Abstract
Background
With aging and menopause, which are associated with decreases in ovarian steroids such as 17β-estradiol (E2), women might experience negative psychological symptoms, including anxiety and depression. Some women use E2-based therapies to alleviate these symptoms, but E2 has been associated with trophic effects that might increase vulnerability to some steroid-sensitive cancers, such as breast cancer, in both premenopausal and postmenopausal women.
Objective
This study investigated the relationships between the possible beneficial effects of E2 on anxiety and depressive behaviors concurrent with trophic effects using an animal model of E2 decline and replacement.
Methods
Dose-dependent effects of E2 on affective, sexual, and motor behavior of young adult rats were studied. Ovariectomized (OVX) rats were administered the chemical carcinogen 7,12-dimethylbenz(a) anthracene (DMBA) 1.25 mg or inactive vehicle (vegetable oil; control) by gavage. E2 (0.03 or 0.09 mg/kg) or vehicle was administered subcutaneously 44 to 48 hours before assessments of anxiety (light–dark transition), depression (forced swim test), sexual (lordosis), and motor (activity monitor) behaviors. Fourteen weeks after carcinogen exposure, E2 concentrations in plasma and brain regions (cortex, hippocampus, and hypothalamus) were determined. Incidences and numbers of tumors and uterine weight were analyzed.
Results
Administration of E2 (0.09 mg/kg) was associated with significant increases in antianxiety-like behavior in the light–dark transition task, antidepressant-like behavior in the forced swim test, and physiologic circulating and central E2 concentrations compared with E2 (0.03 mg/kg) and vehicle. Compared with vehicle, E2 (0.9 > 0.3 mg/kg) was associated with significant increases in lordosis and uterine weight. Administration of DMBA was associated with significant increases in the incidences and numbers of tumors; this effect was augmented by E2 administration.
Conclusion
Based on the findings in this rat model, the hypothesis that E2 may be effective in reducing anxiety and depressive behaviors and enhance sexual behavior in OVX rats, concurrent with trophic effects in the periphery, was supported. Moderate physiologic levels of E2 might have beneficial effects on affective and sexual behaviors in female rodents, but regimens including E2 might increase tumorigenic capacity.
Keywords: estrogen, anxiety, depression, menopause, breast cancer
INTRODUCTION
The population is aging, and a trend toward better health care in industrialized nations has resulted in greater longevity, particularly in women.1 Despite the increase in longevity, the age at onset of menopause has remained relatively stable, producing a situation in which many women are living one third to half of their lives in a postmenopausal, 17β-estradiol (E2)-deficient state.1 Cessation of E2 production has been associated with physiologic symptoms (eg, hot flushes, night sweats, genital dryness) and changes in psychological measures (eg, cognition, anxiety, mood), resulting in decreased quality of life.2 Some women respond favorably to E2-based treatments of these symptoms; however, in the years following the publication of the findings from the large, randomized, controlled Women’s Health Initiative, the benefits of E2-based treatments in relation to their potential risks have been questioned.3–6 As a result, women and their physicians have begun to reconsider their treatment options for alleviating symptoms associated with the decrease in E2. The potential risks of E2 therapies, as well as the known proliferative effects of E2 in reproductive tissues such as the breast,7 have dampened enthusiasm for using E2 in the treatment of mood and affective disorders. A history of greater exposure to E2, such as occurs related to parity, early age at menarche, obesity, and late-onset menopause, increases breast cancer risk.8–12 A relationship between high serum E2 concentrations and increased breast cancer risk has been found.12–15 For example, in one study, women with high plasma E2 concentrations (≥12 pg/mL) had a relative risk of 1.91, whereas women with lower E2 concentrations (6–7 pg/mL) had a relative risk of 1.17.14 Bilateral oophorectomy has been associated with reduced endogenous E2 levels and breast cancer risk in some premenopausal women,16,17 and administration of exogenous E2 in postmenopausal women has been found to increase breast cancer risk.18–20
In addition to modulating trophic effects in the body, E2 from endogenous and/or exogenous sources might alter mood and anxiety in women throughout the life span and might contribute to the gender disparity that favors men for anxiety and mood disorders. Among adults, there are clear gender differences in mood and anxiety disorders: at least twice as many women are diagnosed with anxiety or depression compared with men.21 These gender differences emerge after the onset of puberty, which is associated with cyclic changes in E2 concentrations in women but not men. E2 concentrations decrease after menopause, when there is cessation of these variations in E2.2,22 Young women in their teens to ~50s (or when menopause begins) are uniquely predisposed to changes in anxiety and mood that occur with natural fluctuations in E2 concentrations (ie, premenstrual syndrome), premenstrual dysphoric disorder, and postpartum depression.23,24 Although the gender differences in anxiety and depressive disorders are typically attenuated with aging, there can be an increased incidence of depression and/or anxiety symptoms coincident with the decline in E2 levels (ie, postmenopausal) in some women. However, it appears that the perimenopausal period may be a particularly sensitive time for changes in affect with aging.25–29
In a cross-sectional study in 265 older (mean age, 74.6 years) postmenopausal women, Beck Depression Inventory scores were significantly inversely related to plasma E2 concentrations; women with greater indices of depression had significantly lower E2 concentrations.30 These data support the hypothesis that although E2 levels may contribute to changes in affect, there may be individual differences in this response, with some women responding poorly to E2 decline and/or fluctuations. Moreover, E2-based treatments might reduce anxiety and improve mood scores when administered in perimenopausal and/or postmenopausal women.31–33 However, there are some inconsistencies in the clinical literature,22,34,35 particularly regarding the benefit of E2-based treatments in postmenopausal women.
