Abstract
Mutations have been identified in a non-canonical Wnt signalling cascade (the planar cell polarity pathway) in several mouse genetic models of severe neural tube defects. In each of these models, neurulation fails to be initiated at the 3-4 somite stage, leading to an almost entirely open neural tube (termed craniorachischisis). Studies in whole embryo culture have identified a defect in the morphogenetic process of convergent extension during gastrulation, preceding the onset of neural tube closure. The principal defect is a failure of midline extension, both in the neural plate and axial mesoderm. This leads to an abnormally wide neural plate in which the elevating neural folds are too far apart to achieve closure. In this chapter, we provide details of several experimental methods that can be used to evaluate convergent extension in cultured mouse embryos. We describe analytical methods that can reveal the abnormalities that characterise neurulation-stage embryos with defective planar cell polarity signalling, in particular the loop-tail (Lp; Vangl2) mutant.
Keywords: mouse, neurulation, whole embryo culture, DiI labelling, electroporation, convergent extension, neural tube defects, craniorachischisis, Vangl2, loop-tail
1. INTRODUCTION
In the mouse embryo, neural tube closure initiates at the hindbrain-cervical boundary (Closure 1) at embryonic day (E) 8.5 (day 22-23 of gestation in humans) and then progresses bi-directionally into the brain and down the spine. Subsequent closure initiation events occur at the forebrain-midbrain boundary (Closure 2) and at the rostral extremity of the forebrain (Closure 3). Closure progresses simultaneously along the spinal region, culminating in closure of the posterior neuropore (PNP) at E10.5 (26-30 days in humans), marking the completion of primary neurulation. For a review of mammalian neurulation, see (1).
Failure of any of these closure events results in an open neural tube defect (NTD). The most severe form of NTD, craniorachischisis, arises when the embryo fails to achieve Closure 1 and combines anencephaly of the midbrain and hindbrain with an entirely open spinal region. While more than 200 genes have been described as necessary for successful neural tube closure in mice (2), the perturbation of a minority of these genes leads to craniorachischisis. To date, all of the well-established models of craniorachischisis have been found to lack functional components of the planar cell polarity (PCP) pathway, establishing a striking connection between the initial event of primary neurulation and non-canonical Wnt signalling.
Closure 1 fails in a number of mutant mice including loop-tail (Lp; Vangl2) (3) (4), circletail (Crc; Scrb1) (5), crash (Crsh; Celsr1) (6) and protein tyrosine kinase 7 (Ptk7) (7), as well as in mice doubly mutant for dishevelled 1 (Dvl1) and Dvl2 (8), for Dvl2 and Dvl3 (9), and for frizzled 3 (Frz3) and Frz6 (10). In each of these single or double mutants, the neural plate lacks the normal, well-defined midline bending point and instead displays an abnormally broad floor plate precursor. Although the neural folds form and elevate towards the midline, the increased distance between them precludes apposition and fusion. In the Lp (Vangl2) mouse, it has been shown that this genetic requirement for PCP signalling is mediated through convergent extension (CE) cell movements in the midline neural plate and underlying axial mesoderm, prior to Closure 1. Vangl2 mutants display a cell-autonomous CE defect, whereby the midline fails to narrow and extend, resulting in the widely-spaced neural folds (11,12).
The Lp (Vangl2) mutation also increases susceptibility to other NTDs, in addition to craniorachischisis, particularly when in double mutant combinations (13). For example, Lp interacts genetically with mutations in both Ptk7 (7) and grainyhead-like-3 (Grhl3) (14) to produce spina bifida, and with collagen triple helix repeat containing 1 (Cthrc1) to produce exencephaly (15). It is not yet understood why a combination of Vangl2 with other mutants can produce such variable phenotypes. In particular, it is unclear whether the genetic interactions represent defects of convergence and extension. If so, this would implicate CE cell movements as an essential feature of later neurulation events, as well as their recognised key role in gastrulation, pre-Closure 1.
The aim of this chapter is to describe some techniques that can be used with early mouse embryos to assess convergence and extension, and initiation of neural tube closure, in PCP mutants. We describe the process of dissecting and culturing E7.5 pre-neurulation stage embryos, combined with the labelling of the midline either by focal injection of DiI into the node or by electroporation of a green fluorescent protein (GFP) expressing vector into the neural plate. We discuss how to evaluate the distinguishing features of Closure 1 failure by examining defective CE and axial extension in Lp mutants, and by morphological examination of the floor plate and notochord.
