Abstract
Objective
Diabetes mellitus causes bone marrow (BM) microangiopathy. This study aimed to investigate the mechanisms responsible for BM endothelial dysfunction in diabetes mellitus.
Methods and Results
The analysis of differentially expressed transcripts in BM endothelial cells (BMECs) from type-1 diabetic and nondiabetic mice showed an effect of diabetes mellitus on signaling pathways controlling cell death, migration, and cytoskeletal rearrangement. Type-1 diabetic-BMECs displayed high reactive oxygen species levels, increased expression and activity of RhoA and its associated protein kinases Rho-associated kinase 1/Rho-associated kinase 2, and reduced Akt phosphorylation/activity. Likewise, diabetes mellitus impaired Akt-related BMEC functions, such as migration, network formation, and angiocrine factor-releasing activity, and increased vascular permeability. Moreover, high glucose disrupted BMEC contacts through Src tyrosine kinase phosphorylation of vascular endothelial cadherin. These alterations were prevented by constitutively active Akt (myristoylated Akt), Rho-associated kinase inhibitor Y-27632, and Src inhibitors. Insulin replacement restored BMEC abundance, as assessed by flow cytometry analysis of the endothelial marker MECA32, and endothelial barrier function in BM of type-1 diabetic mice.
Conclusion
Redox-dependent activation of RhoA/Rho-associated kinase and Src/vascular endothelial cadherin signaling pathways, together with Akt inactivation, contribute to endothelial dysfunction in diabetic BM. Metabolic control is crucial for maintenance of endothelial cell homeostasis and endothelial barrier function in BM of diabetic mice.
Keywords: bone marrow, diabetic microangiopathy, endothelial dysfunction, oxidative stress, RhoA
Atypical form of microangiopathy, consisting of microvascular rarefaction and endothelial barrier dysfunction, contributes to the pathogenesis of retinopathy, nephropathy, neuropathy, cardiomyopathy, and foot ulcers in patients with diabetes mellitus.1 Our group was the first to describe a new form of microangiopathy in the bone marrow (BM) of diabetic animal models.2 Microvascular disease threatens stem cell viability through reduced nutrition and perfusion, and increased oxidative stress. In addition, the marrow vascular niche acts as a controller of stem cell mobilization and a source of trophic factors instrumental to proper hematopoiesis.3-6 An impoverished vascular niche might fail to accomplish these crucial functions with detrimental consequences for stem cell homeostasis and cardiovascular repair.7
Glycemic control is crucial for prevention of cardiovascular events, and especially effective in reducing the risk of microvascular complications. However, it remains unknown whether improved control of hyperglycemia by insulin replacement prevents BM microangiopathy. Furthermore, the mechanisms underpinning BM endothelial dysfunction remain poorly understood.
The present study investigates the signaling pathways implicated in diabetes mellitus–induced BM microangiopathy. Results newly show that diabetes mellitus causes redox-dependent activation of small guanosine triphosphatases (GTPases), phosphorylation of vascular endothelial cadherin (VE-cadherin), and reorganization of cytoskeletal proteins leading to increased permeability to macromolecules and passive efflux of BM mononuclear cells (BM-MNCs). Furthermore, the diabetic endothelium exhibits reduced Akt activity and impairment of Akt-related functions, including migration, network formation, and angiocrine factor-releasing activity. Importantly, endothelial barrier dysfunction is rescued by the metabolic control of diabetes mellitus.
Materials and Methods
Animal Procedures
Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals8 and with approval of the British Home Office. Type-1 diabetes mellitus (T1D) was induced in male CD1 mice (Charles River, Margate, Kent, UK) by streptozotocin.9 Age-matched male CD1 mice injected with the streptozotocin vehicle served as controls. Diabetes mellitus was monitored by measurements of glycaemia at fast and glycosuria.
Insulin Implants
Four weeks after induction of diabetes mellitus, mice were randomized to receive continuous insulin supplementation, through subcutaneous implants (LinBit, LinShin, Toronto, Canada), at the rate of 0.1 unit/implant per day or vehicle. The number of insulin implants was titrated according to the mouse body weight, according to manufacturer’s instructions. Glycaemia was monitored every 4 weeks, whereas glycosuria was assessed at 2 weeks after diabetes mellitus induction and reassessed at the end of the study (Figure I in the online-only Data Supplement).
Cell Cultures
Human BM endothelial cells (hBMECs) were kindly provided by Prof van der Schout (Sanquin University, Amsterdam, The Netherlands) and cultured as described previously.10 In selected experiments, cells were cultured in normal glucose (5 mmol/L D-glucose) or high glucose (HG; 15 mmol/L and 25 mmol/L D-glucose) for 96 hours before use in expressional and functional studies. Equivalent concentrations of L-glucose were used as osmotic control.
BMECs were also isolated from T1D mice (14–18 weeks after diabetes mellitus induction) and age-matched nondiabetic controls, as described previously.2 Purity was assessed by flow cytometry detection of the endothelial marker MECA32, which was consistently expressed by >90% of the isolated cells.
Infection of BMECs
BMECs were infected separately with 2 different adenoviruses: an adenovirus carrying constitutively active myristoylated Akt and an adenovirus carrying the dominant negative form of RhoA.11,12 AdNull (empty vector Ad66) was used as control. BMECs were grown in complete medium in 10-cm petri dishes until 60% to 70% confluent, and then infected overnight with adenoviral vectors at 200 multiplicity of infection for both adenoviruses. The medium was then changed and cells were used for experiments after 2 days.
Illumina Gene Array
An Illumina bead-array interrogating 24 600 murine transcripts was performed on T1D and control BMECs (4 biological replicates each). Microarray expression data are available at the NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE14035.
Differentially expressed genes were associated with biofunction networks and canonical pathway using Ingenuity Pathway Analysis software core analysis (Ingenuity Systems, Redwood City, CA, www.ingenuity.com). Transcripts with expression changes at false discovery rate (q-value) <0.05, induced or repressed >1.25-fold, were associated with biological functions in Ingenuity Knowledge database, and the association was tested for statistical significance as described below.
Flow Cytometry Staining of BMECs
Freshly harvested BM cells were washed with ice-cold Hank balanced salt solution containing 0.5% bovine serum albumin and 0.02% sodium azide. To recognize endothelial cells (ECs), BM cells were stained with anti–MECA-32 biotin-conjugated antibody (BD Biosciences, Oxford, UK), followed by streptavidin–allophycocyanin–conjugated secondary antibody.
Detection of Oxidative Stress Markers
HBMECs were seeded on 6-well plates, cultured until reaching confluence, and then assessed for reactive oxygen species (ROS) levels by flow cytometry detection of MitoSox red (Invitrogen, Carlsbad, CA), a mitochondria-specific hydroethidine-derivative fluorescent dye, or DCF (Molecular Probes, Paisley, UK), a marker of total oxidative stress. Four separate experiments in triplicates were analyzed and averaged.
In addition, BMECs were seeded in 8-chamber slide wells and stained for 45 minutes with MitoTracker Red Cm-H2ROS (500 nmol/L) at 37°C. Cells were fixed with paraformaldehyde, and pictures were taken with identical settings for all conditions.