Some limitations of E2-based treatments of anxiety and depressive processes that have been found in clinical studies22,34,35 have been further explored using animal models. A typical approach to determining the effects of E2 decline and replacement in animal models is to surgically remove the main endogenous source of E2, the ovaries (using ovariectomy [OVX]), and replace them with various concentrations of E2 that mimic levels to which the animal would be exposed naturally during its life span. This approach has been tested in rodent and nonhuman primate models. 34,36 In 2 such studies, anxiety and depressive behaviors were increased in OVX rats compared with young proestrous rats with high physiologic E2 concentrations.34,37 Additional studies in OVX rats37,38 or mice37,39,40 found that the administration of an E2 regimen that produced acute, moderate proestrus-like E2 concentrations was associated with decreases in anxiety and depressive behaviors.37–40 However, in other rodent models, an E2 regimen that produced lower and/or higher circulating E2 did not have congruous antianxiety-like and antidepressant-like effects.34,38 Differences in anxiety and depression can also be observed based on age, due to potential changes in receptor responsiveness and behavioral alterations that occur with aging.34,37 Given that the risk for negative trophic effects (ie, steroid-induced cancers) are a concern in not only older, menopausal women but also in some young women, this study investigated the relationship between the potential beneficial effects of E2 on the brain (eg, affective and sexual processes) and potential negative proliferative effects in the body (eg, tumors, uterine weights).
MATERIALS AND METHODS
All methods used were approved by the Institutional Animal Care and Use Committee at the University at Albany–State University of New York (SUNY), Albany, New York, and were carried out in accordance with accepted standards of humane animal use.41
Animals and Housing
Forty-six adult (age, ~8 weeks) female rats weighing 200 to 300 g were obtained from Taconic Farms (Germantown, New York) or Charles River Laboratories (Wilmington, Massachusetts). Rats were group-housed (3–5 per cage) in polycarbonate cages (45 × 24 × 21 cm) that contained woodchip shavings for bedding, in a temperature-controlled room (21° ± 1°C) in the Laboratory Animal Care Facility at the Life Sciences Research Building at the University at Albany–SUNY. Rats were maintained on a 12-hour reversed light–dark cycle (lights off at 8 am), with rodent chow and tap water available ad libitum.
Procedures
Ovariectomy
All rats underwent OVX under anesthesia induced using xylazine (12 mg/kg; Bayer Corporation, Shawnee Mission, Kansas) and ketamine (60 mg/kg; Fort Dodge Animal Health, Fort Dodge, Iowa). Bilateral dorsolateral incisions were made in the skin and muscle wall, and the ovaries and fallopian tubes were ligatured and removed. The muscle wall was sutured and the skin was closed with a suture or staple and surgical adhesive. Rats recovered for 1 week after surgery, with daily monitoring of postoperative condition.
DMBA or Vehicle Administration
After a 1-week recovery from OVX, 20 rats received a single administration of the chemical carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) (Sigma Chemical Co., St. Louis, Missouri), and 26 rats were given an inactive control substance (vegetable oil). DMBA 1.25 mg was mixed with vegetable oil (based on a pilot study42) and administered by gavage (curved, 16G, 3-inch length feeding needle with a 3-mm diameter ball on the end).
E2 Administration
Starting 2 days after DMBA exposure, rats were injected with 0.03-mg/kg E2 (Steraloids Inc., Newport, Rhode Island) (DMBA exposed, 7 rats; control, 9 rats) or 0.09-mg/kg E2 (DMBA exposed, 6; control, 9) in vegetable oil vehicle, or vegetable oil vehicle only (control), subcutaneously once per week for 14 weeks.
Behavioral Testing
Behavioral testing was conducted weekly at 44 to 48 hours after E2 or vehicle injection, as per previously published investigations.38,43 Rats were assessed for anxiety, depressive, sexual, and motor behaviors using the tasks described subsequently. The order in which rats were tested was identical in all animals. Behavioral data were collected by trained observers, blinded to the hypothesized outcome of the study, and simultaneously recorded using a video camera and/or video tracking system (Any-maze, Stoelting, Inc., Wood Dale, Illinois).
Light–Dark Transition Task
Rats were tested in the light–dark transition task as described elsewhere.44 Briefly, rats were placed on the illuminated side of a 2-chambered box (30 × 40 × 40 cm) that had white walls and floor. The dark side of the chamber was painted black and not illuminated. During the 5-minute task, the time rats spent on the light versus the dark side of the chamber was recorded. More time spent on the light side of the chamber indicated antianxiety-like behavior.
Forced Swim Test
The forced swim test was performed as described elsewhere.38 Rats were placed in a cylindrical chamber (45 cm h × 20 cm diameter; Stoelting) that contained 30-cm-deep, 30°C tap water. The times spent struggling, swimming, and immobile were recorded during the 10-minute task. A decreased time spent immobile, and an increased time spent swimming or struggling, indicated antidepressive-like behavior.