2. MATERIALS
2.1. Embryo dissection
Dulbecco’s Modified Eagle’s Medium containing 25 mM HEPES (DMEM, stored at 4 °C) (Gibco, UK) and supplemented with 10% fetal calf serum (FCS). This should be made fresh as required and warmed to 37°C for use during embryo collection and dissection. Inclusion of HEPES ensures maintenance of pH when used on the open bench.
Watchmakers’ forceps (number 5, Dumont, Switzerland). These should be precisely sharpened using fine emery paper, to allow accurate dissection without applying mechanical stress to the embryo. Dip forcep tips in alcohol and briefly flame-sterilise using a spirit burner before use.
Dissecting scissors. Flame sterilise before use.
Zeiss SV6 or similar stereomicroscope with transmitted light stage.
2.2. DiI Injection into the node
DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, Cell Tracker™ CM-DiI, Molecular Probes, USA): 1 mg dissolved in 25 μl dimethyl sulphoxide, then diluted ten-fold in 3 mol/l sucrose. Store aliquots at −20°C, protected from light. Thaw aliquot before use and the DiI solution can then be stored at 4°C, protected from light, for several days.
Petri dish, 55 mm diameter, containing 4% agarose (in PBS), 2-3 mm deep.
Mouth pipetting tube.
Glass micropipette needles, pulled on a Flaming-Brown horizontal pipette puller (Sutter Instrument Co., USA).
2.3. Electroporation of GFP-expressing vector into neural plate
DNA solution containing GFP-expression vector. For example, pCAβ-mGFP6 in which GFP expression is driven by the chick β-actin promoter under influence of the cytomegalovirus enhancer (16) has proven effective in our hands (11). Prepare a solution for electroporation to contain 2.5 mg/ml DNA, 0.1% Fast Green in PBS-DEPC.
Petri dish containing agarose, glass micropipette needles and mouth pipetting tube, as above.
A pair of gold 5 mm point electrodes (BTX model 508, Harvard Apparatus) attached to a BTX ECM830 electroporator.
2.4. Whole embryo culture
Rat serum. This should be stored in small aliquots (1-5 ml) at −20°C and thawed immediately before use (see Note 1).
Culture tubes (30 ml, plastic; Nunc) and Millipore filter (0.45 μm pore size).
Glisseal silicone grease (Borer Chemie, Switzerland).
Gas mixture (see Note 2).
Roller culture incubator (37°C; B.T.C Engineering, Cambridge).
2.5. Assessment of embryo phenotype and gene expression analysis after culture
Epifluorescence stereomicroscope (e.g. Leica MZ FLIII) with digital camera.
Phosphate buffered saline treated with diethyl pyrocarbonate (PBS-DEPC) and autoclaved.
Glass microscope slides for flat mounting.
Eyepiece graticule for making linear measurements.
Ice-cold paraformaldehyde (PFA, 4%; dissolved in PBS-DEPC) for fixation. This should be stored as aliquots at −20°C and thawed immediately before use with warming to ensure all PFA is dissolved.
Standard reagents and materials for in situ hybridisation on whole mount embryos or on histological sections.
2.6. Examination of morphology and gene expression patterns in embryo sections
Small glass embedding moulds (Agar Scientific).
Histoclear (National Diagnostics).
Paraffin wax (Thermo Scientific). This should be melted at 56°C prior to sectioning.
Two old pairs of forceps for heating and orientation of specimens.
Paper strips for pencil labelling of wax blocks.
Agarose (2% in PBS). Dissolve in microwave or water bath and keep warm.
Small plastic embedding moulds (Thermo Scientific)
Razor blade or scalpel for trimming wax and agarose blocks after embedding.
Hematoxylin and eosin for counterstaining.
3. METHODS
The ability to culture mouse embryos prior to and during neurulation allows a number of experimental studies and chemical manipulations which would not otherwise be possible in a mammalian system. For successful cultures, carefully dissected embryos and high quality rat serum are essential. Here we describe two methods for vitally labelling mouse embryos in order to study CE: DiI labelling by microinjection into the node tissue, and electroporation of a GFP-expression vector into the neural plate.
3.1. Embryo dissection
Dissect the uterus from the pregnant female and place into a 55 mm petri dish containing pre-warmed dissecting medium (DMEM + 10% FCS). Place the dish on the stereomicroscope stage, using transmitted (under-stage) illumination. It is generally best to use the ‘dark field’ adjustment for the early stages of dissection, and change to ‘bright field’ for later stages when the embryo has been removed.
