucose, NG) or HG (25 mmol/L D-glucose, HG) for 48 hours. B, Bar graphs showing mRNA levels of (i) FOXO3a, (ii) CDKN1A/p21, and (iii) CDKN1B/p27kip1 after pre–miR-155 or control SCR transfection. N=5 healthy donors per group assayed in duplicate. C, Colony forming unit (CFU) assay of CD34pos cells. (i) Representative images of CFU-granulocytes, erythroid, macrophage, megakaryocyte (CFU-GEMM), CFU-granulocyte, macrophage (CFU-GM), and CFU-erythroid (CFU-E) colonies. ii, Immunocytochemical characterization of CFU-isolated cells by staining for myeloperoxidase (MPO), a marker for granulocytes, CD68, a marker of macrophages, and glyphorin, a marker of erythrocytes. Arrows in CFU-GEMM point at MPOpos granulocytes. Nuclei are stained blue with hematoxylin. iii, Bar graph showing the effect of miR-155 overexpression on number (top) and area (bottom) covered by colonies. N=5 healthy donors per group. *P<0.05 and **P<0.01 versus SCR HG, §P<0.05 and §§P<0.01 versus SCR NG. Data represent means±SEM.
Discussion
To the best of our knowledge, this is the first study showing the damaging effect of diabetes mellitus on BM SCs and their microvascular environment in human subjects. We demonstrate for the first time the presence of microangiopathy in BM of diabetic patients. Furthermore, we screened a large spectrum of SCs/PCs and mature hematopoietic cells in BM and PB by flow cytometry. Results newly document that the BM of diabetic patients is depleted of hematopoietic and proangiogenic PCs but not of differentiated hematopoietic cells. Finally, we show that both diabetes mellitus and HG alter the miR-155/FOXO3a/p21/p27kip1 signaling pathway, which represents a crucial mechanism controlling human CD34pos-PC renewal and differentiation. Importantly, forced expression of miR-155 inhibits the inductive effect of HG on the FOXO3a/p21/ p27kip1 trio and reduces differentiation of CD34pos-PCs in a colony forming unit assay.
Diabetic Microangiopathy in Human BM
A prominent feature of diabetic BM consists of substitution of the hematopoietic component with adipose tissue. Interestingly, the effect of diabetes mellitus on marrow composition was independent of background factors, with exception of fat accumulation which was also predicted by the combination of duration of diabetes mellitus and body mass index. Although additional investigation is needed to interpret the relationship between fatty infiltration of the marrow and obesity, evidence indicates that BM adipocytes could contribute to inhibit hematopoiesis in an obese model of T2DM.48
We found a direct correlation between vascular rarefaction and marrow remodeling, in line with the concept that a reduction of nurturing vasculature is detrimental for SC homeostasis. Of note, regression analysis indicates that, besides grouping factor, both duration of diabetes mellitus and hypertension are good predictors of capillary and sinusoid rarefaction, whereas arteriole density could be predicted only by variables inherent to diabetes mellitus, that is, disease duration and fasting glucose. The relationship between glycemic control and hypertension in the development of microangiopathy in peripheral organs is well documented. In CLI patients, who showed the most striking microvascular remodeling, analyses were performed on the proximal part of the amputated femoral bone which contains healthy tissue. We cannot exclude that the different anatomic source, in addition to ischemia, might have an impact on the incremental reduction of vascularity observed in this category.
Impact of Diabetes Mellitus on PC Abundance
Comparing marrow aspirates from 10 nondiabetic and 10 diabetic subjects, Fadini et al5 reported a significant reduction of CD34pos cells in the latter group. Extending those data, we observed a decrease in both CD34posCD133pos and CD34posKDRpos PCs in BM of diabetic patients. In contrast, lineage committed hematopoietic cells, including B and T lymphocytes, were similarly abundant or even increased, as in the case of NK subpopulation. Altogether, these findings newly indicate a disparity in the depleting effect of diabetes mellitus on different hematopoietic cell lineages, which might contribute to the altered spectrum of circulating cells. Importantly, the dominant influence of diabetes mellitus on these cellular endpoints was confirmed by analysis of associated risk factors and confounding variables.