Sexual Receptivity
Rats were tested for sexual behavior in a polycarbonate chamber (50 × 25 × 30 cm) using methods described elsewhere.43 Briefly, the behaviors of female rats when mounted by a sexually experienced male rat were recorded for 10 minutes or 10 mounts, whichever occurred first. The frequency of lordosis (lordosis quotient [LQ]) and intensity of lordosis (lordosis ratings [LR]) were quantified by rating dorsiflexion on a scale of 0 to 3 (with 3 corresponding to the greatest level of dorsiflexion).45
Horizontal Crossing Task
Immediately after testing for sexual receptivity, rats were tested in the horizontal crossing task as per methods described elsewhere.46 Rats were placed for 5 minutes in a 39 × 39 × 30-cm Digiscan Optical Animal Activity Monitor (Accuscan Instruments Inc., Columbus, Ohio), which mechanically recorded the number of horizontal beam breaks that were made as an index of general motor behavior.
Tissue Collection
Euthanization was by rapid decapitation. Trunk blood was collected in chilled test tubes, placed on ice, and centrifuged at 3000g for 10 minutes. Plasma was poured into microfuge tubes and stored at −80°C until immediately prior to radioimmunoassay. Brains were rapidly dissected from the head and placed in a weigh boat on dry ice. Whole brains were stored at −80°C until immediately prior to radioimmunoassay. Rats were palpated and visually inspected to determine the presence of tumors. Tumors were dissected out, weighed, and placed on dry ice. Uteri from rats were dissected out, weighed, and placed on dry ice.
Radioimmunoassay of E2
To address the effects of treatments for E2 concentrations in plasma and brain regions that may mediate the behavioral effects from E2 decline (cortex, hippocampus, and hypothalamus), radioimmunoassay for E2 in these tissues was done using previously reported methods.47 Plasma and brains were thawed on ice. Brain regions were dissected out, such that the frontal region of the cortex, and the entire hippocampus and hypothalamus, were assayed. Dissected brain regions were homogenized with a glass/glass homogenizer in 50% MeOH:1% acetic acid. Homogenates were centrifuged at 3000g, followed by chromatographic separation of the supernatant using Sep-pak cartridges (Waters Corporation, Milford, Massachusetts) equilibrated with 50% MeOH:1% acetic acid. Steroids were eluted with increasing concentrations of MeOH (ie, 50% MeOH followed by 100% MeOH). Solvents were evaporated to dryness in a savant. E2 was extracted from plasma samples using snap-freezing with ether. Immediately before radioimmunoassay setup, samples were reconstituted in 150 µL phosphate assay buffer. The radioactive probes used were E2 NET-317, 51.3 Ci/mmol (purchased from Perkin-Elmer Corporation, Boston, Massachusetts). The E2 antibody (in a 1:40,000 dilution; E#244, Dr. G.D. Niswender, Colorado State University, Fort Collins, Colorado) used typically binds between 40% and 60% of [3H] E2 and bound 54% in the present study. The range of the standard curves, prepared in duplicate, was 0 to 1000 pg for E2. Standards were added to assay buffer followed by the addition of antibody and 3H E2. Total assay volumes were 750 µL for E2. The assay was incubated overnight at 4°C. Separation of bound and free E2 was accomplished by rapidly adding dextran-coated charcoal to assay tubes. Following 20-minute incubation with charcoal, samples were centrifuged at 3000g for 20 minutes, and the supernatant was decanted into a glass scintillation vial with 5-mL scintillation cocktail (Scintiverse BD, Fisher Scientific, Pittsburgh, Pennsylvania). Sample tube concentrations were calculated using the logit-log method of Rodbard and Hutt,48 interpolation of the standards, and correction for recovery with Assay Zap (Biosoft, Cambridge, United Kingdom). The interassay and intra-assay reliability coefficients were 0.05 and 0.06.
Statistical Analyses
Two-way analyses of variance (ANOVAs) were used to determine the effects of E2 dosage and DMBA condition on tumor number, uterine weights, and E2 concentrations. One-way ANOVAs were used to determine the effects of E2 condition for behavioral end points. If main effects were found, group differences were determined using the Fisher post hoc test. A P value ≤0.05 was considered statistically significant.
RESULTS
There were dose-dependent effects of E2 (0.09 > 0.03 mg/kg) to significantly increase E2 concentrations in plasma (F2,40 = 8.78; P < 0.01), cortex (F2,40 = 8.05; P < 0.01), hippocampus (F2,40 = 7.34; P < 0.01), and hypothalamus (F2,40 = 3.30; P < 0.01) compared with vehicle. DMBA administration was not associated with significantly altered E2 concentrations (Table I).
Table I.
Concentrations of estradiol (E2) in the plasma, cortex, hippocampus, and hypothalamus in ovariectomized rats administered the chemical carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) or inactive vehicle (no DMBA), followed by weekly priming with E2 or inactive vehicle (control). Values are mean (SEM) pg/mL.
| E2 0.03 mg/kg (n = 16) |
E2 0.09 mg/kg (n = 15) |
Control (n = 15) |
||||
|---|---|---|---|---|---|---|
| Tissue | DMBA | No DMBA | DMBA | No DMBA | DMBA | No DMBA |
| Plasma | 19.8 (4.1)* | 14.3 (4.7) | 43.9 (19.2)* | 41.3 (13.2) | 3.9 (1.0) | 3.6 (0.8) |
| Cortex | 3.9 (1.9)* | 3.8 (1.0) | 12.0 (3.9)* | 9.3 (2.3) | 3.9 (1.3) | 3.0 (1.7) |
| Hippocampus | 4.6 (1.6)* | 6.1 (1.5) | 8.4 (2.2)* | 7.3 (1.2) | 2.9 (0.8) | 2.1 (0.7) |
| Hypothalamus | 3.6 (1.3) | 3.6 (0.8) | 7.3 (2.6)* | 6.3 (2.1) | 3.0 (1.3) | 2.8 (1.2) |
P ≤ 0.01 versus control.