Trim away fat and blood vessels from the mesometrial surface of the uterus (Fig. 1A).
Beginning at the mesometrial surface, gently open the uterus at the site of each implantation in turn by creating a hole in the uterine wall. Expand this hole carefully until the decidual swelling protrudes from the elastic uterine wall. Steady the uterus with one pair of forceps while using the other to grip across the width of the uterus next to the decidua, and then gently ‘milk it out’ of the hole. Repeat this process until all of the decidual swellings have been removed from the uterus (Fig. 1B). Transfer the decidual swellings to a fresh dish of dissecting medium.
Open each decidua starting at the anti-mesometrial (fluffy) end (Fig. 1B), to reveal the trophoblast layer which envelops the embryo (Fig. 1C). Leave the ectoplacental cone intact and, should the mural trophoblast tear open, take care not to damage the underlying yolk sac. Gently release the conceptus from within the swelling by peeling away the decidual debris (Fig. 1D). Transfer all conceptuses to a fresh dish of dissecting medium.
Next, remove the mural trophoblast together with the thin, elastic Reichert’s membrane, which is almost invisible beneath the trophoblast. Grasp the trophoblast with both pairs of forceps in order to tear open the membrane and remove it from the outside of the yolk sac. If successful, the trophoblast layer will come off cleanly in a sheet (Fig. 1E). However, if it detaches in pieces then this indicates that Reichert’s membrane is still intact. As before, it is important not to rupture the yolk sac.
Finally, trim Reichert’s membrane up to the edge of the ectoplacental cone (see Note 3). Once all the embryos have been dissected to this point (Fig. 1F), they are ready to be injected with DiI, electroporated, or put straight into culture (see Note 4).
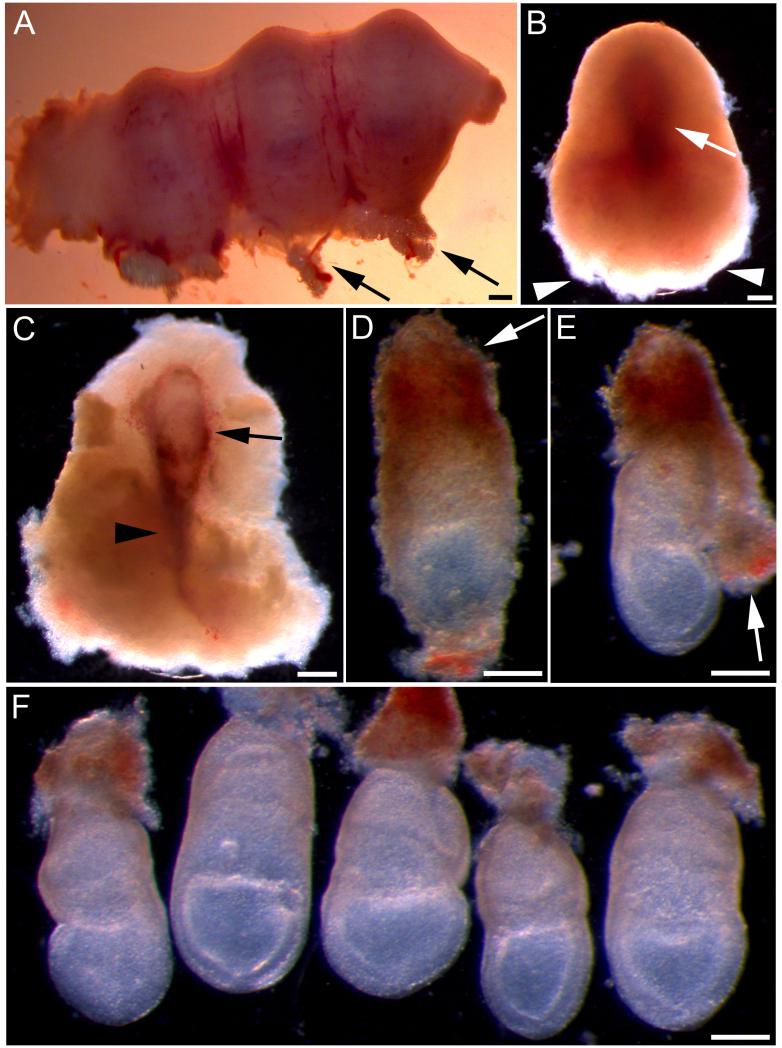
Fig. 1. Dissection of E7.5 mouse embryos for whole embryo culture.
