Abstract
Rationale
Circulating proangiogenic cells (PACs) support postischemic neovascularization. Cardiovascular disease and diabetes mellitus impair PAC regenerative capacities via molecular mechanisms that are not fully known. We hypothesize a role for microRNAs (miRs). Circulating miRs are currently investigated as potential diagnostic and prognostic biomarkers.
Objective
The objectives were the following: (1) to profile miR expression in PACs from critical limb ischemia (CLI) patients; (2) to demonstrate that miR-15a and miR-16 regulate PAC functions; and (3) to characterize circulating miR-15a and miR-16 and to investigate their potential biomarker value.
Methods and Results
Twenty-eight miRs potentially able to modulate angiogenesis were measured in PACs from CLI patients with and without diabetes mellitus and controls. miR-15a and miR-16 were further analyzed. CLI-PACs expressed higher level of mature miR-15a and miR-16 and of the primary transcript pri–miR-15a/16-1. miR-15a/16 overexpression impaired healthy PAC survival and migration. Conversely, miR-15a/16 inhibition improved CLI-PAC–defective migration. Vascular endothelial growth factor-A and AKT-3 were validated as direct targets of the 2 miRs, and their protein levels were reduced in miR-15a/16–overexpressing healthy PACs and in CLI-PACs. Transplantation of healthy PACs ex vivo–engineered with anti–miR-15a/16 improved postischemic blood flow recovery and muscular arteriole density in immunodeficient mice. miR-15a and miR-16 were present in human blood, including conjugated to argonaute-2 and in exosomes. Both miRs were increased in the serum of CLI patients and positively correlated with amputation after restenosis at 12 months postrevascularization of CLI type 2 diabetes mellitus patients. Serum miR-15a additionally correlated with restenosis at follow-up.
Conclusions
Ex vivo miR-15a/16 inhibition enhances PAC therapeutic potential, and circulating miR-15a and miR-16 deserves further investigation as a prognostic biomarker in CLI patients undergoing revascularization.
Keywords: angiogenesis, diabetes mellitus, ischemia, microRNAs, microRNA-15a, microRNA-16, proangiogenic cells
Peripheral artery disease can evolve to critical limb ischemia (CLI), a life-threatening condition characterized by pain at rest and tissue loss with ulcer and gangrene.1 Once CLI occurs, blood flow (BF) must be restored by either percutaneous angioplasty or surgical revascularization. Both options are too often not feasible, and thus amputation may be the only remedy, especially in diabetic patients.2
Therapeutic stimulation of the angiogenesis process represents a novel strategy to support postischemic BF recovery, wound closure, and tissue regeneration. Bone marrow (BM)–derived proangiogenic cells (PACs), which were previously known as early endothelial progenitor cells (EPCs), have been implicated in both native and therapeutically guided angiogenesis.3 PACs derive from mononuclear cells by culture enrichment.4 The real nature, antigenic definition, and proangiogenic mechanisms of PACs are still debated. Notwithstanding, their capacity to promote angiogenesis in ischemic tissue is accepted.3 The pioneer clinical trial Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) showed initial therapeutic promises of PACs enriched from either the BM or the peripheral blood (PB) of patients with acute myocardial infarct.5 Furthermore, a recent meta-analysis of 37 clinical trials has revealed that autologous transplantation of BM-derived cells is a feasible, relatively safe, and potentially effective therapeutic strategy for CLI patients.6 The efficacy of this cell therapy approach needs further consolidation by large, randomized, placebo-controlled, double-blind studies, including the ongoing injection of autologous CD34-positive cells for critical limb ischemia (ACT34-CLI),7 bone marrow outcome trial in critical limb ischemia (BONMOT-CLI),8 and rejuvenating endothelial progenitor cells via transcutaneous intra-arterial supplementation (JUVENTAS).9
Despite the encouraging evidence from early clinical trials, the regenerative potential of PACs derived from patients with tissue ischemia and diabetes mellitus (DM) is reduced, and the underpinning molecular mechanisms are not fully clarified.10–12 Here, we aimed to analyze the contribution of microRNAs (miRs) to PAC dysfunction.
miRs are small (21–25 nucleotides in their mature forms) noncoding RNAs capable of posttranscriptionally inhibiting gene expression.13,14 Importantly, each miR regulates the expression of several target genes, with the possibility to modulate multiple pathways. Several miRs control postischemic angiogenesis acting at different levels.15 Furthermore, miRs influence the therapeutic potential of human embryonic stem cell–derived endothelial progenitor cells and of pericyte progenitor cells16,17 on their transplantation in mouse models of peripheral or myocardial ischemia.
In this study, after an initial expressional screening for 28 angiogenesis-related miRs in PACs from CLI patients with and without type 2 DM (T2DM), we focused our attention on miR-15a and miR-16. In Homo sapiens, 2 miR-15/16 clusters exist: miR-15a/miR-16-1 and miR-15b/miR-16-2 (at 13q14.2 and 3q25.33, respectively). miR-15a and miR-16 share a portion of their seed sequence (ie, the sequence that binds to the 3′ untranslated region of the targeted mRNAs) with 5 other miRs, including miR-503 and miR-424.18 We previously showed that miR-503 impairs angiogenesis in the setting of CLI and diabetes mellitus.19 In addition, Chamorro et al20 demonstrated that miR-16 and miR-424 inhibit in vitro endothelial function and angiogenesis by modulating the expression of VEGF-A, VEGF kinase insert domain receptor (KDR) and fibroblast growth factor receptor-1 (FGF-R1). Furthermore, Hullinger et al21 showed that miR-15 inhibition protects against cardiac ischemic injury.
Here, we show that CLI increases miR-15a and miR-16 levels in PACs. Furthermore, the 2 miRs impair PAC survival and migration toward chemoattractants. Conversely, ex vivo inhibition of miR-15a and miR-16 empowers PACs, increasing their therapeutic potential when transplanted in an immunocompromised mouse limb ischemia model. Finally, the levels of circulating miR-15a and miR-16 are increased in CLI patients, and correlates with restenosis and post-restenosis amputation 12 months after revascularization.
Methods
An expanded Methods section is available in the Online Data Supplement.
Clinical Study
The data reported here were produced to meet the secondary objective (namely, to identify new molecular mechanisms responsible for the known functional impairment of PACs in CLI) of the clinical trial Diabetic Foot and Vascular Progenitor Cells (NCT0126958) developed at MultiMedica-Milan with a consecutive series of consented patients. Nonischemic and nondiabetic volunteers were recruited external to this trial to serve as controls. The characteristics of the human populations who donated blood for this study are reported in Online Table I. Import of human samples, storage, and use at the University of Bristol were approved by the UK National Research Ethic Service South West (REC-11/SW/0093).
PAC Culture
PACs were prepared from PB mononuclear cells, as described.22
miR Expressional Analyses
Mature miR expression was measured using Taqman-validated polymerase chain reaction primers and normalized to either the small nuclear RNA U6 (snU6) (for cells and exosomes) or the synthetic Caenorhabditis elegans miR-39 (Cel-miR-39) (Qiagen; for serum and plasma). When necessary, standard curves for miR quantification were used.
PAC Transfection
PACs were transfected with pre-miR mimics (pre–miR-15a and pre–miR-16), miR inhibitors (anti–miR-15a and anti–miR-16), or scramble negative control (Applied Biosystems) at a final concentration of 50 nmol/L.