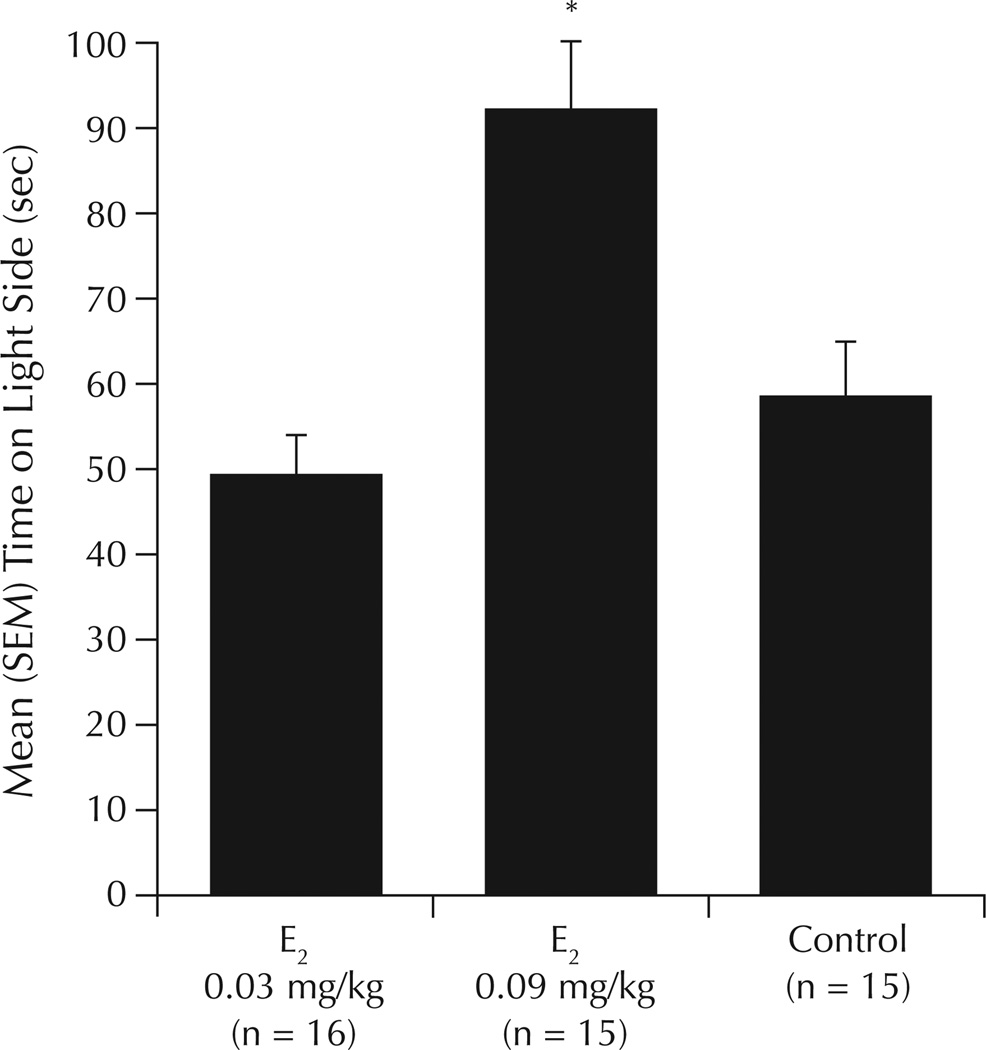
There was a significant main effect of E2 to increase time spent on the light side of the light–dark transition chamber (F2,43 = 3.31; P = 0.05) compared with vehicle (Figure 1). Compared with vehicle, 0.09-mg/kg E2 was associated with significantly increased time spent on the light side of the chamber.
Figure 1.
Time spent on the light side of the light–dark transition chamber in ovariectomized rats administered the chemical carcinogen 7,12-dimethylbenz(a)anthracene or inactive vehicle, followed by weekly priming with estradiol (E2) or inactive vehicle (control). *P ≤ 0.05 versus control.