(A) Part of pregnant uterus containing three implantation sites. Arrows indicate fat and blood vessels at the mesometrial surface. (B) Decidual swelling after removal from the uterus. The outline of the embryo can be seen as a darker region (arrow). The next stage of dissection should begin from the broad, fluffy end (arrowheads). (C) Decidual swelling dissected open to reveal the conceptus, encased in trophoblast (arrow). Arrowhead indicates the ectoplacental cone. (D) Conceptus after being released from the decidual swelling. Trophoblast cells can be seen on the surface and the ectoplacental cone remains intact (arrow). (E) Reichert’s membrane and the overlying trophoblast (arrow) have been partially removed by peeling away from the embryonic region. (F) Five dissected embryos after complete removal of Reichert’s membrane. The yolk sacs remain intact and the embryos are now ready to be cultured. Scale bars represent: 400 μm in A-C; 200 μm in D-F.
3.2. Injection of DiI into the node
Transfer the dissected embryos to a 55 mm agarose petri dish containing fresh dissecting medium. Create a small well in the agarose into which the embryo can be orientated, so as to be viewed from its posterior surface (Fig. 2A). Lodge it firmly in place to facilitate injection.
Place a drop of DiI solution onto a petri dish lid or piece of parafilm. Break the injecting needle to create a micropipette. The orifice diameter should be just wide enough to allow DiI solution to be drawn up into the needle, but small enough that the solution does not leak out when the needle is placed into the dissecting medium (see Note 5). Attach the injecting needle to the mouth pipette tube and draw up a small amount of DiI (e.g. 0.5 μl).
Insert the needle into the embryonic node, allowing it to just penetrate through into the amniotic cavity. Gently release the DiI while slowing withdrawing the needle.
Repeat for the remaining embryos. It is not necessary to accurately control and standardise the volume of DiI which is injected into each embryo; simply ensure that the node is sufficiently labelled. To check for the correct localisation of DiI prior to culture, embryos can be examined under the light stereomicroscope, in which case a thin line of pink-labelled cells should be seen (Fig. 2B). When viewed using a fluorescence stereomicroscope a strong DiI signal should be seen in the node (Fig. 2C). Do not worry if some DiI solution can also be seen dispersed within the amniotic cavity (as in Fig. 2B).
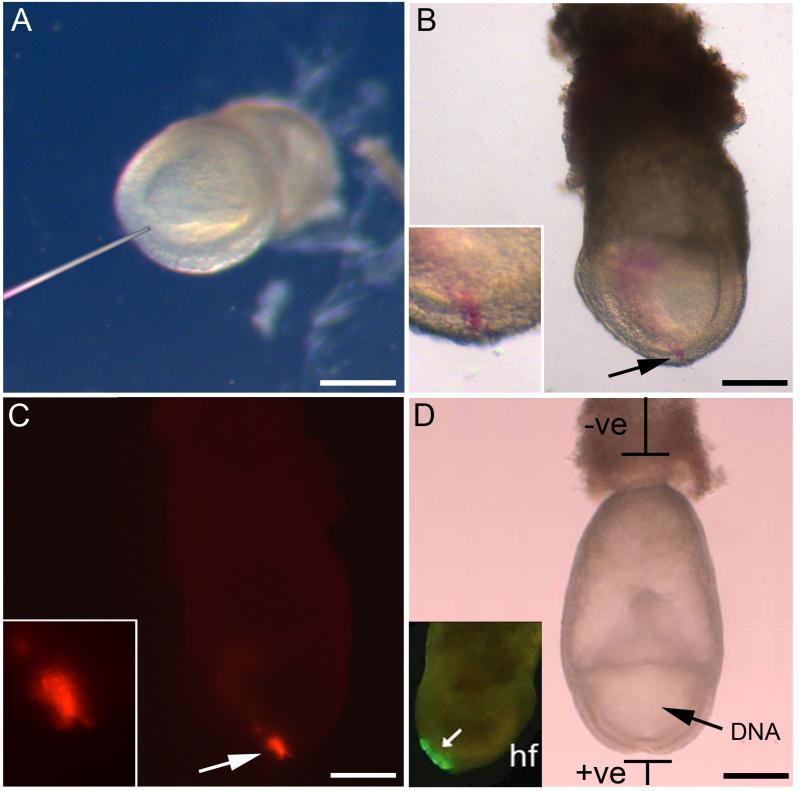
Fig. 2. DiI labelling and GFP electroporation using E7.5 embryos.