Diabetes Mellitus Impinges on Master Molecular Regulators of Hematopoiesis
The present study highlights new molecular mechanisms that may account for HSC depletion in diabetes mellitus. Results of cell cycle analysis show that freshly sorted CD34pos-PCs from BM of diabetic patients are held in G1 phase, the typical restriction checkpoint where HSCs are cycle-arrested to prevent accrual of DNA damage. Moreover, diabetic BM cells show increased apoptosis, as assessed by immunohistochemistry and cell cycle analysis.
FOXO transcription factors are typically involved in enforcing cell cycle checkpoints in hematopoietic cells with DNA damage.27 A potential mediator of FOXO-induced cell cycle arrest as well as of irreversible progression toward cell apoptosis is p27kip1, a cyclin-dependent kinase inhibitor that reportedly reduces proliferation and survival of HSCs.49 We found that FOXO3a and downstream mediators, p21 and p27kip1, are remarkably upregulated in CD34pos-PCs from BM of diabetic patients, with these transcriptional changes being associated with reduction of cell viability. In vitro experiments challenging CD34pos cells with HG confirm that glucose is sufficient to activate the proapoptotic signaling pathway in healthy HSCs.
MiRs regulate major cellular processes, including metabolism, apoptosis, and differentiation and also participate in the pathogenesis of human diseases.50–52 Seminal studies using conditional deletion of Dicer, which disrupts miR processing, revealed critical roles of miRs during development of hematopoietic cell lineages.53,54 In particular, miR-155 regulates early stages of hematopoiesis through inhibition of multiple genes implicated in HSC survival and differentiation, such as the human FOXO3a gene.22 Current knowledge, however, is mainly centered on the implication of miR-155 in inflammation and cancer.55 Intriguingly, a recent study showed that circulating levels of miR-155 are lower in patients with coronary artery disease compared with healthy controls, with an additive diminishing effect of diabetes mellitus.56 It remains unknown what is the source of circulating miR-155 and whether a reduction of miR-155 expression in BM-derived cells can contribute to this defect.
Here, we report for the first time that miR-155 expression is reduced in BM CD34pos cells from diabetic patients and inversely correlated with levels of its validated target FOXO3a. Importantly, the inhibitory effect on miR-155 and the increase of FOXO3a, p21 and p27kip1 by diabetes mellitus were independent of other background factors and all replicated by challenging healthy CD34pos cells with HG. To establish causality, we next asked whether miR-155 would prevent the effects of HG. Results indicate that forced expression of miR-155 is able to reverse the HG-induced upregulation of FOXO3a, p21, and p27kip1. Furthermore, miR-155–transduced BM CD34pos cells formed fewer myeloid and erythroid colonies compared with scramble-transfected controls. The latter result replicates data from a previous study showing the ability of miR-155 in blocking differentiation in models of human hematopoiesis.22 It remains unknown whether the described miR-dependent mechanism is implicated in other deficiencies of diabetic CD34pos cells, including unresponsiveness to granulocyte colony-stimulating factor. Intriguingly, a recent study in healthy primates showed that granulocyte colony-stimulating factor–mobilized CD34pos cells express higher miR-155 levels compared with nonstimulated or Plerixaformobilized cells.57
Altogether, these findings suggest that deregulation of the miR-155/FOXO3a/p27 signaling pathway might contribute to BM CD34pos cell depletion in diabetes mellitus. More investigation is warranted to establish whether other HSC-associated miRs participate in determining an imbalance between endothelial progenitors and mature hematopoietic cells.
Clinical Implications
In conclusion, this study draws attention to the BM as a primary target of diabetes mellitus-induced damage. Our data suggest that the severity of systemic vascular disease has an impact on BM remodeling. Conversely, more severe BM pathologies can cause (or contribute to) macroangiopathy, through shortage of vascular regenerative cells. Moreover, it should be acknowledged that this study was conducted on aged subjects and that inference to a younger population is uncertain. Further research is warranted to find specific treatments able to preserve BM integrity in patients with diabetes mellitus.