PAC In Vitro Assays
PAC apoptosis was measured by flow cytometry using annexin V and propidium iodide. PAC migration and capacity to support endothelial cell networking on 2-dimensional Matrigel were investigated as described.22 Three-dimensional angiogenesis assay (with conditioned culture medium [CCM] of PACs and human umbilical vein endothelial cells [HUVECs]) was performed by Spheroid assay according to the manufacturer’s instructions (PromoCell). The effect of miR manipulation on PAC growth was measured counting trypan blue–negative cells at 48 hours after transfection.
miR Target Gene Analysis
miR-15a and miR-16 gene targets were identified using MirWalk (http://ma.uni-heidelberg.de/apps/zmf/mirwalk), which allows searching of several independent prediction software programs. The 3′ untranslated region luciferase activity assays were used to validate vascular endothelial growth factor (VEGF)-A and AKT3 as direct targets of each miR. VEGF and AKT3 mRNA and protein levels in PACs were measured (real-time reverse-transcriptase polymerase chain reaction and Western blotting or enzyme-linked immunosorbent assay [ELISA], respectively).
In Vivo Study
Experiments on mice were approved by the UK Home Office and conducted at the University of Bristol in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources.
PACs from healthy donors (described in the Online Table II) were transfected with pre–miR-15a plus pre–miR-16, anti–miR-15a plus anti–miR-16, or negative control. Unilateral hindlimb ischemia was induced in anesthetized immunocompromised CD1-Foxn1nu mice (Charles River, United Kingdom; n=11–14 mice/group).16 Transfected PACs (1×105 cells in 15 μL of culture medium) or fresh cell culture medium was injected in 3 equidistant sites of the ischemic adductor muscle. Postischemic BF recovery was sequentially measured (laser color Doppler). After 2 weeks, mice were euthanized and muscular microvascular density was assessed.16,19,22
Statistical Analyses
Continuous variables were compared between cases and controls by using Student t test and analysis of variance [ANOVA] or by means of the Kruskal-Wallis test if they had a skewed distribution. Categorical variables were compared by means of the χ2 test. Correlations were evaluated by Spearman correlation coefficient. To evaluate the association between miR expression and the risk of events, odds ratios (OR) and their 95% confidence intervals (CIs) were calculated from multiple logistic regression after adjustment for age and sex. Continuous variables that were positively skewed were analyzed on the log-2 scale. Results were considered statistically significant at P<0.05.
Results
CLI Impacts on PAC miR Expression
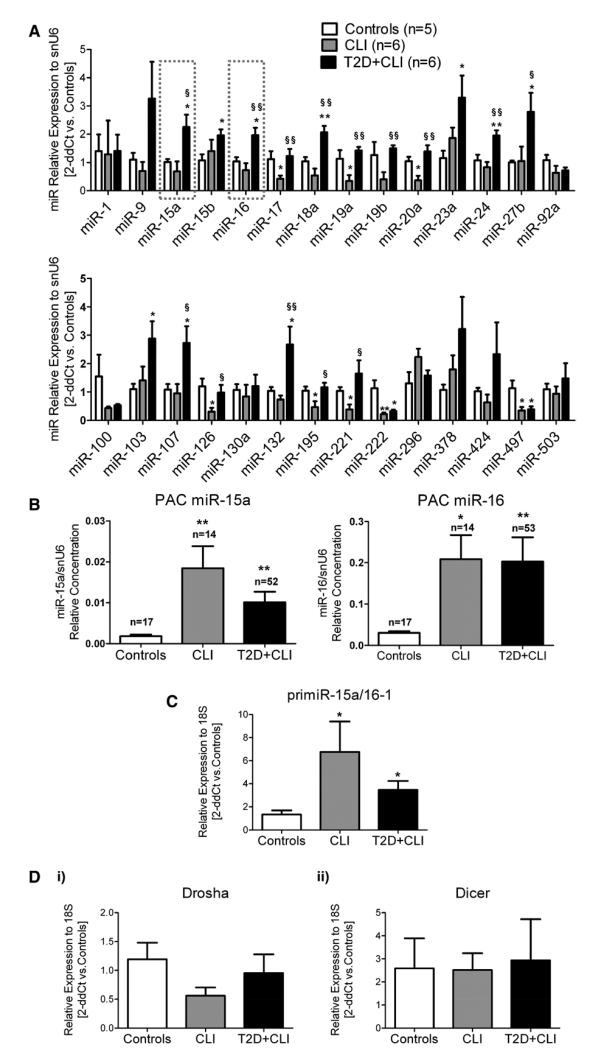
PAC identity was assessed as previously described22 (Online Figure I). Using PACs from small cohorts of healthy donors (n=5) and CLI patients with and without T2DM (n=6 per group) who were randomly selected from larger populations illustrated in Online Table I, we screened the expression of 28 miRs (internal control: snU6) selected because of their potential to modulate angiogenesis (Online Table III). These analyses were performed using the delta delta cycle threshold (2-ddCT) method and without using miR standard curves for quantification. Eighteen of the 28 miRs appeared differentially regulated among groups (Figure 1A). miR-16 and miR-15 have been related to angiogenesis20,23 and ischemia,21 respectively. Furthermore, they belong to the extended miR-16 family that interests us.18,19 Hence, we selected miR-15a and miR-16 for further expressional and functional analyses.
Figure 1. Critical limb ischemia (CLI) affects the expression of microRNAs (miRs) in proangiogenic circulating cells (PACs).
A, Expression of 28 miRs (TaqMan real-time polymerase chain reaction) in PACs from small cohorts of CLI patients with or without type 2 diabetes mellitus (T2D) and in healthy controls. Data were reported to the control group by the 2-ddCt method [internal control: small nuclear RNA u6 (snu6)]. Rectangles indicate miR-15a and miR-16. B, miR-15a (left) and miR-16 (right) relative expression (measured using standard curves for miR and snU6) in PACs prepared from larger cohorts of CLI patients with and without T2D and controls. C, Pri–miR-15a/16-1 PAC expression (at least n=6 donors/group). D, Drosha and Dicer mRNA expression in PACs (at least n=3 donors/group). Data in (C) and (D) are analyzed vs the control group by the 2-ddCt method (internal control: mRNA18s). All data are mean±SEM. *P<0.05 and **P<0.001 vs healthy controls. §P<0.05 and §§P<0.001 vs CLI without T2D.
miR-15a and miR-16 Expression in PACs From Healthy and Ischemic Subjects
Relative expression of miR-15a and miR-16 was measured again in PACs prepared from larger cohorts and analyzed using miR standard curves. We confirmed that miR-15a and miR-16 were increased in CLI-PACs, but without further difference induced by T2DM (Figure 1B). Furthermore, hypoxia, which mimics ischemia in vitro, increased miR-15a and miR-16 in healthy PACs (Online Figure II). Levels of miR-15a and miR-16 in PACs were directly correlated (Spearman correlation coefficient=0.6601; P<0.0001). This can be reconciled with the fact that miR-15a and miR-16-1 are clustered and, hence, transcribed together as a primary transcript (pri–miR-15a/16-1). In fact, pri–miR-15a/16-1 expression was increased in CLI-PACs (Figure 1C). The enzymes Drosha and Dicer are essential for miR maturation. Drosha cleaves pri-miRs to pre-miRs (precursor miRs), and Dicer cleaves pre-miRs to mature miRs.15 We found that PACs express both enzymes at mRNA level, without difference among groups (Figure 1D). The sum of the aforementioned evidence suggests that PACs are able to transcribe and process miR-15a and miR-16. In addition, as shown in Figure 2B, PACs are able to secrete the 2 miRs embedded in exosomes, an emerging class of microvesicles of endosomal origin involved in the transport of protein and mRNA with implications in neovascularization.24,25 This allows for speculating a role of PAC-derived miR-15a and miR-16 in cell-to-cell communications during angiogenesis, as well as their contribution to the pool of PB circulating miRs.