In Vitro Permeability Assay
HBMECs and BMECs were seeded onto transwell inserts (pore size: 0.3 μm) coated with 0.5 μg/mL fibronectin, and grown until they reached confluence. Then, the media was replaced in the lower chamber and fresh media containing 70 kDa fluorescein isothiocyanate-labeled dextran was added to the upper chamber. The increase in fluorescence in the lower chamber was evaluated at different time points over 16 hours, with identical exposure settings using FLUOstar Optima fluorimeter (Berthold Technologies Wildbad, Germany). Three independent experiments in triplicate were analyzed and averaged.
In Vivo Permeability Assay
Mice were intracardially injected with a 500 μL bolus of 70 kDa fluorescein isothiocyanate-labeled dextran (200 μM), followed after 3, 5, or 7 minutes by an equal bolus of 70 kDa tetramethylrhodamine isothiocyanate-labeled dextran (n=3 mice each time point). Animals were euthanized 30 seconds after the last injection. Fluorescein isothiocyanate and tetramethylrhodamine isothiocyanate fluorescence was measured using a FLUOstar Optima fluorimeter, with identical exposure settings for all conditions in plasma and in bone flushes.
Data were plotted as mg fluorescein isothiocyanate (IF) in BM interstitium per mg BM tissue (W) against time. Three independent experiments were carried out for each time point and condition.
Transendothelial Migration of BM-MNCs
Transendothelial migration of BM-MNCs was assessed using transwell cell culture inserts equipped with 3-μm pore size filters (BD Biosciences) using stromal cell–derived factor-1 as the chemoattractant. BM-MNCs from T1D and control mice were labeled with chloromethyl-dyalkilcarbocyanine (Life Technologies, NY), and then added to the top compartment (3×105 cells per well in 300 μL). After 16 hours incubation at 37°C, nonmigrated cells on the upper side of the membrane were removed by scraping. All inserts were fixed for 10 minutes in methanol and mounted on slides counterstained with 4′,6-diamidino-2-phenylindole. Three separate experiments in triplicates were analyzed and averaged.
BMEC Migration
Migration of cultured BMECs was studied, as described previously.13 Briefly, BMECs were seeded on the upper part of 24-transwell plate filters coated with fibronectin. The lower wells contained basal medium supplemented with vascular endothelial growth factor A (100 ng/mL, R&D, Abingdon, UK). After 8 hours incubation, BMECs migrated to the lower part of the filter membrane were counted. Five random fields per each filter were evaluated at ×200 magnification using a fluorescent microscope (Olympus). Four separate experiments in triplicate were performed.
Matrigel Assay
BMECs (3×104 in a total volume of 100 μL Dulbecco’s modified Eagle’s medium) were added on top of 100 μL gelified, growth-enriched Matrigel (BD Biosciences, Oxford, UK) in each well of 8-well chamber slides. After 8 hours at 37°C, gels were washed gently with sterile PBS and fixed with 2% paraformaldehyde, and then mounted with glycerol. Three samples per group were analyzed in triplicate to compute the cumulative tube length of the endothelial network.
Immunohistochemistry and Immunocytochemistry
Paraffin-embedded sections (5 μm thick) of BM from T1D and control mice (n=6 per group) were used for in situ identification of vascular structures (Alexa Fluor 568-conjugated isolectin B4 [Invitrogen]) expressing phosphorylated VE-cadherin (VE-cad pY731 or pY658 rabbit polyclonal antibodies [Invitrogen]). A goat antirabbit Alex488 was used as secondary antibody (Invitrogen). All samples were counterstained with 4′,6-diamidino-2-phenylindole. Microphotographs were taken with a Leica SP5 confocal imaging system (Leica, Milton Keynes, UK) at ×400 magnification.
To study cytoskeletal rearrangements, BMECs were stained with rhodamine phalloidin (Sigma). Five images per field were captured at ×200 magnification using a fluorescent microscope (Olympus, Southend on Sea, UK).
Quantitative PCR
Total RNA was isolated from murine BMECs (RNeasy, Qiagen, Manchester, UK), and RNA quality confirmed using the RNA Nano LabChip in a bioanalyzer (Agilent, Stockport, UK). RNA was reverse transcribed (Sensiscript reverse transcriptase, Qiagen, Crawley, UK) and quantitative PCR was performed in a LightCycler480 (Roche). Primers for PCR amplification for housekeeping gene, 18S rRNA, used were: forward, GATCTGCTCATTTGCTGCCG; reverse, GTGTGGTGGCTGATACATTTGAT. The ΔCt obtained was used to find the gene relative expression according to the formula: relative expression=2−ΔΔCt, where ΔΔCt is equal to ΔCt of a given gene in experimental group subtracted by the ΔCt of the same gene in control group. The analyses were performed on at least 4 samples per time and repeated 3 times. Primers used are detailed in Table I in the online-only Data Supplement.
Assay for Akt Activity
Protein preparations (25 μg) from BMECs of T1D and control mice were assessed for Akt activity using the Kinase Activity Assay Kit, according to the manufacturer’s instructions (Enzo Life Sciences, Exeter, UK). Three independent experiments in triplicate were performed.
Assay for Rho Activity
GTP-bound active Rho was assessed by pulldown assays, according to the manufacturer’s instructions (Millipore, Billerica, MA).
Western Blot
The analysis of protein expression was performed on lysates from confluent hBMECs and BMECs using phosphospecific antibodies against antiphospho Ser 1177 endothelial nitric oxide synthase (eNOS) (Cell signaling technologies), NADPH oxidase isoform 2 (BD Biosciences, Oxford, UK), VE-cadherin-Y731, VE-cadherin-Y658, and Pyk2-Y402 (Cell Signaling Technologies, MA), antibodies raised against respective total proteins and a monoclonal antibody for recognition of tubulin. Blots were analyzed with an enhanced chemiluminescence detection system (Amersham Biosciences, Buckinghamshire, UK).
Immunoprecipitation
Membrane samples were obtained, as described previously,14 in lysis buffer, 20 mmol/L Tris-HCL, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L ethylene glycol tetraacetic acid, 1% Triton-X, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerophosphate, 1 mmol/L Na3VO4, 1 μg/mL leupeptin. Cav1 was immunoprecipitated using anti-Caveolin-1 (Cav-1) antibody (Abcam) and protein A (Santa Cruz). After washing of the immune complexes in wash buffer, 20 mmol/L Tris-HCl, 137 mmol/L NaCl, 1% Triton-X, 2 mmol/L EDTA, the complexes were run on a SDS-PAGE gel and blotted for total eNOS (Cell Signaling Technologies). Samples incubated with nonimmune rabbit IgG, instead of anti–Cav-1 antibody, were used as controls.
Data Analysis and Statistical Procedures
Values are presented as mean±SEM. If data failed to pass normality and equal variance tests, a nonparametric analysis was applied and results were expressed as median with 5 to 95 percentile distribution. Multiple groups were compared by parametric 2-way ANOVA, followed by Bonferroni post t test, 1-way ANOVA, followed by Bonferroni Multiple Comparison test, or nonparametric ANOVA on ranks, followed by Tukey pairwise comparison or Dunnett test for multiple comparisons against a single control group. Comparison of 2 groups was performed by paired or unpaired Student t test. In gene array studies, the right-tailed Fisher exact test was used to evaluate the probability that the association of differentially expressed genes and biological functions or canonical pathways is because of chance. The significance of the association between the data set and a given canonical pathway was also measured as the ratio of the number of differentially expressed genes in a pathway and the total number of genes in the same pathway. A P value <0.05 was considered significant.