(A) Embryo held in place for DiI injection by positioning the ectoplacental cone in agarose. The micro-injection needle indicates the site of injection at the node. (B) Bright field image of embryo after DiI injection into the node. A pink line of labelled cells can be seen (arrow and inset). (C) Fluorescence image of embryo in B. Arrow indicates the DiI-labelled node (enlarged in inset). (D) Diagram showing the method for electroporating the neural plate of E7.5 embryos. DNA is injected into the amniotic cavity (arrow), after which current is passed between the electrodes, with the anode positioned adjacent to the node region. Inset (reproduced with permission from (11)): embryo 4 h after electroporation, showing early GFP expression in the caudal neural plate (arrow). Headfold indicated by hf. Scale bars represent 200 μm in A-D.
3.3. Electroporation of GFP-expression vector into the neural plate
Place embryos in PBS-DEPC in a 55 mm agarose petri dish on the stereomicroscope stage and inject the DNA solution containing Fast Green into the amniotic cavity using a hand-held glass micropipette (Fig. 2D). Injection is continued until the amniotic cavity swells slightly.
Place the embryo immediately between the gold point electrodes attached to the electroporator, with the ventral midline of the caudal embryonic region next to the anode (Fig. 2D).
Pass the current (5 pulses, 50 msec, 15 V), and then repeat for each embryo to be electroporated. Place embryos into culture immediately afterwards.
3.4. Whole embryo culture
Prepare the rat serum in advance of DiI injection or embryo electroporation. Thaw aliquots by warming to 37°C and then pass through a 0.45 μm Millipore filter. In general we culture two E7.5 embryos per ml of serum, although this will vary depending on the length of culture and embryonic stage.
Prepare the culture tubes by smearing the outer rim with a small amount of silicone grease to create an airtight seal.
Pipette the required volume of serum into the culture tubes. Gas the serum for one minute with the appropriate mixture by attaching a pasteur pipette via plastic tubing to the cylinder and gently blowing the gas on to the inner wall of the tube. The surface of the serum should gently ripple without bubbling.
Leave the culture tubes at 37°C (at least 15 min) until the embryos are ready. Embryos should be placed into culture as soon as possible after DiI injection or electroporation.
Gently transfer the embryos into the serum using a Pasteur pipette, ensuring that a minimal amount of dissecting medium is introduced into the culture tube.
Gas the serum again and place the tubes into the roller culture incubator. Cover the lid to protect from light.
Culture for 18-20 hours (i.e. overnight), rolling. Re-gas every 6-12 hours (See Note 6).
3.5 Assessment of Embryonic Phenotype after Culture
E7.5 embryos cultured for 18-20 hours will not yet have developed a functioning yolk sac circulation, which is a later indicator of health following a period in culture. Therefore, to evaluate the success of the cultures at this earlier stage, transfer the embryos to fresh, warm dissecting medium and examine their overall appearance. The yolk sac should look intact and round, with a smooth surface, and contain a suitably-sized embryo at the early somite stage (Fig. 3A,B). A good strategy is to arrange for a second litter of age-matched embryos to be available for dissection just prior to harvesting embryos from culture. Then compare the ‘in vivo’ embryos with those from culture. Detailed methods for scoring the morphology of embryos at E8.5 are available: (17) (18).
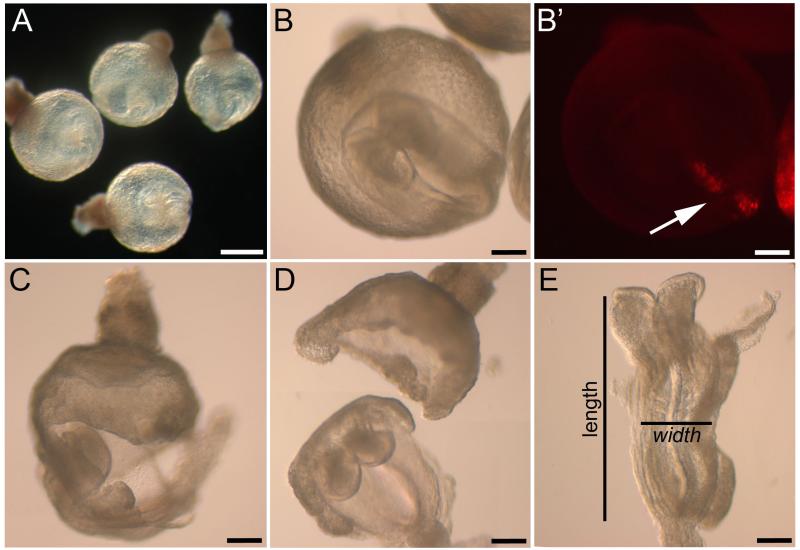
Fig. 3. Dissection and assessment of embryonic length to width ratio after culture.