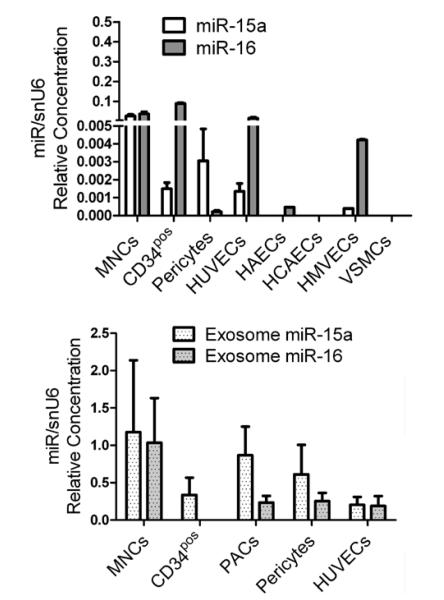
Figure 2. Expression of microRNA (miR)-15a and miR-16 in bone marrow, vascular cells, and exosomes.
Bar graphs of relative miR-15a and miR-16 expression by (A) circulating nonselected mononuclear cells (MNCs), CD34pos MNCs, and vascular cells, that is, pericytes, human umbilical vein endothelial cells (HUVECs), human microvascular endothelial cells (HMVECs), human coronary artery endothelial cells (HCAECs), and human aorta endothelial cells (HAECs; at least n=3/cell type). B, Exosomes purified from corresponding cell-conditioned medium (n=4 cell replicates). Data in (A) and (B) are relative expression (measured using standard curves for miR and snU6).
Expression and Secretion of miR-15a and miR-16 by Different Cell Populations
We additionally measured the expression of miR-15a and miR-16 in several cell populations that are relevant for neovascularization, including unfractionated PB mononuclear cells, CD34pos mononuclear cells, several endothelial cell lines, vascular smooth muscle cells (VSMCs), and pericytes. Figure 2A shows different levels of miR-15a and miR-16 among these cell types. Interestingly, the 2 miRs and especially miR-15a seemed enriched in the exosomes prepared from the CCM of the tested cell populations (Figure 2B).
Forced miR-15a/16 Overexpression Impairs PAC Survival and Migration
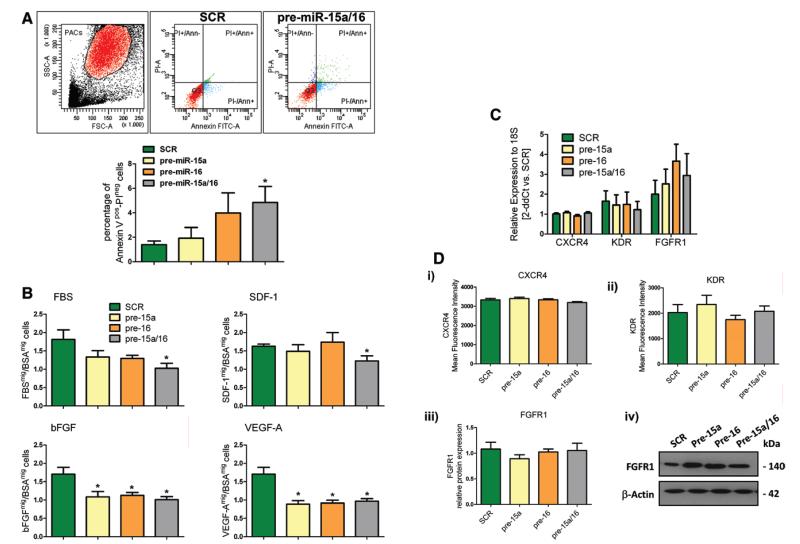
To investigate the functional relevance of miR-15a and miR-16, PACs from healthy donors were transfected with pre–miR-15a and pre–miR-16 (control: scramble). Efficacy of pre-miR transfection is shown in Online Figure IIIA. PACs overexpressing both miRs showed increased apoptosis (Figure 3A) and reduced capacity to migrate toward fetal bovine serum (unspecific stimulus), stromal cell–derived factor-1α (SDF-1α), basic FGF, and VEGF-A (Figure 3B i – 3B iv). However, single overexpression of either miR-15a or miR-16 inhibited migration to basic FGF and VEGF-A only (Figure 3B iii and 3B iv). We assessed the potential regulation of SDF-1α receptor CXC chemokine receptor, type 4 (CXCR4), FGF-R1, and KDR by pre-miR transfection of PACs. Different from what was reported by Chamorro-Jorganes et al20 in HUVECs, miR-15a/16 overexpression did not affect the expression of these receptors in PACs (Figure 3C and 3D). Next, to study whether miR-15a and miR-16 alter the capacity of PACs to collaborate with ECs in the angiogenic process, we evaluated the impact of PACs transfected with both pre-miRs on the capillary-like network formation from HUVECs seeded on Matrigel. However, we could not observe any significant difference induced by miR-15a/16 overexpression in PACs (Online Figure IVA i and IVA ii). The CCM of miR-15a/16–overexpressing PACs also failed to affect HUVEC networking on Matrigel (control: medium of PACs transfected with scramble; data not shown). Similarly, negative results were observed when the CCM from healthy PACs overexpressing pre-miRs or scramble was used to stimulate HUVECs in the 3-dimensional spheroid angiogenesis assay (Online Figure IVB)
Figure 3. Functional effects of microRNA (miR)-15a and miR-16 overexpression in control proangiogenic circulating cells (PACs).
A, Apoptosis expressed as percentage of annexin Vpos/PIneg cells in healthy PACs (n=5/group). B, Migration (in transwell chamber assay) of PACs toward fetal bovine serum (FBS), stromal cell–derived factor-1α (SDF-1α), basic fibroblast growth factor receptor (bFGF), or vascular endothelial growth factor (VEGF)-A (n=5 donors/group assayed in triplicate). C, CXC chemokine receptor, type 4 (CXCR4), kinase insert domain receptor (KDR), and FGF receptor-1 (FGFR1) mRNA levels (Taqman real-time reverse-transcriptase polymerase chain reaction) in transfected PACs. D, CXCR4, KDR, and FGFR1 protein levels: (i) CXCR4 and (ii) KDR mean fluorescence intensity (measured by flow cytometry, n=6/group); (iii and iv) FGFR1 protein levels measured by Western blot (n=9/group); (iv) representative Western blot bands. All data are expressed as mean±standard error of the mean. *P<0.05 vs PACs transfected with scramble negative control (SCR).