Results
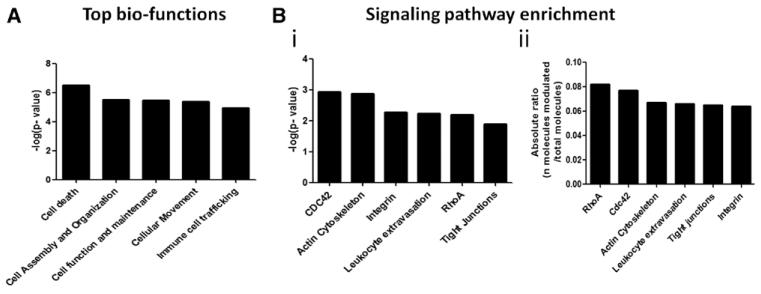
To determine the mechanisms underlying BM endotheliopathy, we performed an Illumina gene array on primary BMECs isolated from T1D (18 weeks from diabetes mellitus induction) and age-matched nondiabetic mice. Of 792 transcripts with expression changes at false discovery rate (q value) <0.05, 448 were induced or repressed >1.25-fold. Table II in the online-only Data Supplement shows the list of differentially expressed genes within canonical pathways. Among top-ranked functions, Ingenuity Pathway Analysis showed a highly significant effect of diabetes mellitus on signaling pathways associated with cellular death, assembly, organization, trafficking, and inflammation (Figure 1A).
Figure 1.
Ingenuity Pathway Analysis of transcription-associated biofunctions and signaling pathways. A, Bar graph showing −log probability values of canonic biological functions associated with expressional changes induced by diabetes mellitus in bone marrow endothelial cells (BMECs). B, Bar graphs showing −log probability values (i), and enrichment of genes (ii) in signaling pathways modulated by diabetes mellitus in BMECs.
Functional enrichment analysis identified small GTPases (RhoA and CDC42), actin cytoskeleton dynamics, integrin, leukocyte extravasation, and tight junctions, as the signaling pathways most enriched with differentially expressed genes (Figure 1B). Moreover, within the actin cytoskeleton and leukocyte extravasation/vascular permeability signaling pathways, we found that 14 of 209 and 12 of 183 genes, respectively, were modulated by diabetes mellitus (Figure II in the online-only Data Supplement). Actin-related protein 2/3 (nucleation site for actin filaments polymerization), membrane-organizing extension spike protein (moesin, a cross-linker between the endothelial plasma membrane and actin-based cytoskeleton), and the Rho-associated kinase-2 (ROCK2, an activator of moesin through phosphorylation on Thr558) were all upregulated in diabetic BMECs.
Taken together, these gene array data indicate transcriptional alterations compatible with loosened adhesive intercellular contacts and increased endothelial permeability.11
Altered RhoA/ROCK and Akt Activity in Diabetic BM Endothelium
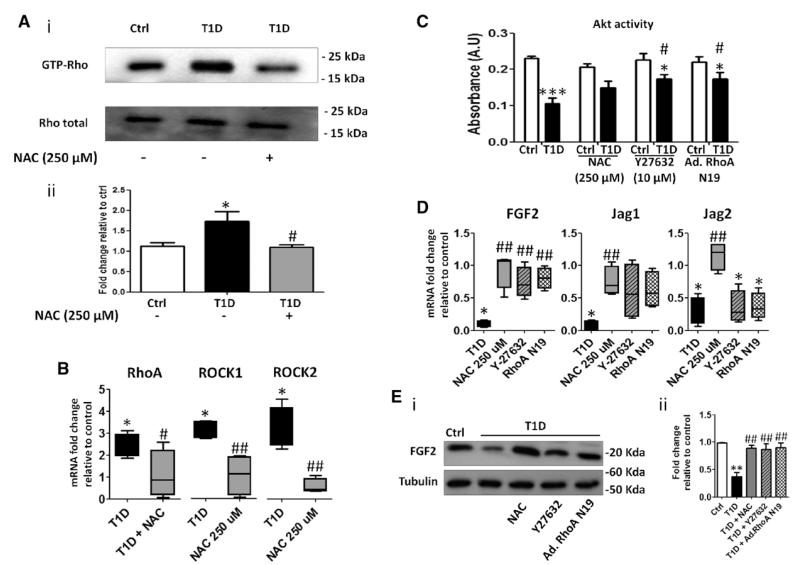
RhoA and ROCK regulate a wide range of cellular functions, including cytoskeletal rearrangement, migration, and proliferation. Using a RhoA–GTP-bound pulldown assay, we found that diabetes mellitus increases Rho activity in BMECs (Figure 2A). It is known that oxidative stress is a potent inducer of RhoA.15-17 Here, we confirm our previous finding of increased oxidative stress at the mitochondrial level in T1D-BMECs (Figure IIIA in the online-only Data Supplement).2 Moreover, we found polyADP-ribose polymerase 1 to be upregulated and transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nfr2) downregulated in T1D-BMECs (Figure IIIB in the online-only Data Supplement). It is known that oxidative stress induces DNA strain breaks, which in turn activate polyADP-ribose polymerase,18,19 whereas Nfr2 exerts antioxidant activity to protect vascular cells from HG-induced damage.19-21 In contrast, the expression of NADPH oxidase isoform 2, another important source of ROS, was similar in BMECs from healthy and diabetic mice (Figure IIIC in the online-only Data Supplement). Thus, oxidative stress in BM endothelium is attributable to increased ROS production in mitochondria and reduced antioxidant defense. In accordance, we found that preconditioning T1D-BMECs with the ROS scavenger, N-acetyl-cysteine (NAC), inhibits RhoA activation (Figure 2A). Furthermore, RhoA upregulation was accompanied by increased ROCK1 and ROCK2 mRNA levels, which was again prevented by NAC (Figure 2B).
Figure 2.
Rho-mediated Akt inactivation. A, Rho activity was assessed by pulldown assay in control (Ctrl) and type-1 diabetic (T1D) bone marrow endothelial cells (BMECs) in the presence or absence of N-acetyl-cysteine (NAC). Representative blots (i) and bar graph illustrating average results (ii). B, RhoA, Rho-associated kinase (ROCK) 1, and ROCK2 mRNA expression was evaluated by quantitative PCR. All data are expressed as fold change relative to control. C, Akt activity was assessed in control and diabetic BMECs in the presence or absence of NAC, ROCK inhibitor Y27632, or BMECs infected with adenovirus carrying the dominant negative form of RhoA. Bar graph showing that both NAC, RhoA knockdown, and Y27632 rescue Akt impairment in diabetic BMECs. D, The expression of Akt-related angiocrine factors fibroblast growth factor 2, JAGGED1 (Jag1), and JAGGED2 (Jag2) was assessed by quantitative PCR. E, Protein expression of fibroblast growth factor 2 was also assessed by Western blot. Data are expressed as mean±SEM. *P<0.05; **P<0.01; ***P<0.001 vs Ctrl; #P<0.05; and P<0.01 vs T1D. Each experiment was performed in triplicate.