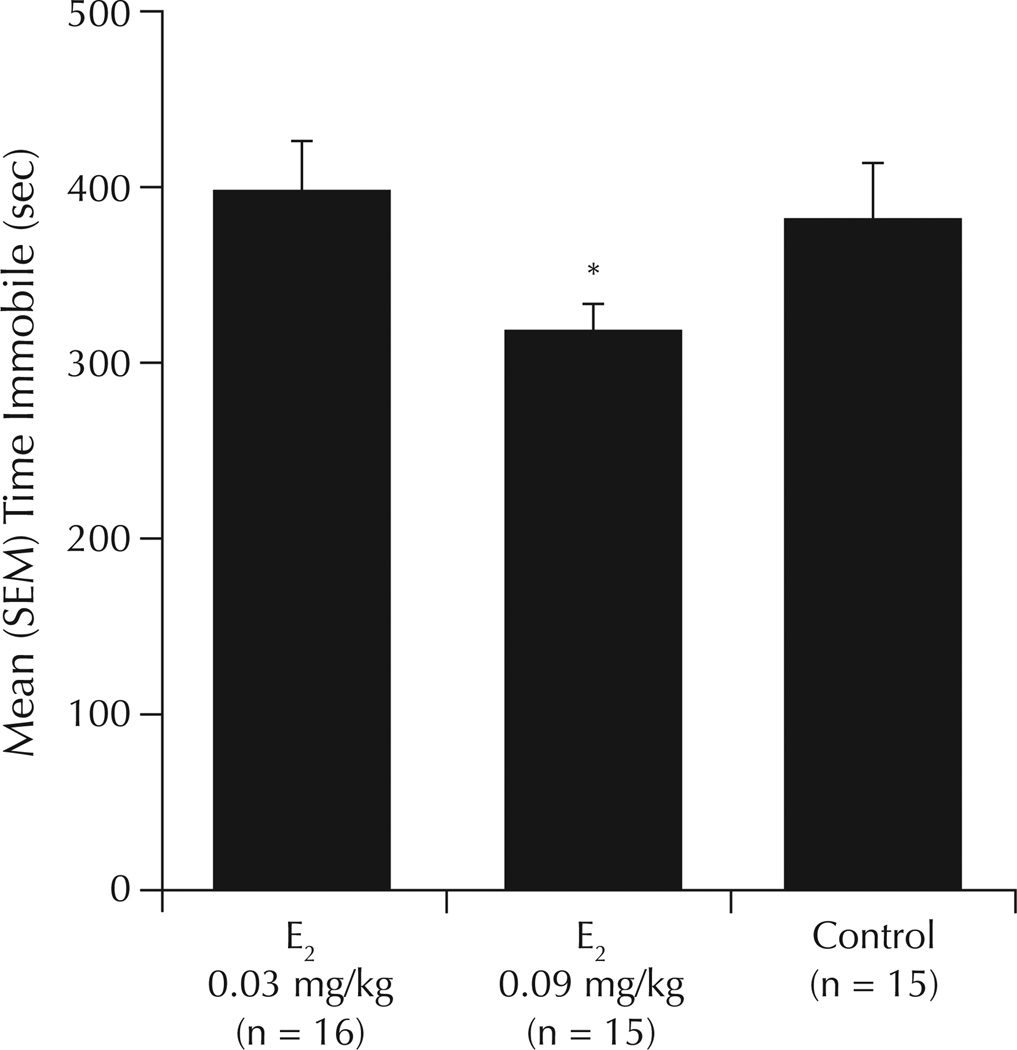
There was a significant main effect of E2 to increase time spent struggling (F2,43 = 4.53; P = 0.02) and decrease time spent immobile (F2,43 = 3.54; P = 0.04) in the forced swim test compared with vehicle (Figure 2). Compared with vehicle, E2 0.09 mg/kg was associated with significantly increased time spent struggling (P ≤ 0.05) (Table II) and decreased time spent immobile in the forced swim test (Figure 2). There were no significant differences in swimming duration (Table II).
Figure 2.
Time spent immobile in the forced swim test in ovariectomized rats administered the chemical carcinogen 7,12-dimethylbenz(a)anthracene or inactive vehicle, followed by weekly priming with estradiol (E2) or inactive vehicle (control). *P ≤ 0.05 versus control.
Table II.
Behavioral measures in ovariectomized rats administered the chemical carcinogen 7,12-dimethylbenz(a)anthracene or inactive vehicle, followed by weekly priming with estradiol (E2) or inactive vehicle (control). Values are mean (SEM).
| Outcome Measure | E2 0.03 mg/kg (n = 16) | E2 0.09 mg/kg (n = 15) | Control (n = 15) |
|---|---|---|---|
| Forced swim test: time spent struggling, sec | 143.9 (13.2) | 203.8 (20.9)* | 140.1 (15.4) |
| Forced swim test: time spent swimming, sec | 65.3 (18.3) | 90.3 (16.3) | 80.5 (21.4) |
| Lordosis quotient | 25.2 (3.6)† | 38.2 (3.1)† | 7.8 (3.0) |
| Activity monitor: no. of beam breaks made | 695.4 (102.8) | 917.4 (119.5) | 842.5 (106.3) |
P ≤ 0.05 versus control.
P ≤ 0.01 versus control.