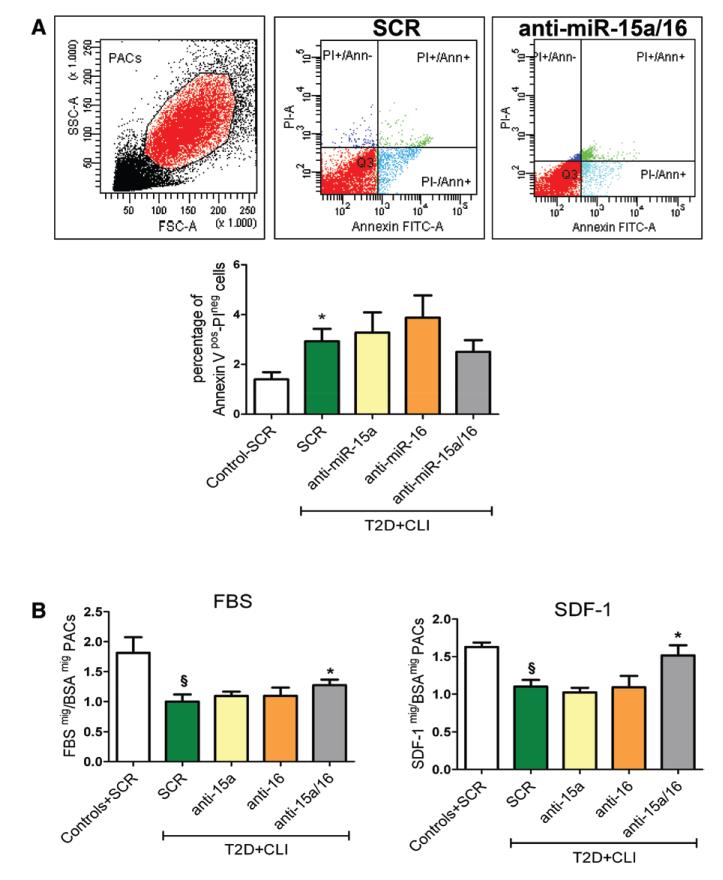
Inhibition of miR-15a/16 Rescues the Impaired Migratory Capacity of PACs From CLI-T2DM Patients
Based on the results obtained in healthy PACs by increasing miR-15a/16, we hypothesized that lowering miR-15a and miR-16 levels could improve the functional capabilities of patient-derived PACs. Thus, T2DM-CLI PACs were transfected with anti–miR-15a and anti–miR-16. As controls, we used diseased and healthy PACs transfected with scramble. After confirmation of miR-15a and miR-16 efficient reduction by anti-miRs (Online Figure IIIB), we assayed PAC apoptosis, migratory capacity, and proangiogenic activity. As expected, apoptosis was higher in diseased PACs (Figure 4A). This defect could not be corrected by anti–miR-15a/16 intervention. As expected, diseased PACs showed impaired migration to fetal bovine serum or SDF-1α. Importantly, this could be improved by anti–miR-15a/16 (Figure 4B). CXCR4 mRNA and protein expression were similar in healthy and diseased PACs (Online Figure VA and VB, respectively). Hence, the decreased capacity of diseased PACs to migrate toward SDF-1α does not depend on changes in receptor expression. As shown in Online Figure IVA iii and IVA iv, T2DM-CLI PACs could not compare with healthy PACs in the support offered to HUVECs in forming endothelial networks on Matrigel. However, anti–miR-15a/16 did not increase the proangiogenic capacity of diseased PACs. Furthermore, the CCM of T2DM-CLI PACs showed impaired capacity to stimulate HUVEC sprouting in the spheroid assay, which could not be rescued by anti–miR-15a/16 (Online Figure IVB). Finally, PAC growth in culture was not affected by disease or forced changes in miR-15a and miR-16 expression (Online Figure VI).
Figure 4. MicroRNA (miR)-15a and miR-16 inhibition improves type 2 diabetes mellitus (T2D) and critical limb ischemia (CLI)- proangiogenic circulating cell (PAC) migration.
Untreated T2D-CLI PACs were compared with control healthy PACs for their apoptosis rates and then transfected with anti–miR-15a, anti–miR-16, their combination, or scramble (SCR). SCR healthy PACs served for reference. A, PAC apoptosis measured as percentage of annexin V+/PI− cells (n=4/group); *P<0.05 vs SCR healthy PACs. B, PAC migration toward fetal bovine serum (FBS) and stromal cell–derived factor-1α (SDF-1α); PACs from n=5 subjects/group, each assayed in triplicate); §P<0.05 vs SCR healthy PACs; *P<0.05 vs SCR-T2D-CLI PACs. All data are expressed as mean±standard error of the mean.
VEGF-A and AKT3 Are Direct Targets of Both miR-15a and miR-16
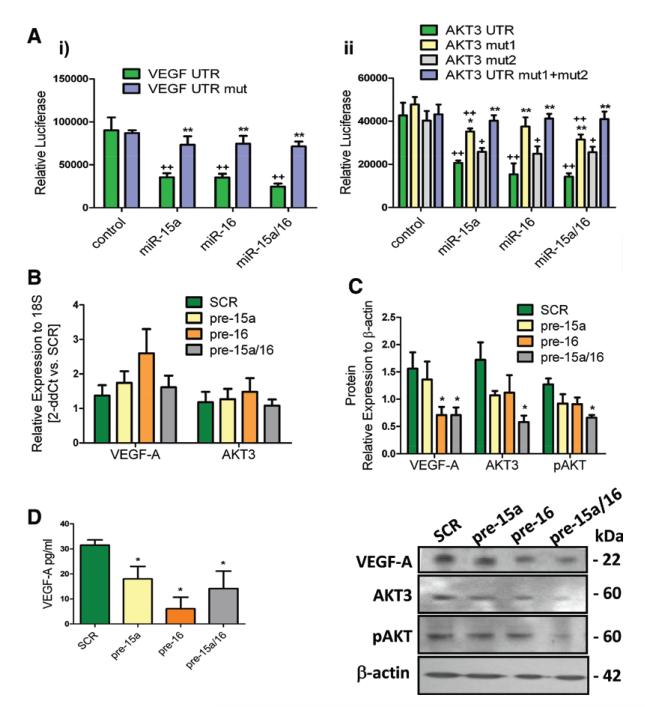
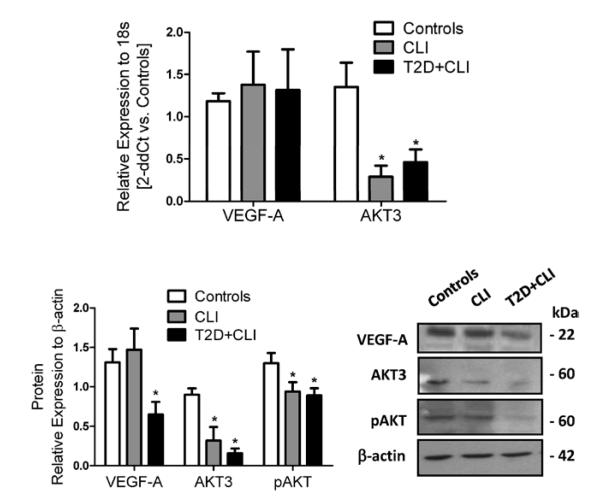
VEGF-A was predicted as direct target of both miR-15a and miR-16 (by 6/9 prediction software searched using miRwalk). AKT-3 was predicted as direct target of miR-15a (7/9 software) and miR-16 (6/9 software). We validated that VEGF-A and AKT-3 are direct targets of miR-15a and miR-16 using 3′ untranslated region luciferase assays (Figure 5A). The significant decrease in luciferase activity in the presence of miR-15a and miR-16 confirmed the binding to the 3′ untranslated region of VEGF-A and AKT3. Furthermore, this effect was reverted by mutations in the putative miR-binding sites. In control PACs, pre–miR-15a/16 did not change AKT3 and VEGF-A mRNA expression (Figure 5B) but reduced intracellular AKT3 and VEGF-A protein levels (Figure 5C) and secreted VEGF-A (Figure 5D). The active, Ser(473)-phosphorylated, form of AKT was also decreased (Figure 5C), pointing to a deregulation of AKT-associated signaling pathway induced by miR-15a and miR-16. Expression of AKT1 and AKT2 was not affected by pre–miR-15a/16 (data not shown). Next, we sought confirmation of similar miR-15a/16–associated changes in patient-derived PACs. Importantly, PACs from CLI patients with/without T2DM showed lower RNA and protein expression of AKT-3 and VEGF-A. Phospho-AKT was also decreased (Figure 6A and 6B).