The influence of activated RhoA on Akt is controversial, with reports indicating that RhoA/ROCK causes induction22 or suppression of Akt activity in ECs.23 We found that Akt activity is remarkably depressed in diabetic BM endothelium. Notably, this deficit was partially reverted by NAC, the ROCK inhibitor Y27632, or by transfecting cells with adenovirus carrying the dominant negative form of RhoA (Figure 2C), thus suggesting that small GTPase activation by oxidative stress is responsible for Akt inhibition.
Akt activation in ECs reportedly induces the release of angiocrine factors that support BM stem cell expansion.3 Several of these angiocrine substances, such as fibroblast growth factor 2, JAGGED1, and JAGGED2, were downregulated in diabetic BMECs, but restored after antioxidant treatment (Figure 2D and 2E). The ROCK inhibitor Y27632 and RhoA knockdown recovered fibroblast growth factor 2, but not JAGGED1 and JAGGED2 mRNA expression (Figure 2D).
Rescue of Endothelial Dysfunction by ROCK Inhibition or Akt Activation
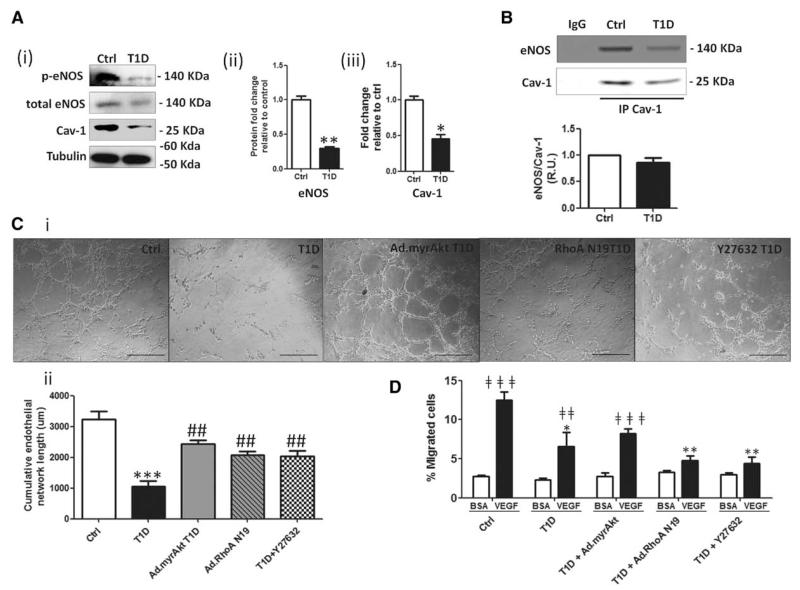
We next investigated whether an altered RhoA–Akt axis has specific consequences for the BMEC function-type. Akt is a potent inducer of eNOS activity, which synthesizes nitric oxide, a key molecule in EC function. In total membrane fractions from T1D BMECs, we observed a decrease in eNOS phosphorylation as well as a reduction in Cav-1 expression. Cav-1 negatively regulates eNOS by directly interacting with it. Immunoprecipitation of Cav-1 confirmed that Cav-1 and eNOS interact both in Ctrl and T1D BMECs (Figure 3A and 3B). Taken together, these data suggest a reduced nitric oxide (NO) availability in diabetic cells.
Figure 3.
Rho-associated kinase (ROCK) inhibition or Akt activation rescues endothelial dysfunction. A, Endothelial nitric oxide synthase (eNOS) activity (ii) along with Caveolin-1 (Cav-1) (iii) expression were measured by western blot (WB) (i) in membrane fractions. B, Diabetes mellitus impairs eNOS activation and interaction with Cav-1, as demonstrated by Western blot for eNOS in Cav-1 immunoprecipitates (IP Cav-1). Nonimmunogenic IgG was used as control. C, Representative microphotographs (scale bars 100 μm; i) and bar graph (ii) showing network-forming activity of control and diabetic bone marrow endothelial cells (BMECs). The deficit of network formation in diabetic BMECs was rescued by ROCK inhibition with Y27632 or transfection with constitutively active Akt or adenovirus carrying the dominant negative form of RhoA. Images were captured 16 hours after BMEC seeding. D, Bar graph showing percentage of migrated BMECs after stimulation with vascular endothelial growth factor A or vehicle (BSA). Diabetic BMECs showed impaired migratory capacity toward vascular endothelial growth factor A that was rescued, in part, by constitutively active Akt, but not by ROCK inhibition or adenovirus carrying the dominant negative form of RhoA infection. Data are expressed as mean±SEM. *P<0.05; **P<0.01; **P<0.001; ***P<0.001 vs Ctrl; #P<0.05; ##P<0.01 vs type-1 diabetic (T1D); ‡‡P<0.01; and ‡‡‡P<0.001 vs BSA. Each experiment was performed in triplicate.
We next investigated the effect of Akt activation (by transfection of BMECs with a constitutively active form), of RhoA knocking down (by a transfection with a dominant negative form), and of pharmacological ROCK inhibition with the compound Y27632. Successful transduction of cells by adenovirus carrying constitutively active myristoylated Akt and adenovirus carrying the dominant negative form of RhoA was documented by Western blot for Akt and Rho activity assay (Figure IIID and IIIE in the online-only Data Supplement).
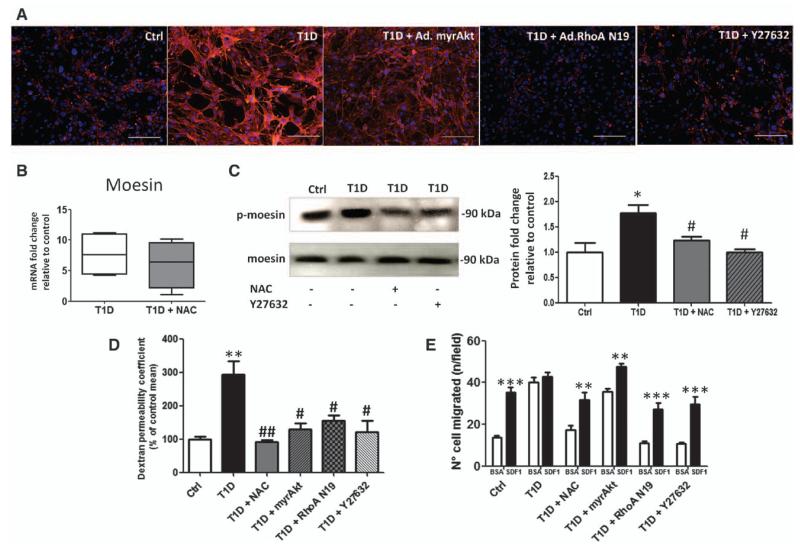
In a network formation assay on matrigel, T1D-BMECs showed reduced tube-formation capacity, which was restored by constitutively active Akt, adenovirus carrying the dominant negative form of RhoA, or ROCK inhibition (Figure 3C). In addition, T1D-BMECs displayed a reduced migratory response to vascular endothelial growth factor A, with this defect being partially recovered by Akt activation, but not by RhoA/ROCK inhibition (Figure 3D). ROS are known to induce the rearrangement of F-actin stress fibers and cell contraction via RhoA–ROCK activation and phosphorylation of moesin,24 resulting in increased endothelial permeability.25,26 We asked whether this mechanism is activated in T1D-BMECs. Accordingly, we found that diabetes mellitus triggers the formation of F-actin stress fibers in BMECs, which is reduced by ROCK inhibition and to a lesser extent by Akt activation (Figure 4A). Furthermore, moesin mRNA (Figure 4B) and protein phosphorylation levels (Figure 4C) were increased in T1D-BMECs, with the latter effect being blunted by NAC and ROCK inhibitor Y27632.