(A) Healthy embryos at early somite stage, following 18-20 h in culture. (B, B’) Cultured embryo viewed from the ventral surface (rostral to upper left). Arrow in B’ indicates DiI-labelled cells extending along the midline. (C,D) Removal of the yolk sac and ectoplacental cone prior to analysis in cultured embryos. (E) Flat mounted wild type embryo showing the method for measuring length to width ratio. Scale bars represent: 400 μm in A, 200 μm in B-E.
Gently open the yolk sac and underlying amnion using forceps and remove from around the embryo (Fig. 3C,D). If the yolk sac is to be kept for genotyping, first remove and discard the remains of the ectoplacental cone which may have maternal blood contamination. Rinse the yolk sac in PBS-DEPC and place into an eppendorf tube.
To flat mount for photography, rinse the embryo in PBS-DEPC and place onto a glass microscope slide. Using forceps, orientate the embryo so that it lies dorsal side up. Carefully soak up some of the PBS from the edge of the droplet with a tissue, until the surface tension ‘pulls’ the embryo into a flat position (Fig. 3E, see Note 7). The developmental stage can now be determined by counting the somites.
One characteristic of Lp (Vangl2) mutants is a reduction in the embryonic length to width (L:W) ratio due to failure of axial extension. To assess this phenotype, the total length and width of the embryo can be measured after flat mounting using an eyepiece graticule, or later from a photograph (Fig. 3E).
To evaluate CE and axial extension, examine the DiI-labelled or GFP-electroporated flat mount embryos under the fluorescence stereomicroscope. In wild type DiI-labelled embryos, red-fluorescent cells should be observed along the axial midline (Fig. 3B,B’; Fig. 4A,A’), representing labelled notochord and floor plate which extend rostral to the node. Similarly, in wild type GFP-electroporated embryos, green-fluorescent cells should be visible in the midline neural plate from the caudal site of electroporation rostrally into the future brain region (Fig. 4C,D). In contrast, in a large proportion of Lp homozygous mutants, labelled cells will persist caudally at the injection or electroporation site due to defective midline extension (Fig. 4B,B’ and E). In Lp heterozygotes a variable, intermediate phenotype is generally seen.
If the embryos are to be processed further, they can be fixed flat on the microscope slide by replacing the PBS with a drop of 4% PFA (the fixative and length of fixation depends on the type of further analysis to be carried out).
After fixation, rinse the embryos in ice-cold PBS-DEPC, dehydrate through a graded series of methanol washes and store in 100% methanol at −20°C.
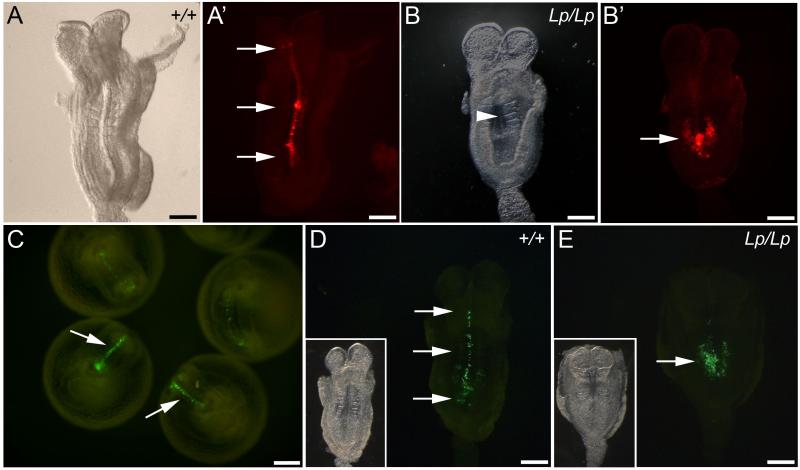
Fig. 4. Assessment of PCP phenotype in DiI-labelled and GFP-electroporated embryos after culture.