Figure 5. MicroRNA (miR)-15a and miR-16 directly target AKT3 and vascular endothelial growth factor (VEGF)-A.
A, Bar graphs showing average luciferase assay data obtained in human embryonic kidney (HEK) 293 cells transfected with plasmid carrying the 3-untranslated region (UTR) sequence for (i) VEGF-A wild type or its mutated form (mut) and (ii) AKT3 wild type and 2 mutated forms (mut1 and mut2) or their combination (mut1+mut2) (n=4; *P<0.05 and **P<0.01 vs scramble (SCR) control; +P<0.05 and ++P<0.01 vs nonmutated corresponding to 3′-UTR). B, VEGF-A and AKT3 mRNA levels (Taqman real-time reverse-transcriptase polymerase chain reaction) in healthy proangiogenic circulating cells (PACs) transfected with pre–miR-15a, pre–miR-16, their combination, or SCR; (n=9/group). C, Results of VEGF-A, AKT3, and phospho-AKT (pAKT) protein analysis (left) by Western blot (n=4/group) and representative Western blot bands (right). D, VEGF-A content (measured by enzyme-linked immunosorbent assay) in the conditioned medium of transfected PACs (n=6/group). Data are mean±standard error of the mean. *P<0.05 vs SCR.
Figure 6. AKT3 and vascular endothelial growth factor (VEGF)-A are altered in proangiogenic circulating cells (PACs) from critical limb ischemia (CLI) patients.
A, VEGF-A and AKT3 mRNA levels in PACs of patients with CLI with and without type 2 diabetes mellitus (T2D) and healthy PAC control (n=6 donors/group). B, Results of VEGF-A, AKT3, and phospho-AKT (pAKT) protein analysis (left) by Western blot (at least n=3/group) and representative Western blot bands (right). Data are expressed as mean±standard error of the mean. **P<0.001 and *P<0.05 vs healthy PACs.
miR-15a and miR-16 Modulate the Therapeutic Potential of PACs in a Mouse Model of Limb Ischemia
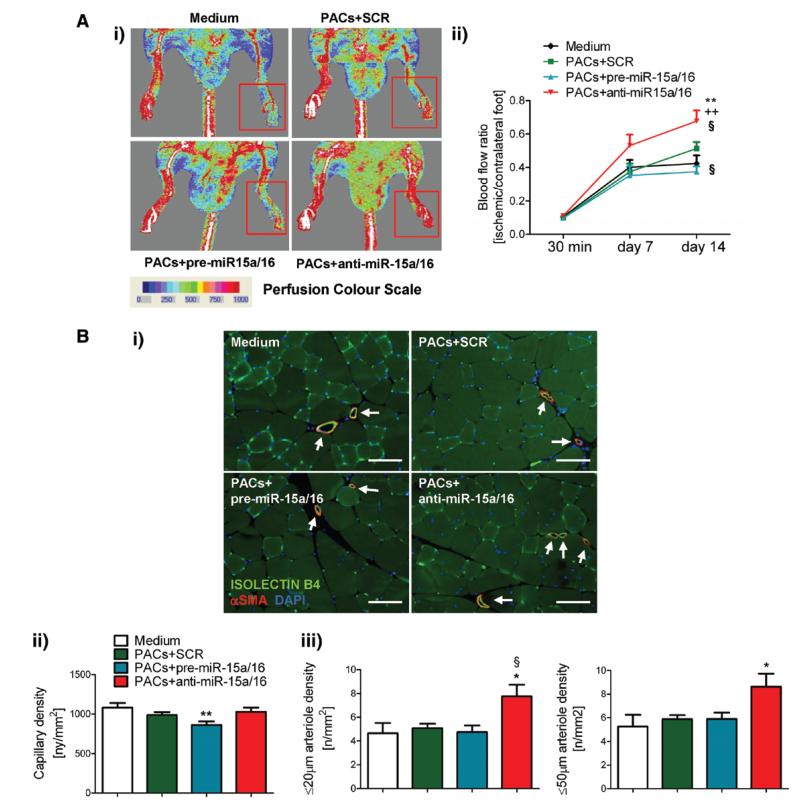
Next, we sought in vivo confirmation of the in vitro evidence suggesting a role for miR-15a and miR-16 in controlling PAC functions. Control PACs were engineered ex vivo to either increase or reduce the expression of miR-15a and miR-16 and then were transplanted into the ischemic adductor muscles of nude mice. Control groups consisted of mice receiving PACs transfected with scramble or no cells (substituted by the fresh cell medium). As shown in Figure 7, anti–miR-15a/16 PACs increased BF recovery and arteriole density in the ischemic adductor. In contrast, mice transplanted with pre–miR-15a/16 PACs showed impaired BF recovery and reduced muscular capillary number in comparison with mice given scramble-transfected PACs.
Figure 7. Ex vivo microRNA (miR)-15a/16 manipulation affects the in vivo proangiogenic potential of proangiogenic circulating cells (PACs).
Nude mice (n=11–14/group) with unilateral limb ischemia received engineered PACs or fresh cell medium in the ischemic adductor muscle. A, Postischemic blood flow (BF) recovery: (i) representative color laser Doppler flowmetry images at 14 days from surgery (red squares delimit the area of measurement in the ischemic foot). ii, Time course of BF recovery (calculated as the ratio of BF in ischemic to contralateral foot). B (i), Representative ischemic muscle sections stained with the endothelial marker isolectin B4 (green fluorescent) and an antibody for α-smooth muscle actin (red fluorescent) to identify smooth muscles cells in the arterial wall (scale bars, 100 μm); (ii) bar graphs showing capillary and arteriole (≤20 μm and ≤50 μm diameter) density in ischemic adductor muscles at 2 weeks postischemia. Data are expressed as mean±SEM. **P<0.01 and *P<0.05 vs cell medium; §P<0.05 vs scramble PACs; ++P<0.01 vs pre–miR-15a/16 PACs.