Figure 4.
Rho-associated kinase (ROCK) inhibition and Akt activation rescues diabetes mellitus-induced endothelial barrier dysfunction in bone marrow endothelial cells (BMECs). A, Microphotographs (scale bars 100 μm) showing BMECs stained for F-actin with rhodamine-associated phalloidin. Diabetes mellitus induces actin fiber activation, which is blunted by ROCK inhibitor Y27632, adenovirus carrying constitutively active myristoylated Akt or adenovirus carrying the dominant negative form of RhoA. B and C, Representative blots and graphs showing the effect of diabetes mellitus on mRNA expression (B) and the phosphorylation state of moesin (C) in BMECs. Upregulation of moesin is blunted by N-acetyl-cysteine (NAC) and ROCK inhibition. D, Bar graph showing the effect of diabetes mellitus on endothelial permeability using fluorescein isothiocyanate-labeled 70-kDa dextran. The increase in endothelial permeability is blunted by NAC, ROCK inhibition, constitutively active Akt or the dominant negative form of RhoA. E, Bar graph showing BM-MNC transendothelial migration after exposure to stromal cell–derived factor-1 (directed migration) or PBS (vehicle, spontaneous migration). Spontaneous transmigration was enhanced and directed migration suppressed by diabetes mellitus. These alterations were prevented by treating BMECs with NAC and ROCK inhibitors. Constitutively-active Akt improves directed migration without reducing the exaggerated spontaneous migration of bone marrow mononuclear cells (BM-MNCs). Data are mean±SEM. *P<0.05; **P<0.001; ***P<0.01 vs Ctrl; #P<0.05; and ##P<0.01 vs type-1 diabetic (T1D). Each experiment was performed in triplicate.
We next asked whether ROS- and ROCK-dependent activation of BMEC cytoskeleton translates into increased endothelial permeability and barrier dysfunction. Size-selective assessment of paracellular permeability was performed using fluorescently labeled dextran. Figure 4D shows that the T1D-BMEC monolayer is more permeable to dextran compared with BMECs from healthy mice. This increased permeability was prevented by NAC, myristoylated Akt, and RhoA/ROCK inhibition. The presence of endothelial barrier dysfunction was further assessed using a transendothelial migration assay on BM-MNCs. Results confirm our previous findings indicating that spontaneous transendothelial migration of BM-MNCs is increased in the presence of diabetic BMECs compared with control BMECs, whereas directed migration toward stromal cell-derived factor-1 is abolished.2 Moreover, we newly show that endothelial barrier function is rescued, in part, by ROS scavenging and RhoA/ROCK inhibition (Figure 4E). In contrast, Akt activation did not reduce the increased basal migration of BM-MNCs, but restored responsiveness to stromal cell–derived factor-1.
Altogether, these data indicate that the Rho/ROCK–Akt axis plays a crucial role in the functional alterations of diabetic BMECs.
HG Increases BMEC Permeability Through VE-Cadherin Phosphorylation
We next investigated the direct effect of HG on BMEC permeability. To this end, we established an in vitro model consisting of hBMECs cultured in normal (5 mmol/L, normal glucose) or high (15 and 25 mmol/L, HG) D-glucose for 96 hours. ROS levels were augmented by progressive increases of glucose concentration, as assessed by flow cytometry detection of MitoSox and 2′,7′-dichlorofluorescein-2A. The ROS production was brought back to control levels totally by catalase treatment, and partially reduced by superoxide inhibitor and antioxidant diethyldithiocarbamate (Figure IVA in the online-only Data Supplement). Moreover, HG alters hBMEC permeability in a dose-dependent manner, as assessed in an in vitro assay using 70 kDa dextran (Figuve IVB in the online-only Data Supplement). The increase in permeability was completely reversed by treating hBMECs with NAC or catalase; however, neither the hydroxyl scavenger MCI-186 nor diethyldithiocarbamate modified the effect of HG on permeability. The inhibition of detoxifying chain at superoxide level suggests that this ROS, and the ones generated as peroxynitrite, can trigger molecular changes leading to increased permeability.
ROS reportedly modifies the activity of several tyrosine kinases.27 Among them, Src increases vascular permeability through phosphorylation of VE-cadherin, a key component of EC adherens junctions.28 We found that HG increases the phosphorylation of VE-cadherin at Y731 and Y658, which are binding sites for β-catenin and p120, respectively. Moreover, VE-cadherin phosphorylation was prevented by both NAC treatment and Src inhibition, suggesting a pivotal role of Src kinase in adherens junction disassembly through a redox-sensitive mechanism (Figure VA and VB in the online-only Data Supplement). Of note, the HG–induced increase in permeability was reverted by Src inhibitor SU6656 (Figure IVB in the online-only Data Supplement). Another redox-sensitive kinase controlling adherens junctions is represented by the prolyne-rich kinase 2 (Pyk2), which has the same targets as Src. In accordance, the active phosphorylated form of Pyk2 was increased in hBMECs under HG. This effect was totally prevented by NAC (Figure VC in the online-only Data Supplement).
In addition, we found that the proapoptotic and proinflammatory redox-sensitive kinases p3829 and c-Jun N-terminal kinases30 are activated in both HG-treated hBMECs and T1D-BMECs. This effect was reversed by NAC and catalase. Finally, the MAPK kinase kinase, MEK1, which control angiogenesis and proliferation in ECs, was found increased in HBMECs treated with HG, but not in diabetic cells (Figure VI in the online-only Data Supplement).