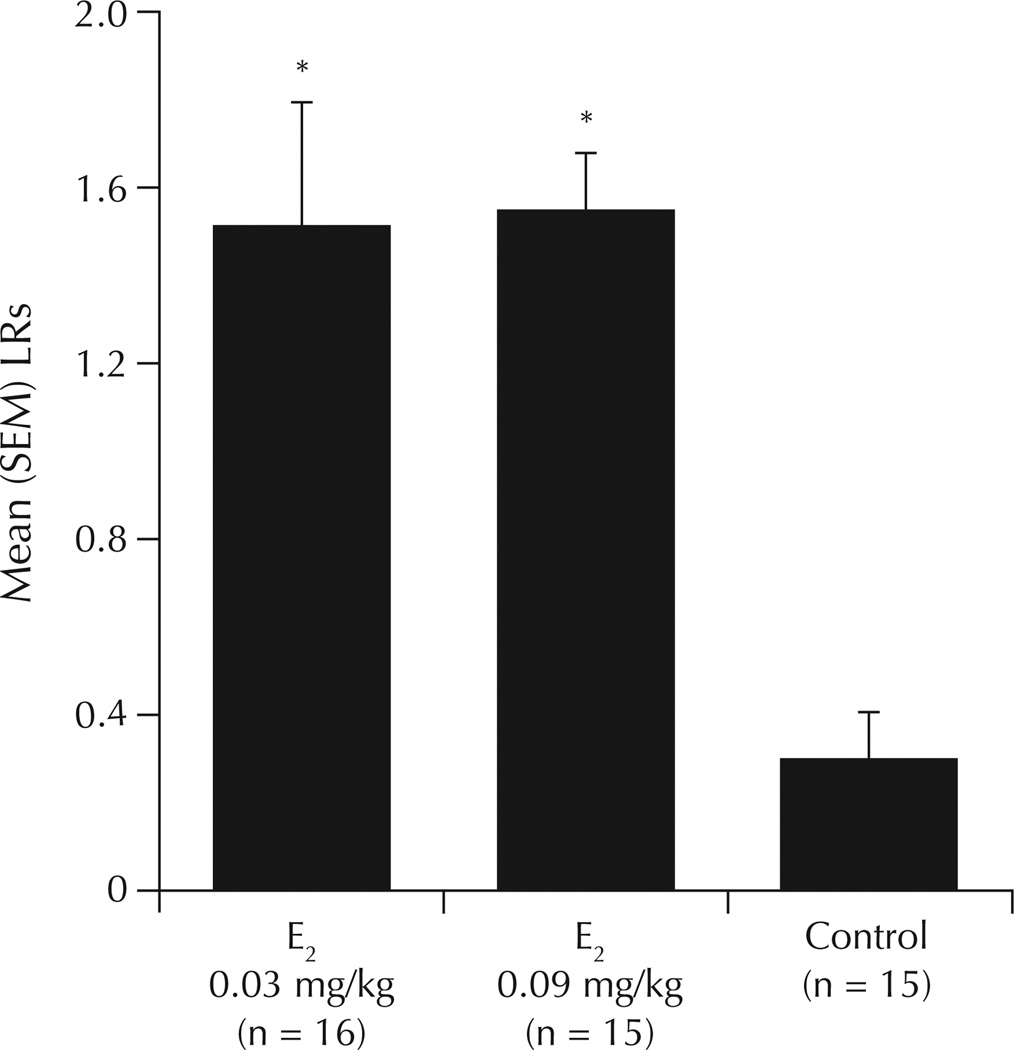
There was a significant main effect of E2 to increase LQ (F2,43 = 23.03; P = 0.01) (Table II) and LR (F2,43 = 16.13; P = 0.01) (Figure 3) compared with vehicle. Compared with vehicle, E2 (0.03 and 0.09 mg/kg) was associated with significant increases in LQ and LR.
Figure 3.
Lordosis ratings (LRs) in ovariectomized rats administered the chemical carcinogen 7,12-dimethylbenz(a)anthracene or inactive vehicle, followed by weekly priming with estradiol (E2) or inactive vehicle (control). *P = 0.01 versus control.
There were no significant differences in the numbers of beam breaks made in the activity monitor (Table II).
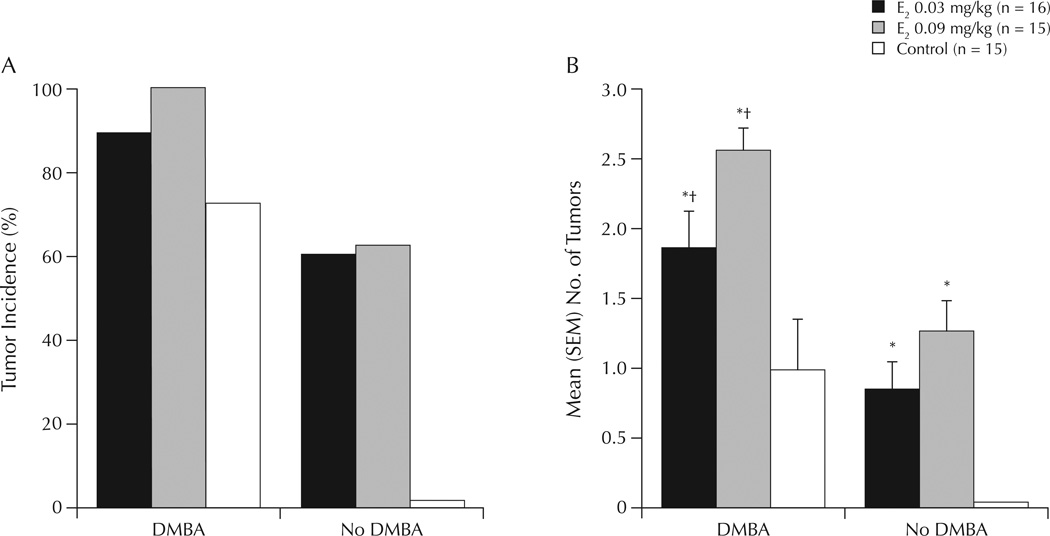
There was a significant main effect of DMBA administration to increase mean numbers of tumors compared with vehicle (F1,40 = 20.38; P < 0.01). DMBA 1.25 mg was associated with significantly increased mean tumor weight compared with vehicle (P < 0.01). Both dosages of E2 were associated with significantly increased mean numbers of tumors compared with vehicle (F2,40 = 8.72; P < 0.01) (Figure 4).