Circulating miR-15a and miR-16
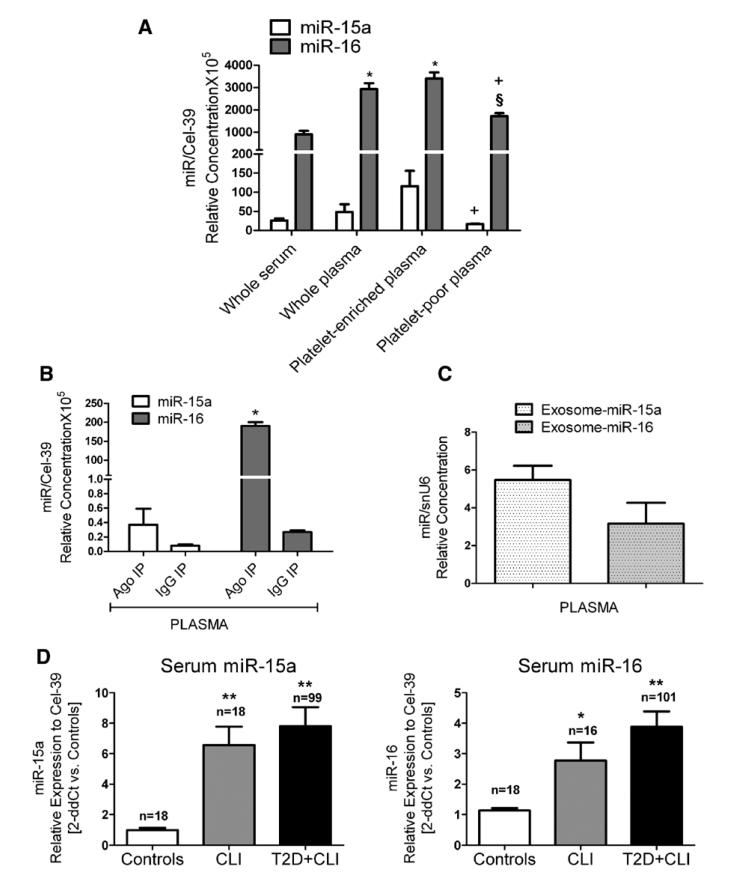
As shown in Figure 8A, both miR-15a and miR-16 were detectable in the PB of nonischemic and nondiabetic subjects, especially in platelet-rich plasma. Furthermore, the 2 miRs also were found in the plasma as part of exosomes (Figure 8B) and conjugated to argonaute-2 (Figure 8C), which are regarded as 2 transport systems preserving miR stability. Our aforementioned clinical trial that has supported the PAC study was designed to prepare serum only. Interestingly, miR-15a and miR-16 were increased in the serum of CLI patients with and without T2DM (Figure 8C). No correlation was found between miR-15a or miR-16 levels in PACs and serum (data not shown).
Figure 8. Circulating microRNA (miR)-15a and miR-16.
miR-15a and miR-16 relative expression (measured using standard curves for the 2 miRs and Cel-39) in (A) serum and fractions of plasma of healthy (n=4; *P<0.05 vs whole serum, § vs whole plasma, and + vs plateletenriched plasma) (B) immunoprecipitated (IP) fractions of healthy donor plasma with argonaute-2 (Ago-2) protein and IgG negative control, respectively (n=4; *P<0.05 vs IgG negative control). C, miR-15a and miR-16 relative expression (measured using standard curves for miR and snU6) in exosomes isolated from healthy control plasma (n=4 donors). D, Serum expression of miR-15a (left) and miR-16 (right) measured in critical limb ischemia (CLI) patients with and without type 2 diabetes mellitus (T2D) and controls. Data were reported to the control group by the 2-ddCt method (internal control: Cel-39). Data are mean±SEM. **P<0.001 and *P<0.05 vs healthy controls.
Circulating miR-15a Levels Are Positively Associated With Increased Risk of Adverse Events in T2DM Patients Undergoing Revascularization for CLI
Finally, we conducted statistical analyses to assess the potential value of miR-15a and miR-16 (expressed in either PACs or serum) in predicting adverse events (restenosis and/or amputation) after revascularization. miR expression (both PACs and serum) distribution was similar between CLI cases with and without associated T2DM. Because we could enroll more diabetic than nondiabetic patients in our trial, the T2DM-CLI group (whose characteristics are reported in Online Table III) was used for analysis attempting to correlate miR expression with clinical events. Online Table IV reports the incidence of events recorded at the 12-month follow-up in our study group. As shown in Online Table V, after the adjustment for the patient age and sex, the risk of an amputation after restenosis positively correlated with relative abundance of serum miR-15a (OR, 2.10; P=0.002) and serum miR-16 (OR, 2.07; P=0.012). Serum miR-15a also was positively associated with post-revascularization restenosis considered as first event (OR, 1.28; P=0.04), but serum miR-16 was not (OR, 0.96; P=0.75). In contrast, miR-15a and miR-16 in PACs were not significantly associated with amputation after restenosis or restenosis alone. Noteworthy, for technical and logistic issues, the PAC samples were less numerous than the serum samples (Figures 1B and 8D show sample sizes). Other potential confounding variables, such as the presence of hypertension, neuropathy, coronary artery disease, smoking, statin, and insulin treatment, were not associated with the risk of restenosis in our population (data not shown), and therefore they were not considered as confounders in multivariable analysis.
miR-15a Does Not Affect VSMC Proliferation and Migration
Restenosis involves increased VSMC proliferation and migration.26 Because miR-15a levels were associated with the risk of restenosis, we characterized the effect of miR-15a overexpression on VSMC proliferation and migration. However, miR-15a inhibited VSMC proliferation and migration (Online Figure VI).
Discussion
This study shows for the first time to our knowledge that several miRs are differently expressed in PACs enriched from the PB of patients with CLI. Furthermore, we have identified miR-15a and miR-16 as critical regulators of in vitro survival and migration and in vivo therapeutic potential of PACs. In addition, we provide preliminary evidence that increased circulating levels of miR-15a and miR-16 are associated with higher risk of limb amputation and restenosis in T2DM patients undergoing percutaneous angioplasty for CLI.
miRs have recently emerged as key regulators of endothelial cell functions and angiogenesis.15 In addition, miRs are important for both maintaining stem cell pluripotency and inducing stem cell vascular differentiation.27 Furthermore, we recently showed that miR-132 is essential for therapeutic proangiogenic actions of pericyte progenitor cells.17 A few studies highlight the differential expression of a subset of miRs in PACs in patients with heart disease,28–30 but investigations have not yet been extended to include patients with peripheral vascular diseases, let alone CLI. A recent report shows that miR-126 is downregulated in PACs isolated from diabetic patients without vascular complications.29 In addition, circulating levels of several miRs are modified by diabetes mellitus.31 We found that plasma miR-503 increased in diabetic patients with CLI undergoing limb amputation. Notwithstanding, no other miR has been measured in the circulation of CLI patients. These facts outline the novelty and importance of this study.