Redox-Dependent Activation of VE-Cadherin in BMEC Leads to Endothelial Barrier Dysfunction in T1D Mice
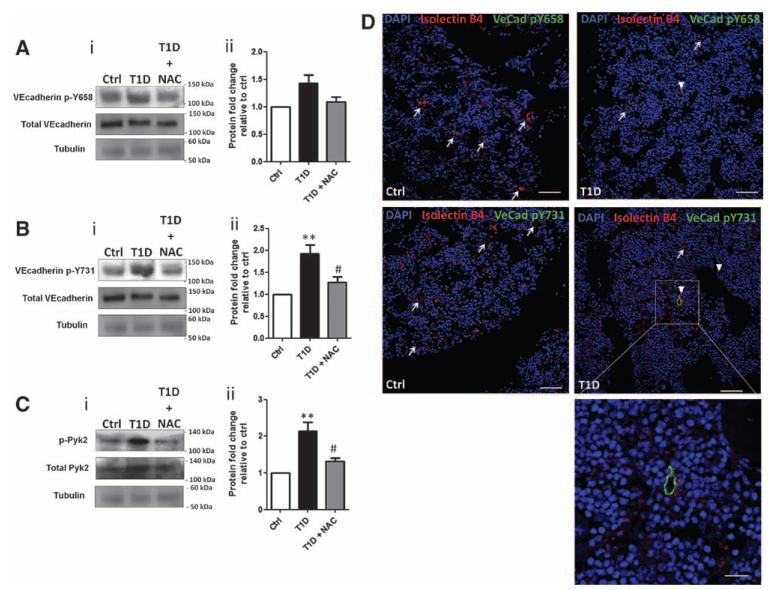
We next asked whether phosphorylation events associated with VE-cadherin activation occur in BMECs from diabetic mice. As for HG-treated hBMECs, phosphorylation of VE-cadherin and Pyk2 was increased in diabetic murine BMECs, but reduced by NAC (Figure 5A–5C). Fluorescence microscopy demonstrated in situ phosphorylation of VE-cadherin in BM vascular cells of T1D mice (Figure 5D and Figure VII in the online-only Data Supplement).
Figure 5.
Vascular endothelial cadherin (VE-cadherin) phosphorylation is increased in diabetic bone marrow endothelial cells (BMECs). A–C, Representative Western blots (i) and bar graphs (ii) showing phosphorylation of VE-cadherin pY658 (A) and VE-cadherin p731 (B) and pPyk2 (C) in BMECs. D, Immunostaining for residues pY658 (top) and pY731 (bottom) of VE-cadherin (arrowhead) and isolectin B4 (arrow) in bone marrow (femura) of type-1 diabetic (T1D) and control nondiabetic mice (scale bars: main pictures 80 μm; enlargement 20 μm). Data are mean±SEM. **P<0.01 vs Ctrl; and #P<0.05 vs T1D. Each experiment was performed in triplicate.
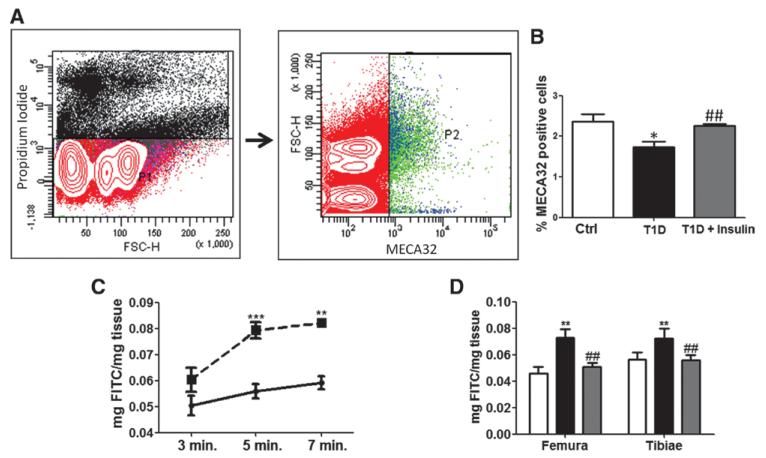
Finally, we assessed the abundance of BMECs by flow cytometry of MEC32-positive cells and BM endothelial barrier function in vivo using a double tracer technique. We found that MECA-32–positive ECs are reduced in BM of T1D mice (Figure 6A and 6B). Moreover, vascular permeability is increased by diabetes mellitus (Figure 6C and 6D), which was confirmed at different times from diabetes mellitus induction (Figure VIIIA and VIIIB in the online-only Data Supplement). To verify whether the observed changes can be contrasted by metabolic control, we treated diabetic animals with insulin implants. Of note, insulin replacement resulted in maintenance of BMECs abundance and normalization of vascular permeability (Figure 6A–6D). Furthermore, in vitro insulin treatment of BMECs was capable of reducing VE-cadherin phosphorylation at site Y731. Conversely, p-Pyk2 seemed not to be affected by insulin (Figure VIIIA and VIIIB in the online-only Data Supplement).
Figure 6.
Diabetes mellitus increases vascular permeability in bone marrow of type-1 diabetic (T1D) mice. A, Gating strategy for flow cytometry assessment of MECA-32–positive bone marrow endothelial cells (BMECs). B, Insulin replacement restores the abundance of MECA-32–positive ECs in bone marrow (BM) of T1D mice. C, Line graph showing the concentration of fluorescein isothiocyanate-labeled dextran in femoral BM of T1D (dotted line) and control mice (full line), at different time points from dextran injection. D, Bar graph showing restoration of proper vascular permeability in BM of T1D mice given insulin implants (grey bars) in comparison with untreated T1D mice (black bars). Reference value of nondiabetic control group (white) is also shown. Data are mean±SEM. *P<0.05; **P<0.01 and vs Ctrl; and ##P<0.01 vs T1D. Each experiment was performed in triplicate.
Discussion
This study provides new mechanistic insights into BM endothelial dysfunction induced by diabetes mellitus. BMECs from T1D mice showed a spectrum of functional alterations, including defects in angiocrine activity, migration, network formation, and permeability. Endothelial dysfunction can be traced back to mitochondrial oxidative stress triggered by high levels of glucose and alteration of the RhoA/ROCK/Akt signaling pathway. Moreover, BMEC availability and endothelial barrier dysfunction were confirmed in vivo and corrected by insulin.
RhoA controls several cellular function, including migration, angiogenesis, and apoptosis.31-33 In ECs, this Ras-like protein is committed to the formation of stress fibers through its effector ROCK.34 In recent years, RhoA has gained attention in the field of diabetes mellitus,15,35,36 being acknowledged as a primary target for oxidative stress or advanced glycation end products, and as an initiator of a series of transcriptional and posttranscriptional events leading to endothelial dysfunction.12,37,38 Here, we newly demonstrate that diabetes mellitus increases RhoA expression and activity, as well as the mRNA levels of ROCK isoforms in diabetic BMECs. ROCK1 activation is involved in permeability changes under inflammatory conditions,39 whereas ROCK2 contributes to the increase in adhesion molecules via nuclear factor-κB p65.40 Activation of moesin by ROCK-mediated phosphorylation induces rearrangement of the actin cytoskeleton and cell contraction instrumental to endothelial permeability.41 Importantly, we found that moesin is transcriptionally upregulated and phosphorylated in BMECs of T1D mice, leading to the activation of stress fibers and increased permeability to MNCs and macromolecules. These effects were prevented by the ROS scavenger and ROCK inhibitor, thus delineating a causal association between oxidative stress, RhoA/ROCK activation, stress fiber contraction, and endothelial barrier dysfunction.