(A,A’) Bright field and fluorescence images of flat mounted wild type embryo. DiI-labelled cells extend rostral to the node (arrows in A’), indicating normal CE and axial elongation. (B,B’) Bright field and fluorescence images of a loop-tail homozygous mutant. The embryo displays failure of Closure 1 (arrowhead in B indicates open neural tube) and very limited midline extension of DiI-labelled cells (arrow in B’). (C) Wild type embryos cultured for 18-20 h following electroporation of a GFP-expression vector into the caudal neural plate. GFP-labelled cells extend along the midline (arrows). (D,E) Fluorescence (inset: bright-field) images of embryos following GFP electroporation. The wild type embryo (D) displays marked extension of GFP-labelled cells along the neural plate midline (arrows), whereas the loop-tail homozygous embryo (E) has undergone minimal axial extension of GFP-positive cells (arrow). Scale bars represent: 200 μm in A-E.
3.6. Examination of morphology and gene expression in neurulation-stage embryos
Further evidence of defective CE in Lp embryos is provided by the abnormally wide floor plate precursor and notochord (12). To examine this aspect of the phenotype, transverse sections of the cultured embryos can be cut and counterstained with hematoxylin and eosin. An alternative approach is to perform whole mount in situ hybridisation for sonic hedgehog (Shh) mRNA, a marker of notochord (and floor plate following neural tube closure). The protocol for whole mount in situ hybridisation is as described previously (19), based on the technique of Wilkinson (20). After developing, embryos should be re-fixed and dehydrated to methanol for embedding in paraffin wax (for microtome sectioning), or rinsed in PBS for agarose embedding (for vibratome sectioning). Preparation of transverse histological sections then enables study of floor plate and notochordal morphology.
Paraffin wax embedding:
Remove the embryos from −20°C and wash twice in 100% ethanol for 30 minutes, rocking at room temperature (RT).
Transfer each embryo to a separate glass mould, remove the ethanol and fill the well with Histoclear. Leave at RT for 20 minutes.
Wash again with Histoclear, this time for 20 minutes in a 60°C oven.
Incubate in three changes of molten paraffin wax, 45 minutes at 60°C each, to allow penetration (see Note 8).
At the end of the final wash, view the glass well under a stereomicroscope. Using heated forceps to keep the wax molten, gently move the embryo around until a solid layer of soft wax has formed at the base of the well. Orientate the embryo for transverse sectioning by gently positioning it in the soft layer as desired. Insert a paper label and allow the remaining wax to set overnight at RT.
Remove the solid wax block from the mould by placing at −20°C until it can be easily pushed out.
Trim the block to produce a cube which can then be attached to a microtome chuck using a small amount of molten wax. Place on ice for 30 minutes before securing to the microtome for sectioning. Cut 7-12 μm thick sections.
Agarose embedding:
8. Wash embryos in PBS (or rehydrate to PBS if they have been fixed and dehydrated to 100% methanol after in situ hybridisation).
9. Pour some warm agarose (2%; dissolved in PBS) into a plastic embedding mould.
10.Transfer an embryo to the mould (in minimal PBS).
11.As the agarose begins to polymerise, use forceps to move the embryo to the centre of the mould (briefly placing on ice speeds up the polymerisation process). For transverse sectioning, the embryo should be positioned lying on its side.
12.When the embryo remains in position, place the well on ice to allow the agarose to set completely.
13.Remove the agarose block from the well and cut to size. Remember that, when attached to the vibratome, the first sections will be cut from the top of the block, so ensure that the desired region of the embryo is closest to this side.
ACKNOWLEDGEMENTS
The authors’ research on convergent extension is supported by the Wellcome Trust and Medical Research Council.
Footnotes
Prepare serum for culture by withdrawing blood from the abdominal aorta of male rats terminally anaesthetised using diethyl ether or isofluorane. The blood is immediately centrifuged (5 min at 1,000 × g) to pellet the red cells, then allowed to clot so that the serum can be extracted by gently squeezing the fibrin clot. After heat inactivation, the serum from different rats is pooled to produce a batch of uniform quality. All embryos in an experiment should be exposed to the same serum batch during culture. For a more detailed description of serum preparation, see (21).
The required composition of the gas mixture varies depending on the embryonic stage. When culturing E7.5-E9.5 embryos use: 5% O2, 5% CO2 and 90% N2. For E9.5-E10.5 use: 20% O2, 5% CO2 and 75% N2. For embryos older than E10.5 use: 40% O2, 5% CO2 and 55% N2.