Increased miR-15a levels in PACs and serum of CLI patients and in PACs cultured under hypoxia are in line with the higher miR-15 expression found in the ischemic pig and mouse myocardium by Hullinger et al.21 We additionally found pri–miR-15a/16-1 and mature miR-16 expression to be increased by CLI in PACs. Recent studies described an active transcriptional regulation of miR-15a/16-1 and miR-15b/16-2 by E2F1 and E2F3.32 Interestingly, E2F1 acts as a negative regulator of postischemic angiogenesis by inhibiting VEGF-A expression.33
Guided migration of PACs from the BM to the PB and then from the PB to the ischemic site is essential for the cells to exert their regenerative functions. Both ischemia and diabetes mellitus compromise PAC migratory capacity.22,34 Thus, it is significant that miR-15a and miR-16 negatively regulate PAC migration toward a series of chemotactic stimuli, including basic FGF, VEGF-A, and SDF-1α, which are produced by limb muscles in response to ischemia.35,36 In PACs, miR-15a/16 did not reduce FGF-R1, KDR, or CXCR4 expression, which suggests that the impairment in migration was dictated by post-receptor mechanisms, possibly including deficit in AKT-3 leading to altered phospho-Akt levels.37 Different from what we observed in PACs, HUVECs overexpressing miR-16 showed reduced levels of both FGF-R1 and KDR.20 Furthermore, in the same study, HUVEC proliferation was inhibited by pre–miR-16. At difference, PAC growth is not affected by miR-16. These discrepancies can be explained with the differences in cells used in the 2 studies. For example, HUVECs have a higher in vitro proliferative capacity than PACs.38 Both studies show that pre–miR-16 downregulates VEGF-A. Nonetheless, the paracrine proangiogenic activities of PACs were not influenced by manipulating miR-15a or miR-16. It is possible that the impact of the 2 miRs on VEGF-A alone is not sufficient to alter the proangiogenic property of the PAC secretome, which is composed of a plethora of factors.
Transplantation of PACs ex vivo–manipulated with pre–miR-15a/16 and anti–miR-15a/16 influenced the postischemic recovery of mice with limb ischemia. From the aforementioned in vitro data, we can speculate that the therapeutic benefit achieved by miR-15/16 inhibition in PACs derives from improved survival and migratory capacities of the cells and not from changes in paracrine actions. Taken together, our PAC data allow proposing that miR therapeutic could be a meaningful strategy to empower PACs before autologous transplantation in patients with CLI. Furthermore, ex vivo miR therapeutics should help in minimizing off-target and side effects, thus facilitating the entrance of small RNA-targeting approaches in the arena of clinical vascular medicine.
A limitation of our study is that the age and sex differences between healthy and diseased donors may introduce a bias in the interpretation of the miR screening data. However, the results we obtained after manipulating miR-15a and miR-16 in PACs and the in vivo confirmation of the crucial role of these miRs in controlling PAC functions strengthen our hypothesis that they are new potential targets for autologous cell therapy in CLI patients.
The interest toward the use of miRs as diagnostic and prognostic biomarkers is growing. Here, we have found that miR-15a and miR-16 correlate with adverse events at 12-month follow-up in T2DM patients who have undergone percutaneous angioplasty to correct CLI. However, this preliminary finding must be confirmed in larger patient populations, including subjects of different ethnic backgrounds and those affected by several comorbidities. In fact, differences in patient characteristics could alter a series of processes that determine circulating miR values. These processes would include miR transcription, maturation, release from cells to the circulation, and uptake from the circulation by other cells, as well as excretion. Furthermore, the data on circulating miR-15a and miR-16 leave several questions open, including their cellular sources, the stimuli triggering and the mechanisms permitting their extracellular release, their form of transportation, and their putative function at local (ischemic muscles?) or at distance communicators.39 We have attempted to investigate some of these points, and here we provide evidence that miR-15a and miR-16 are differently expressed in BM-derived cells (including PACs) and vascular cell types. In culture, some of these cells release exosomes containing miR-15 and miR-16. Importantly, exosomes prepared from the plasma of healthy donors contain both miRs. Among the 2 miRs, miR-15a seems less expressed in cells, but is enriched in exosomes. This is in line with the level of miR-15 in plasma exosomes. The release of miR-15a–containing and miR-16–containing exosomes from a cell type when cultured in vitro suggests the hypothesis that in vivo cells of the same lineages may contribute to the pool of circulating miR-15a and miR-16. Notwithstanding, additional efforts are necessary to track the cell/tissue origin of PB circulating exosomes. At difference with miR-15a, miR-16 is also present in the healthy blood as conjugated to argonaute 2. These preliminary data should foster more research into the mechanisms and significance of in vivo miR release from cells and tissues and the impact of diseases. Noteworthy, miR-16 was found to be prevalently expressed in plasma, which could have influenced the evaluation of its biomarker potential in our patients (of whom only serum was available). This additionally suggests the convenience of simultaneous collection of different blood derivates when studying miR potential as novel biomarkers.
Supplementary Material
Novelty and Significance.
What Is Known?
Proangiogenic circulating cells (PACs) can contribute to postischemic vascular repair.
MicroRNAs (miRs) inhibit the expression of their target genes by acting at the posttranscriptional level.
miRs play essential roles in vascular development and repair.
Hypoxia and ischemia regulate the expression of some miRs (including miR-15a).
What New Information Does This Article Contribute?
miR-15a and miR-16 impair PAC survival and migration in vitro. Ex vivo inhibition of the 2 miRs enhances the therapeutic potential of human PACs in a mouse model of limb ischemia.
miR-15a and miR-16 levels are increased in PACs and serum prepared from patients with critical limb ischemia.
Serum levels of miR-15a and miR-16 are positively associated with postrevascularization restenosis and amputation in patients with critical limb ischemia.
New therapeutic approaches for critical limb ischemia are urgently needed. Cell therapies have been tested in clinical studies with promising results, but disease-related molecular defects might limit the success of cell transplantation. Here, we show that critical limb ischemia affects the expression of several angiogenesis-related miRs in PACs, including miR-15a and miR-16 that were found to be upregulated. Importantly, both miRs diminish PAC survival and function, possibly by reducing the expression of their target genes vascular endothelial growth factor-A and AKT-3. Furthermore, we found that nonviral delivery of anti–miR-15a and anti–miR-16 oligonucleotides improves the capacity of human PACs to promote blood flow recovery and angiogenesis in ischemic limb muscles. Finally, we provide evidence that serum miR-15a and miR-16 levels are increased by critical limb ischemia, and they are positively associated with adverse events after revascularization in a cohort of 122 patients. Although validation in a larger cohort of patients is needed, the findings of this study suggest that miRs could be biomarkers for the prediction of clinical outcomes in patients with critical limb ischemia.
Acknowledgments
A. Caporali is a British Heart Foundation (BHF) Intermediate Research Fellow, S. Shantikumar is a BHF PhD student, and C. Emanueli is a BHF Senior Research Fellow. I. Floris is a Bristol visiting PhD student from the University of Sassari (sponsored by the Sardinia Region Higher Education office). We thank Professor Alaistar Poole (Bristol) for important advice.
Sources of Funding The study was supported by the British Heart Foundation (BHF), Diabetes UK, the Italian Ministry of Health (Diabetic Foot project RF-MUL-2007–633361), the Italian Lombardy Region (International Scientific and Technological Cooperation project 16928), and the British National Institute of Health Bristol Biomedical Research Unit in Cardiovascular Medicine.
Non-standard Abbreviations and Acronyms
- BF
blood flow
- BM
bone marrow
- Cel-miR-39
Caenorhabditis elegans microRNA-39
- CXCR4
CXC chemokine receptor, type 4
- CLI
critical limb ischemia
- FGF-R1
fibroblast growth factor receptor-1
- KDR
kinase insert domain receptor
- HUVEC
human umbilical vein endothelial cell
- miR
microRNA
- PAC
proangiogenic cell
- PB
peripheral blood
- SDF-1α
stromal cell–derived factor-1α
- snU6
small nuclear RNA u6
- T2DM
type 2 diabetes mellitus
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cell
Footnotes
Disclosures None.