Supplementary Material
Novelty and Significance.
What Is Known?
Diabetes mellitus is associated with reduced levels of circulating progenitor cells.
Studies in diabetic animal models indicate the presence of microangiopathy in bone marrow endangering resident stem cell integrity.
A restricted spectrum of microRNAs (miRs) that regulate self-renewal is expressed in hematopoietic stem cells.
What New Information Does This Article Contribute?
In patients with diabetes mellitus, there is microangiopathy in the bone marrow, which is associated with incremental vascular damage in the presence of critical limb ischemia.
The bone marrow of diabetic patients is depleted of hematopoietic and proangiogenic progenitor cells but not differentiated hematopoietic cells.
Hyperglycemia inhibits miR-155, thereby releasing the activity of proapoptotic pathway involving FOXO3a/p21.
Although remodeling of blood vessels in peripheral organs has been extensively studied, little is known about the impact of common diseases on the marrow vascular niche, which is crucial for stem cell homeostasis and mobilization. Therefore, we investigated the effect of diabetes mellitus on bone marrow stem cells and their nurturing vasculature in humans. Results show a profound remodeling of the vascular niche, which is mainly replaced by fat, especially in patients with critical ischemia. Stem cell depletion did not preclude a regular abundance of mature hematopoietic cells, suggesting a defect in stem cell self-renewal. Investigation of underpinning mechanisms revealed that hyperglycemia inhibits a master regulator of hematopoietic cell fate, miR-155, thereby resulting in an unbalance between proliferation and differentiation. This was corrected by forced expression of miR-155. Our findings advance current understanding of pathological mechanisms leading to collapse of the vascular niche and reduced availability of proangiogenic cells. These data provide a key for interpretation of diabetes mellitus-associated defect in stem cell mobilization. In addition, our study reveals a negative circuitry, normally contrasted by a hematopoietic miR-responsible for pauperization of the marrow regenerative potential.
Acknowledgments
Sources of Funding This study was supported by a grant from the British Heart Foundation (BHF) entitled Bone marrow dysfunction alters vascular homeostasis in diabetes. C. Emanueli is a BHF senior basic research fellow. In addition, the study was supported by a grant from the Cariplo Foundation (Ref. 2011-0566).
Nonstandard Abbreviations and Acronyms
- BM
bone marrow
- CLI
critical limb ischemia
- EC
endothelial cell
- HG
high glucose
- HSC
hematopoietic stem cell
- KDR
kinase insert domain receptor
- miR
microRNA
- NK
natural killer lymphocytes
- PB
peripheral blood
- PC
progenitor cell
- SC
stem cell
- T2DM
type 2 diabetes mellitus
Footnotes
Disclosures None.