Diabetic endotheliopathy is characterized by an alteration in the phosphorylation state and activity of several kinases. We have previously reported that diabetic BMECs have higher phosphorylation levels of VE-cadherin (at tyrosine 731, the β-catenin–binding site) and Pyk2 (at tyrosine 402, which is the autophosphorylation site for Pyk2) compared with control BMECs.2 Here, we newly report that HG-induced oxidative stress causes phosphorylation of VE-cadherin via the redox-sensitive kinases Src and Pyk2, thereby favoring the disassembly of adherens junctions and BM-MNC extravasation. Furthermore, we found that both diabetes mellitus and HG trigger the phosphorylation of apoptosis-related kinases, such as p38 and c-Jun N-terminal kinases, in human and murine cells. The redox-sensitive MAPK kinase kinase, MEK1, which in turn activates extracellular-signal-regulated kinases 1/2 exerts a modulatory control of angiogenesis.42 We found that in vitro exposure of hBMECs to HG increases the phosphorylation of MEK1, however, MEK1 levels were similar in BMECs from diabetic or nondiabetic mice. Thus, this specific pathway seems to be particularly sensitive to acute increases in glucose levels. We also observed a differential effect of various antioxidants on vascular permeability. The alteration of endothelial barrier function diabetes mellitus is more likely to depend on the formation of peroxynitrite, which is an activator of the RhoA/ROCK pathway, whereas redox-sensitive kinases are triggered by an increase in hydrogen peroxide production.
Another hallmark of BM endotheliopathy consists of Akt inactivation. NAC, RhoA dominant negative transfection, and ROCK inhibitor Y27632 were able to rescue Akt activity, suggesting an intertwined relationship between redox-dependent activation of RhoA–ROCK and Akt suppression. In fact, either inhibiting ROCK or enhancing Akt activity rescued diabetes mellitus–induced dysfunctions, including migratory and angiogenic defects, and increased permeability. Akt seems to be crucial for BMECs to manifest a migratory phenotype, as Akt inactivation in diabetes mellitus reduces their migratory and network-forming capacity, whereas Akt reactivation rescues both defects. In accordance with this hypothesis, we observed an impairment in eNOS activity. Therefore, the picture that emerges from a joint analysis of molecular and functional readouts is that of a contracted and leaky BM endothelium, incapable of responding to migratory signals as a consequence of dysfunctional Akt. In addition, it has been recently shown that Akt is essential for BMECs to convey self-renewal and differentiation signals to long-term hematopoietic stem cells through the release of angiocrine factors.3,4 We newly report the decreased expression of some Akt-dependent factors in diabetic BMECs, that is, the Notch ligands JAGGED1 and JAGGED2 and the angiogenic factor fibroblast growth factor 2. Further studies are warranted to investigate whether a depressed angiocrine signaling may contribute to BM stem cell depletion in diabetes mellitus.
The increased production of ROS plays a pivotal role in the pathogenesis of diabetes mellitus and the resulting complications. Along with several other tissues (kidney, eye, and nervous system), we have shown that oxidative stress plays a pivotal role in diabetic microangiopathy observed in BM. Thus, our results reinforce the concept that antioxidant administration can be helpful in managing diabetic complications. Indeed, several other investigations have been carried out to evaluate the ability of antioxidants to manage diabetic complications. For instance, NAC, vitamin C, vitamin E, and α-lipoic acid showed positive results in reducing diabetic complications.43-46 However, these previous clinical trials yielded promising yet inconsistent results because of the lack of data regarding optimal antioxidant concentration required to manage diabetic complications, with the lowest side effects possible.47 For this reason, it is important to understand the molecular mechanisms triggered by oxidative stress in different tissues so that a systemic antioxidant approach can be combined with a more tailored one; for example, ROCK inhibitors have already given promising results in in vitro study tissues other than BM.15,48,49
In summary, the present study highlights a molecular network responsible for endothelial barrier dysfunction in BM and identifies candidate mechanistic targets for rectification of the dysfunctional phenotype (Figure IX in the online-only Data Supplement). Importantly, insulin replacement exerts significant protection of BM vasculature. The notion that insulin is a potent inducer of Akt,50 and an inhibitor of RhoA in vascular cells,51 confirms the validity of the proposed molecular network.
BM-specific microangiopathy might have relevant clinical consequences. First, microvascular rarefaction endangers BM stem cell viability through reduction of perfusion and suspension of paracrine trophic signaling. Second, plasma extravasation is particularly harmful for a tissue like the marrow that is contained in nonexpandable bone. Third, barrier dysfunction might impinge on the release of stem cells, as illustrated by experiments showing exaggerated spontaneous transendothelial migration and reduced directed migration toward chemoattractants. These considerations call for urgent investigation into the status of BM in patients with complicated diabetes mellitus. Here, we show that hBMECs develop typical molecular and functional alterations when exposed to HG. We have also gathered new evidence that microvascular rarefaction occurs together with hematopoietic tissue remodeling and stem cell depletion in BM of diabetic patients.52 Hence, preserving the fitness of BM microvasculature represents a novel therapeutic target in the management of patients with diabetes mellitus.
Supplementary Material
Sources of Funding
This study was supported with funds from a British Heart Foundation project grant entitled “Bone marrow dysfunction alter vascular homeostasis in diabetes” to Paolo Madeddu.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.300424/-/DC1.
References
- 1.Shami SK, Chittenden SJ. Microangiopathy in diabetes mellitus: II. Features, complications and investigation. Diabetes Res. 1991;2:157–168. [PubMed] [Google Scholar]
- 2.Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G, Kraenkel N, Prezioso L, Emanueli C, Madeddu P. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol. 2010;30:498–508. doi: 10.1161/ATVBAHA.109.200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, Rafii S. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol. 2010;105:703–712. doi: 10.1007/s00395-010-0109-0. [DOI] [PubMed] [Google Scholar]
- 6.Ferraro F, Lymperi S, Méndez-Ferrer S, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 8.National Research Council, Institute for Laboratory Animal Research, National Academies Press . Guide for the care and use of laboratory animals. National Academies Press; Washington, D.C.: 1996. [Google Scholar]
- 9.Gadau S, Emanueli C, Van Linthout S, Graiani G, Todaro M, Meloni M, Campesi I, Invernici G, Spillmann F, Ward K, Madeddu P. Benfotiamine accelerates the healing of ischaemic diabetic limbs in mice through protein kinase B/Akt-mediated potentiation of angiogenesis and inhibition of apoptosis. Diabetologia. 2006;49:405–420. doi: 10.1007/s00125-005-0103-5. [DOI] [PubMed] [Google Scholar]
- 10.Rood PM, Calafat J, von dem Borne AE, Gerritsen WR, van der Schoot CE. Immortalisation of human bone marrow endothelial cells: characterisation of new cell lines. Eur J Clin Invest. 2000;30:618–629. doi: 10.1046/j.1365-2362.2000.00672.x. [DOI] [PubMed] [Google Scholar]