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.111.300418/-/DC1.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, et al. TASC II Working Group Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Faglia E. Characteristics of peripheral arterial disease and its relevance to the diabetic population. Int J Low Extrem Wounds. 2011;10:152–166. doi: 10.1177/1534734611417352. [DOI] [PubMed] [Google Scholar]
- 3.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, Grünwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 6.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Losordo DW. Cell therapy for critical limb ischemia: moving forward one step at a time. Circ Cardiovasc Interv. 2011;4:2–5. doi: 10.1161/CIRCINTERVENTIONS.110.960716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amann B, Lüdemann C, Rückert R, Lawall H, Liesenfeld B, Schneider M, Schmidt-Lucke J. Design and rationale of a randomized, double-blind, placebo-controlled phase III study for autologous bone marrow cell transplantation in critical limb ischemia: the BONe Marrow Outcomes Trial in Critical Limb Ischemia (BONMOT-CLI) VASA. 2008;37:319–325. doi: 10.1024/0301-1526.37.4.319. [DOI] [PubMed] [Google Scholar]
- 9.Sprengers RW, Moll FL, Teraa M, Verhaar MC, JUVENTAS Study Group Rationale and design of the JUVENTAS trial for repeated intraarterial infusion of autologous bone marrow-derived mononuclear cells in patients with critical limb ischemia. J Vasc Surg. 2010;51:1564–1568. doi: 10.1016/j.jvs.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 11.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 12.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caporali A, Emanueli C. MicroRNA regulation in angiogenesis. Vascul Pharmacol. 2011;55:79–86. doi: 10.1016/j.vph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Kane NM, Howard L, Descamps B, Meloni M, McClure J, Lu R, McCahill A, Breen C, Mackenzie RM, Delles C, Mountford JC, Milligan G, Emanueli C, Baker AH. Role of microRNAs 99b, 181a, and 181b in the differentiation of human embryonic stem cells to vascular endothelial cells. Stem Cells. 2012;30:643–654. doi: 10.1002/stem.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporali A, Emanueli C. MicroRNA-503 and the extended microRNA-16 family in angiogenesis. Trends Cardiovasc Med. 2012;21:162–166. doi: 10.1016/j.tcm.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caporali A, Meloni M, Völlenkle C, et al. Deregulation of microR-NA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 20.Chamorro-Jorganes A, Araldi E, Penalva LO, Sandhu D, Fernández-Hernando C, Suárez Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol. 2011;31:2595–2606. doi: 10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, Hare JM, Olson EN, van Rooij E. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinetti G, Fortunato O, Cordella D, Portararo P, Kränkel N, Katare R, Sala-Newby GB, Richer C, Vincent MP, Alhenc-Gelas F, Tonolo G, Cherchi S, Emanueli C, Madeddu P. Tissue kallikrein is essential for invasive capacity of circulating proangiogenic cells. Circ Res. 2011;108:284–293. doi: 10.1161/CIRCRESAHA.110.236786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin KJ, Olsen K, Hamblin M, Zhang J, Schwendeman SP, Chen YE. Vascular endothelial cell-specific microRNA-15a inhibits angiogenesis in hindlimb ischemia. J Biol Chem. 2012;287:27055–27064. doi: 10.1074/jbc.M112.364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS ONE. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenagy RD, Fukai N, Min SK, Jalikis F, Kohler TR, Clowes AW. Proliferative capacity of vein graft smooth muscle cells and fibroblasts in vitro correlates with graft stenosis. J Vasc Surg. 2009;49:1282–1288. doi: 10.1016/j.jvs.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard L, Kane NM, Milligan G, Baker AH. MicroRNAs regulating cell pluripotency and vascular differentiation. Vascul Pharmacol. 2011;55:69–78. doi: 10.1016/j.vph.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Kandic I, Kutryk MJ. Dysregulation of angiogenesis-related microRNAs in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun. 2011;405:42–46. doi: 10.1016/j.bbrc.2010.12.119. [DOI] [PubMed] [Google Scholar]
- 29.Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J Mol Cell Cardiol. 2012;53:64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Xu Q, Seeger FH, Castillo J, Iekushi K, Boon RA, Farcas R, Manavski Y, Li YG, Assmus B, Zeiher AM, Dimmeler S. Micro-RNA-34a contributes to the impaired function of bone marrow-derived mononuclear cells from patients with cardiovascular disease. J Am Coll Cardiol. 2012;59:2107–2117. doi: 10.1016/j.jacc.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res. 2012;93:583–593. doi: 10.1093/cvr/cvr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ofir M, Hacohen D, Ginsberg D. MiR-15 and miR-16 are direct transcriptional targets of E2F1 that limit E2F-induced proliferation by targeting cyclin E. Mol Cancer Res. 2011;9:440–447. doi: 10.1158/1541-7786.MCR-10-0344. [DOI] [PubMed] [Google Scholar]
- 33.Qin G, Kishore R, Dolan CM, et al. Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proc Natl Acad Sci USA. 2006;103:11015–11020. doi: 10.1073/pnas.0509533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kränkel N, Katare RG, Siragusa M, et al. Role of kinin B2 receptor signaling in the recruitment of circulating progenitor cells with neovascularization potential. Circ Res. 2008;103:1335–1343. doi: 10.1161/CIRCRESAHA.108.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egger M, Schgoer W, Beer AG, et al. Hypoxia up-regulates the angiogenic cytokine secretoneurin via an HIF-1alpha- and basic FGF-dependent pathway in muscle cells. FASEB J. 2007;21:2906–2917. doi: 10.1096/fj.06-7440com. [DOI] [PubMed] [Google Scholar]
- 36.van Weel V, Seghers L, de Vries MR, Kuiper EJ, Schlingemann RO, Bajema IM, Lindeman JH, Delis-van Diemen PM, van Hinsbergh VW, van Bockel JH, Quax PH. Expression of vascular endothelial growth factor, stromal cell-derived factor-1, and CXCR4 in human limb muscle with acute and chronic ischemia. Arterioscler Thromb Vasc Biol. 2007;27:1426–1432. doi: 10.1161/ATVBAHA.107.139642. [DOI] [PubMed] [Google Scholar]
- 37.Madeddu P, Kraenkel N, Barcelos LS, Siragusa M, Campagnolo P, Oikawa A, Caporali A, Herman A, Azzolino O, Barberis L, Perino A, Damilano F, Emanueli C, Hirsch E. Phosphoinositide 3-kinase gamma gene knockout impairs postischemic neovascularization and endothelial progenitor cell functions. Arterioscler Thromb Vasc Biol. 2008;28:68–76. doi: 10.1161/ATVBAHA.107.145573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caporali A, Pani E, Horrevoets AJ, Kraenkel N, Oikawa A, Sala-Newby GB, Meloni M, Cristofaro B, Graiani G, Leroyer AS, Boulanger CM, Spinetti G, Yoon SO, Madeddu P, Emanueli C. Neurotrophin p75 receptor (p75NTR) promotes endothelial cell apoptosis and inhibits angiogenesis: implications for diabetes-induced impaired neovascularization in ischemic limb muscles. Circ Res. 2008;103:e15–e26. doi: 10.1161/CIRCRESAHA.108.177386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011;31:2383–2390. doi: 10.1161/ATVBAHA.111.226696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.