References
- 1.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 2.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 3.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 4.Fadini GP, Maruyama S, Ozaki T, Taguchi A, Meigs J, Dimmeler S, Zeiher AM, de Kreutzenberg S, Avogaro A, Nickenig G, Schmidt-Lucke C, Werner N. Circulating progenitor cell count for cardiovascular risk stratification: a pooled analysis. PLoS ONE. 2010;5:e11488. doi: 10.1371/journal.pone.0011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadini GP, Boscaro E, de Kreutzenberg S, Agostini C, Seeger F, Dimmeler S, Zeiher A, Tiengo A, Avogaro A. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care. 2010;33:1097–1102. doi: 10.2337/dc09-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadini GP, Albiero M, Seeger F, Poncina N, Menegazzo L, Angelini A, Castellani C, Thiene G, Agostini C, Cappellari R, Boscaro E, Zeiher A, Dimmeler S, Avogaro A. Stem cell compartmentalization in diabetes and high cardiovascular risk reveals the role of DPP-4 in diabetic stem cell mobilopathy. Basic Res Cardiol. 2013;108:313. doi: 10.1007/s00395-012-0313-1. [DOI] [PubMed] [Google Scholar]
- 7.Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, Beem E, Shaw LC, Li Calzi S, Harrison JK, Tran-Son-Tay R, Grant MB. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–109. [PubMed] [Google Scholar]
- 8.Kränkel N, Katare RG, Siragusa M, et al. Role of kinin B2 receptor signaling in the recruitment of circulating progenitor cells with neovascularization potential. Circ Res. 2008;103:1335–1343. doi: 10.1161/CIRCRESAHA.108.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busik JV, Tikhonenko M, Bhatwadekar A, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206:2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferraro F, Lymperi S, Méndez-Ferrer S, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadini GP, Albiero M, Vigili de Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, Poncina N, Agostini C, Avogaro A. [Accessed January 7, 2013];Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care. 2012 Oct 30; doi: 10.2337/dc12-1084. doi: 10.2337/dc12-1084. http://care.diabetesjournals.org/content/early/2012/10/28/dc12-1084.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiPersio JF. Diabetic stem-cell “mobilopathy”. N Engl J Med. 2011;365:2536–2538. doi: 10.1056/NEJMcibr1112347. [DOI] [PubMed] [Google Scholar]
- 13.Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol. 2010;105:703–712. doi: 10.1007/s00395-010-0109-0. [DOI] [PubMed] [Google Scholar]
- 14.Saito H, Yamamoto Y, Yamamoto H. Diabetes alters subsets of endothelial progenitor cells that reside in blood, bone marrow, and spleen. Am J Physiol, Cell Physiol. 2012;302:C892–C901. doi: 10.1152/ajpcell.00380.2011. [DOI] [PubMed] [Google Scholar]
- 15.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 16.Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G, Kraenkel N, Prezioso L, Emanueli C, Madeddu P. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol. 2010;30:498–508. doi: 10.1161/ATVBAHA.109.200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113:175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 20.Garzon R, Volinia S, Liu CG, et al. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood. 2008;111:3183–3189. doi: 10.1182/blood-2007-07-098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connell RM, Chaudhuri AA, Rao DS, Gibson WS, Balazs AB, Baltimore D. MicroRNAs enriched in hematopoietic stem cells differentially regulate long-term hematopoietic output. Proc Natl Acad Sci USA. 2010;107:14235–14240. doi: 10.1073/pnas.1009798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgantas RW, 3rd, Hildreth R, Morisot S, Alder J, Liu CG, Heimfeld S, Calin GA, Croce CM, Civin CI. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci USA. 2007;104:2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masaki S, Ohtsuka R, Abe Y, Muta K, Umemura T. Expression patterns of microRNAs 155 and 451 during normal human erythropoiesis. Biochem Biophys Res Commun. 2007;364:509–514. doi: 10.1016/j.bbrc.2007.10.077. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M, Kondo E, Takeuchi M, Harashima A, Otani T, Tsuji-Takayama K, Yamasaki F, Kumon H, Kibata M, Nakamura S. miR-155, a modulator of FOXO3a protein expression, is underexpressed and cannot be upregulated by stimulation of HOZOT, a line of multifunctional treg. PLoS ONE. 2011;6:e16841. doi: 10.1371/journal.pone.0016841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 26.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 27.Lei H, Quelle FW. FOXO transcription factors enforce cell cycle checkpoints and promote survival of hematopoietic cells after DNA damage. Mol Cancer Res. 2009;7:1294–1303. doi: 10.1158/1541-7786.MCR-08-0531. [DOI] [PubMed] [Google Scholar]