Figure 4.
(A) Incidence of tumors in experimental rats and (B) number of tumors in ovariectomized rats administered the chemical carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) or inactive vehicle (no DMBA), followed by weekly priming with estradiol (E2) or inactive vehicle (control). *P ≤ 0.01 versus control; †P ≤ 0.05 versus no DMBA.
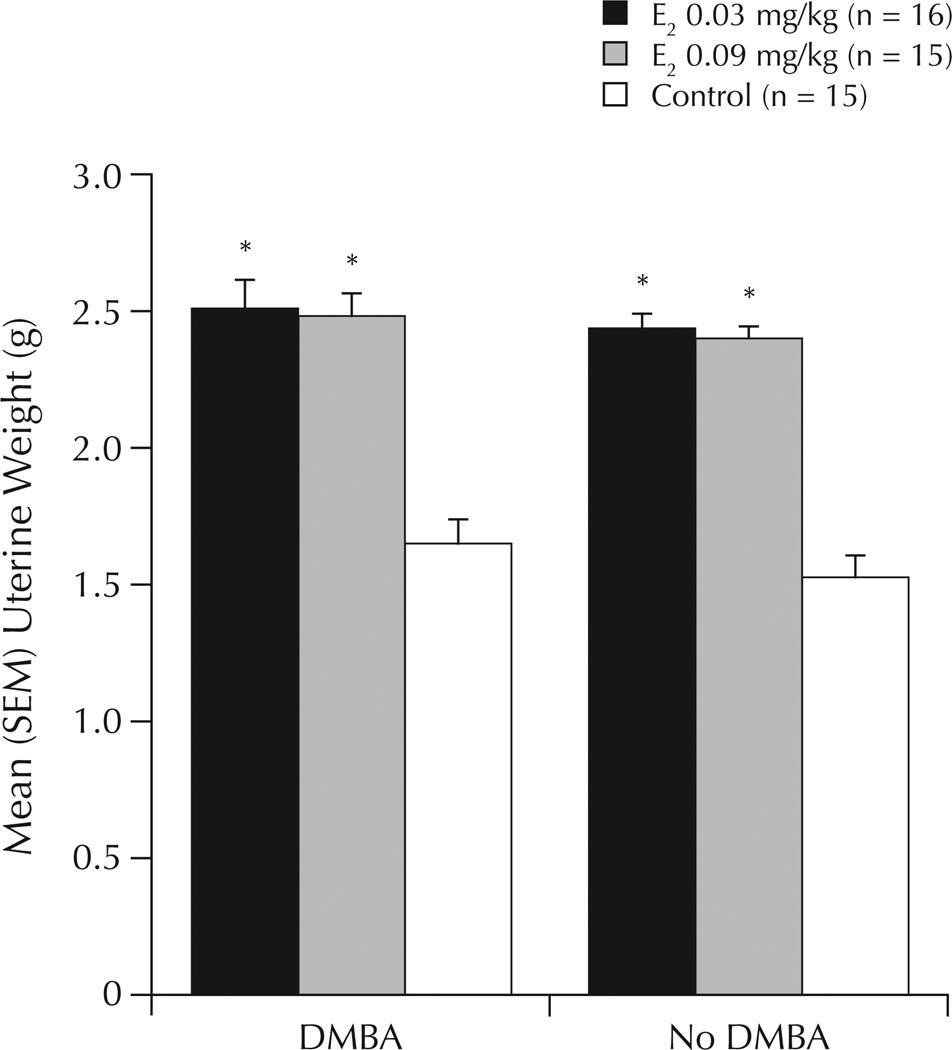
There was a significant main effect of E2 administration to increase uterine weight (F1,40 = 5.40; P < 0.01) (Figure 5). Compared with vehicle, E2 0.03 and 0.09 mg/kg were associated with significantly increased uterine weights (both, by ~40%; P < 0.01).
Figure 5.
Uterine weight in ovariectomized rats administered the chemical carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) or inactive vehicle (no DMBA), followed by weekly priming with estradiol (E2) or inactive vehicle (control). *P ≤ 0.01 versus control.
DISCUSSION
The findings from the present study supported our a priori hypothesis that E2 would have dose-dependent effects on anxiety and depressive behaviors and trophic effects in the periphery in OVX rats. Administration of a moderate dose of E2 (0.09 mg/kg) was associated with significantly decreased anxiety-like behavior, characterized by more time spent in the light of the light–dark transition task, and depressive-like behavior, characterized by decreased immobility on the forced swim task, compared with a lower E2 dose (0.03 mg/kg) and inactive vehicle. Both doses of E2 were associated with significantly enhanced sexual behavior (lordosis in response to mounting by a male rat), but there were no significant differences in general motor behavior (movement in activity chamber) compared with vehicle. Compared with no carcinogen exposure, DMBA administration was associated with significantly increased incidences and numbers of tumors, and this effect was augmented by E2 administration. E2 was associated with significantly increased uterine weight, with the 0.09-mg/kg dose being associated with significantly greater physiologic circulating and brain concentrations of E2 compared with the lower dose (0.03 mg/kg) and vehicle, akin to levels in intact, naturally receptive rats. Together, these data suggest that E2 has dose-dependent beneficial effects on anxiety and depressive behavior in female rodents, but these same regimens can have detrimental trophic effects in the periphery.