Removal of Reichert’s membrane is the most challenging stage of the dissection process. It may be easiest to grasp the membrane at the opposite side to the ectoplacental cone, stretching it away from the embryo slightly so that it can be torn with the other pair of forceps. Reichert’s membrane should be removed as close to the ectoplacental cone as possible as it can become ‘sticky’ during culture, causing the embryo to adhere to the tube surface.
Dissection of E7.5 embryos for culture is a delicate process, requiring extreme care. However it is also important to work as quickly as possible, minimising the length of time between removal of the embryos from the uterus and the start of the culture experiment.
The DiI injection needle should be broken so that its end is smooth and perpendicular to the axis of the needle (not bevelled or jagged). This process is most easily achieved using a microforge (e.g. Narishige, Japan), but can be performed adequately by hand, given practice. It is best to prepare a number of injection needles in advance.
For overnight cultures we find that the embryos develop well if gassed in the early evening and again first thing the following morning. For shorter cultures (around 6 hours) the embryos do not need to be re-gassed after the start of the experiment.
When removing the PBS from the slide, take great care not to soak up the embryo as it will become lost on the tissue.
The wax will quickly solidify at RT, so try to change the washes as quickly as possible. To maintain the temperature of the moulds, it is helpful to place the embryos on a metal tray inside the oven. This can then be used to transport the moulds to and from the wax container. Pasteur pipettes for changing washes should also be kept at 60°C. If the wax solidifies at any point, simply place the mould back into the oven and continue once it has melted.
REFERENCES
- (1).Copp AJ, Greene NDE, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- (2).Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2007;79:187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- (3).Kibar Z, Vogan KJ, Groulx N, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nature Genet. 2001;28:251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- (4).Murdoch JN, Doudney K, Paternotte C, et al. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- (5).Murdoch JN, Henderson DJ, Doudney K, et al. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- (6).Curtin JA, Quint E, Tsipouri V, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1–20. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- (7).Lu X, Borchers AG, Jolicoeur C, et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- (8).Wang J, Hamblet NS, Mark S, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–78. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Etheridge SL, Ray S, Li S, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ybot-Gonzalez P, Savery D, Gerrelli D, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–99. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Greene NDE, Gerrelli D, Van Straaten HWM, et al. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev. 1998;73:59–72. doi: 10.1016/s0925-4773(98)00029-x. [DOI] [PubMed] [Google Scholar]
- (13).Greene NDE, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009;18:R113–R129. doi: 10.1093/hmg/ddp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Stiefel D, Shibata T, Meuli M, et al. Tethering of the spinal cord in mouse fetuses and neonates with spina bifida. J Neurosurg (Spine) 2003;99:206–13. doi: 10.3171/spi.2003.99.2.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Yamamoto S, Nishimura O, Misaki K, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- (16).Yaneza M, Gilthorpe JD, Lumsden A, et al. No evidence for ventrally migrating neural tube cells from the mid- and hindbrain. Dev Dyn. 2002;223:163–7. doi: 10.1002/dvdy.1241. [DOI] [PubMed] [Google Scholar]
- (17).Brown NA. Routine assessment of morphology and growth: scoring systems and measurements of size. In: Copp AJ, Cockroft DL, editors. Postimplantation Mammalian Embryos: A Practical Approach. IRL Press; Oxford: 1990. pp. 93–108. [Google Scholar]
- (18).Van Maele-Fabry G, Delhaise F, Picard JJ. Morphogenesis and quantification of the development of post-implantation mouse embryos. Toxic in Vitro. 1990;4:149–56. doi: 10.1016/0887-2333(90)90037-t. [DOI] [PubMed] [Google Scholar]
- (19).Ybot-Gonzalez P, Copp AJ, Greene NDE. Expression pattern of glypican-4 suggests multiple roles during mouse development. Dev Dyn. 2005;233:1013–7. doi: 10.1002/dvdy.20383. [DOI] [PubMed] [Google Scholar]
- (20).Wilkinson DG. Situ Hybridisation: A Practical Approach. IRL Press; Oxford: 1992. [Google Scholar]
- (21).Copp A, Cogram P, Fleming A, et al. Neurulation and neural tube closure defects. Methods Mol Biol. 2000;136:135–60. doi: 10.1385/1-59259-065-9:135. [DOI] [PubMed] [Google Scholar]