- 28.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 29.Mackie AR, Losordo DW. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38:474–485. [PMC free article] [PubMed] [Google Scholar]
- 30.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 31.Gallacher L, Murdoch B, Wu DM, Karanu FN, Keeney M, Bhatia M. Isolation and characterization of human CD34(−)Lin(−) and CD34(+) Lin(−) hematopoietic stem cells using cell surface markers AC133 and CD7. Blood. 2000;95:2813–2820. [PubMed] [Google Scholar]
- 32.Barcelos LS, Duplaa C, Kränkel N, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski D, Freund M, Steinhoff G. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 34.Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, Van Haute I, Lootens N, Heyndrickx G, Wijns W. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112:I178–I183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 35.Mansour S, Roy DC, Bouchard V, Stevens LM, Gobeil F, Rivard A, Leclerc G, Reeves F, Noiseux N. One-year safety analysis of the COMPARE-AMI trial: comparison of intracoronary injection of CD133 bone marrow stem cells to placebo in patients after acute myocardial infarction and left ventricular dysfunction. Bone Marrow Res. 2011;2011:385124. doi: 10.1155/2011/385124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34−/ CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006;98:e20–e25. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]
- 37.Pelosi E, Valtieri M, Coppola S, Botta R, Gabbianelli M, Lulli V, Marziali G, Masella B, Müller R, Sgadari C, Testa U, Bonanno G, Peschle C. Identification of the hemangioblast in postnatal life. Blood. 2002;100:3203–3208. doi: 10.1182/blood-2002-05-1511. [DOI] [PubMed] [Google Scholar]
- 38.Botta R, Gao E, Stassi G, Bonci D, Pelosi E, Zwas D, Patti M, Colonna L, Baiocchi M, Coppola S, Ma X, Condorelli G, Peschle C. Heart infarct in NOD-SCID mice: therapeutic vasculogenesis by transplantation of human CD34+ cells and low dose CD34+KDR+ cells. FASEB J. 2004;18:1392–1394. doi: 10.1096/fj.03-0879fje. [DOI] [PubMed] [Google Scholar]
- 39.Madeddu P, Emanueli C, Pelosi E, Salis MB, Cerio AM, Bonanno G, Patti M, Stassi G, Condorelli G, Peschle C. Transplantation of low dose CD34+KDR+ cells promotes vascular and muscular regeneration in ischemic limbs. FASEB J. 2004;18:1737–1739. doi: 10.1096/fj.04-2192fje. [DOI] [PubMed] [Google Scholar]
- 40.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 41.Pirro M, Schillaci G, Menecali C, Bagaglia F, Paltriccia R, Vaudo G, Mannarino MR, Mannarino E. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34+ cells of hypertensive patients. J Hypertens. 2007;25:2093–2099. doi: 10.1097/HJH.0b013e32828e506d. [DOI] [PubMed] [Google Scholar]
- 42.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spinetti G, Fortunato O, Cordella D, Portararo P, Kränkel N, Katare R, Sala-Newby GB, Richer C, Vincent MP, Alhenc-Gelas F, Tonolo G, Cherchi S, Emanueli C, Madeddu P. Tissue kallikrein is essential for invasive capacity of circulating proangiogenic cells. Circ Res. 2011;108:284–293. doi: 10.1161/CIRCRESAHA.110.236786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, Sohn YD, Lee MY, Houge MA, Yoon YS. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19:1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- 46.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 47.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 48.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 50.Caporali A, Emanueli C. MicroRNA regulation in angiogenesis. Vascul Pharmacol. 2011;55:79–86. doi: 10.1016/j.vph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res. 2012;93:583–593. doi: 10.1093/cvr/cvr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. microRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther. 2010;125:92–104. doi: 10.1016/j.pharmthera.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Cobb BS, Nesterova TB, Thompson E, Hertweck A, O’Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, Merkenschlager M. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;201:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, Rajewsky K. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132:860–874. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 55.Tili E, Michaille JJ, Wernicke D, Alder H, Costinean S, Volinia S, Croce CM. Mutator activity induced by microRNA-155 (miR-155) links inflammation and cancer. Proc Natl Acad Sci USA. 2011;108:4908–4913. doi: 10.1073/pnas.1101795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 57.Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, Stroncek DF. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009;114:2530–2541. doi: 10.1182/blood-2009-04-214403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.