- 11.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Liu H, Chen B, Li Q, Huang X, Wang L, Guo X, Huang Q. RhoA/ROCK-dependent moesin phosphorylation regulates AGE-induced endothelial cellular response. Cardiovasc Diabetol. 2012;11:7. doi: 10.1186/1475-2840-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangialardi G, Monopoli A, Ongini E, Spinetti G, Fortunato O, Emanueli C, Madeddu P. Nitric oxide-donating statin improves multiple functions of circulating angiogenic cells. Br J Pharmacol. 2011;164(2b):570–583. doi: 10.1111/j.1476-5381.2011.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, Bernatchez P, van Nieuw Amerongen GP, Bonini MG, Skidgel RA, Malik AB, Minshall RD. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell. 2012;23:1388–1398. doi: 10.1091/mbc.E11-09-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, Zandi S, Almulki L, Tayyari F, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58:215–226. doi: 10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, Caldwell RW. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol. 2012;165:506–519. doi: 10.1111/j.1476-5381.2011.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Remessy AB, Tawfik HE, Matragoon S, Pillai B, Caldwell RB, Caldwell RW. Peroxynitrite mediates diabetes-induced endothelial dysfunction: possible role of Rho kinase activation. Exp Diabetes Res. 2010;2010:247861. doi: 10.1155/2010/247861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz HD. Reversible nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase upon serum depletion. Eur J Cell Biol. 2001;80:419–427. doi: 10.1078/0171-9335-00174. [DOI] [PubMed] [Google Scholar]
- 19.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 20.Ungvari Z, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B, Liu S, Miao L, Cai L. Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res. 2012;2012:216512. doi: 10.1155/2012/216512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basile JR, Gavard J, Gutkind JS. Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem. 2007;282:34888–34895. doi: 10.1074/jbc.M705467200. [DOI] [PubMed] [Google Scholar]
- 23.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyper-permeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- 26.Wójciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114(Pt 7):1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 27.Knock GA, Ward JP. Redox regulation of protein kinases as a modulator of vascular function. Antioxid Redox Signal. 2011;15:1531–1547. doi: 10.1089/ars.2010.3614. [DOI] [PubMed] [Google Scholar]
- 28.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 29.Choi YJ, Lim HS, Choi JS, Shin SY, Bae JY, Kang SW, Kang IJ, Kang YH. Blockade of chronic high glucose-induced endothelial apoptosis by Sasa borealis bamboo extract. Exp Biol Med (Maywood) 2008;233:580–591. doi: 10.3181/0707-RM-205. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Wang P, Yu S, Zheng Z, Xu X. Calcium entry mediates hyperglycemia-induced apoptosis through Ca(2+)/calmodulin-dependent kinase II in retinal capillary endothelial cells. Mol Vis. 2012;18:2371–2379. [PMC free article] [PubMed] [Google Scholar]
- 31.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 32.van der Heijden M, Versteilen AM, Sipkema P, van Nieuw Amerongen GP, Musters RJ, Groeneveld AB. Rho-kinase-dependent F-actin rearrangement is involved in the inhibition of PI3-kinase/Akt during ischemia-reperfusion-induced endothelial cell apoptosis. Apoptosis. 2008;13:404–412. doi: 10.1007/s10495-007-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryan BA, D’Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–2065. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- 35.Bach LA. Rho kinase inhibition: a new approach for treating diabetic nephropathy? Diabetes. 2008;57:532–533. doi: 10.2337/db07-1768. [DOI] [PubMed] [Google Scholar]
- 36.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Li Q, Du J, Chen B, Li Q, Huang X, Guo X, Huang Q. Advanced glycation end products induce moesin phosphorylation in murine retinal endothelium. Acta Diabetol. 2012;49:47–55. doi: 10.1007/s00592-011-0267-z. [DOI] [PubMed] [Google Scholar]
- 38.Guo X, Wang L, Chen B, Li Q, Wang J, Zhao M, Wu W, Zhu P, Huang X, Huang Q. ERM protein moesin is phosphorylated by advanced glycation end products and modulates endothelial permeability. Am J Physiol Heart Circ Physiol. 2009;297:H238–H246. doi: 10.1152/ajpheart.00196.2009. [DOI] [PubMed] [Google Scholar]
- 39.Mong PY, Wang Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J Immunol. 2009;182:2385–2394. doi: 10.4049/jimmunol.0802811. [DOI] [PubMed] [Google Scholar]
- 40.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem. 2010;285:12536–12542. doi: 10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, Zhu W, Wu D, Xu A. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28:835–840. doi: 10.1161/ATVBAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 43.Zherebitskaya E, Akude E, Smith DR, Fernyhough P. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes. 2009;58:1356–1364. doi: 10.2337/db09-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iino K, Iwase M, Sonoki K, Yoshinari M, Iida M. Combination treatment of vitamin C and desferrioxamine suppresses glomerular superoxide and prosta-glandin E production in diabetic rats. Diabetes Obes Metab. 2005;7:106–109. doi: 10.1111/j.1463-1326.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 45.Lin J, Bierhaus A, Bugert P, Dietrich N, Feng Y, Vom Hagen F, Nawroth P, Brownlee M, Hammes HP. Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia. 2006;49:1089–1096. doi: 10.1007/s00125-006-0174-y. [DOI] [PubMed] [Google Scholar]
- 46.Mayer-Davis EJ, Costacou T, King I, Zaccaro DJ, Bell RA. Insulin Resistance and Atherosclerosis Study (IRAS). Plasma and dietary vitamin E in relation to incidence of type 2 diabetes: The Insulin Resistance and Atherosclerosis Study (IRAS) Diabetes Care. 2002;25:2172–2177. doi: 10.2337/diacare.25.12.2172. [DOI] [PubMed] [Google Scholar]
- 47.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Peng W, Jian W, Li Y, Li Q, Li W, Xu Y. ROCK inhibitor fasudil attenuated high glucose-induced MCP-1 and VCAM-1 expression and monocyte-endothelial cell adhesion. Cardiovasc Diabetol. 2012;11:65. doi: 10.1186/1475-2840-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma DW, Wang QY, Ma XY, Li J, Guan QH, Fu Y. [The effect of fasudil via Rho/ROCK signaling pathway on the inflammation and fibrosis in human mesangial cells in high glucose medium] Zhonghua Nei Ke Za Zhi. 2011;50:580–584. [PubMed] [Google Scholar]
- 50.Hermann C, Assmus B, Urbich C, Zeiher AM, Dimmeler S. Insulin-mediated stimulation of protein kinase Akt: A potent survival signaling cascade for endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:402–409. doi: 10.1161/01.atv.20.2.402. [DOI] [PubMed] [Google Scholar]
- 51.Begum N, Sandu OA, Duddy N. Negative regulation of rho signaling by insulin and its impact on actin cytoskeleton organization in vascular smooth muscle cells: role of nitric oxide and cyclic guanosine monophosphate signaling pathways. Diabetes. 2002;51:2256–2263. doi: 10.2337/diabetes.51.7.2256. [DOI] [PubMed] [Google Scholar]
- 52.Spinetti G, Cordella D, Fortunato O, Sangalli E, Losa SP, Gotti A, Carnelli F, Rosa F, Riboldi S, Sessa F, Avolio E, Beltrami AP, Emanueli C, Madeddu PR. [Accessed January 15, 2013];Global remodeling of the vascular stem cell niche in bone marrow of diabetic patients: implication of the mir-155/foxo3a signaling pathway. Circ Res. 2012 Dec 18; doi: 10.1161/CIRCRESAHA.112.300598. doi: 10.1161/CIRCRESAHA.112.300598. http://circres.ahajournals.org/content/early/2012/12/18/CIRCRESAHA.112.300598.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.