The current findings are consistent with previously published data on the effects of physiologic dosing of E2 for affective behavior in rodents. In support, proestrous rats, which are naturally sexually receptive and have high physiological E2 levels, have increased antianxiety-like and antidepressive-like behavior compared with rats with lower E2 levels.46,49 In previously published studies, rats administered an E2 regimen (5–10 µg) consistent with physiologic E2 concentrations (similar to that observed in naturally receptive rats) had significantly increased antianxiety and antidepressive behaviors.38–40 In contrast, 2 studies found that low (<5 µg) or high (20–50 µg) doses of E2, or a regimen that would not be expected to significantly increase circulating E2 concentrations at test time to levels observed in naturally receptive rats, generally had no or weak effects to increase antianxiety and antidepressive behavior.34,40 The findings from the present study extend those findings by suggesting that dose-dependent effects of E2 may be specific to affective processes. The present study found that both doses of E2 were associated with significantly increased sexual receptivity in female rats and uterine weight. However, only the dose of E2 that was associated with physiologic E2 concentrations in plasma and brain regions that may modulate the effects of E2 for anxiety and depression (0.09 mg/kg) was associated with significantly increased antianxiety and antidepressive behaviors. Together, these data suggest that E2 regimens consistent with E2 concentrations in naturally receptive/proestrous rats increases antianxiety-like and antidepressive-like behaviors in OVX rats.
The current findings are consistent with those from previously published reports on the effects of E2 on tumorigenic processes in animal models, described as follows. In a study in OVX Noble rats, long-term (13 weeks) treatment with high-dose E2 benzoate (polymeric silicone capsule filled with crystalline E2 benzoate) administered by subcutaneous implantation was associated with mammary gland proliferation at 13 weeks after treatment initiation.50 In that study, palpable tumors first appeared 5 months following the initiation of E2 treatment.50 A study in our laboratory at the University at Albany found significantly increased incidences of tumors at 6 to 8 months of E2 exposure (polymeric silicone capsules filled with crystalline E2) compared with 2 months of exposure or no exposure in OVX rats.42 The present study found that E2 administration once weekly for 14 weeks increased tumor burden (incidence, number) in OVX rats, and that DMBA-induced tumor incidence and number were increased in rats administered E2. Previously published studies in adult female Noble and Sprague-Dawley rats found that DMBA exposure was associated with mammary tumors that were hormone dependent. 51–53 DMBA exposure is typically used in animals to model mammary and ovarian cancers. 53,54 Together, these data support that E2 has trophic effects in animal models of hormone-dependent cancer.
The current findings extend previously reported effects described in the preceding paragraph by concurrently investigating behavioral and tumorigenic processes in a whole-animal model. Although other studies38–40,46–53 have separately investigated the effects of E2 on psychological symptoms and trophic effects, it is important to examine both of these processes. The expression of steroid receptors in the mammary glands of women with breast cancer may be associated with psychological symptoms.55 Studies are investigating the estrogen receptor (ER) and other steroid receptor–mediated mechanisms of these effects.37 Determining the mechanisms of action of E2 in its effects on affective behaviors has great clinical significance. Increasing life expectancy in women, together with a relatively constant age at onset of menopause, has resulted in low or at-nadir endogenous E2 concentrations for one third to half of life in some women. Thus, it is likely that more women will use E2-based therapies to relieve some symptoms, including those neuropsychiatric in nature, associated with E2 decline.56 An important topic of investigation of the therapeutic efficacy of E2 is related to the differential distribution of ERα and ERβ. It is clear that E2 can have trophic effects, and this is most evident in ERα-containing cells in mammary and uterine tissue.57 Importantly, ERβ is more widely distributed in the limbic regions of the brain, such as the hippocampus,58 which may account for some of the beneficial effects associated with this receptor subtype. In our laboratory and others, the functional effects of E2 via actions at ERβ on anxiety behavior34,37,44,59–62 and ERα on sexual behavior62,63 have been determined. E2 might also alter the formation of new neurons in the hippocampus64 or alter spine density,65 thereby enhancing central nervous system plasticity. Thus, it is crucial to discern the receptor mechanisms important in the beneficial versus unwanted proliferative effects of E2.
CONCLUSIONS
The present data suggest that E2 significantly decreased anxiety-like and depressive-like behaviors in female OVX rats in a dose-dependent manner. E2 was also associated with significantly increased physiologic circulating and brain concentrations of E2. The specificity of these effects for anxiety-like and depressive-like behavior was apparent in that the effects occurred with little evidence of dose dependency on sexual and general motor behaviors. Exposure to a chemical carcinogen was associated with significantly increased incidences and numbers of tumors compared with no carcinogen exposure, and E2 at both doses significantly enhanced these effects.
ACKNOWLEDGMENT
This research was supported, in part, by grants from the Department of Defense Congressionally Directed Medical Research Program–Breast Cancer Research Program, National Institute of Mental Health, and the National Science Foundation.
This manuscript was prepared solely by the authors. Assistance, provided by Carolyn Koonce and Danielle Osborne for surgeries, E2-priming, behavioral testing, and tissue collection, is greatly appreciated.
Footnotes
The authors have no other financial support for these studies to disclose.
REFERENCES
- 1.Miniño AM, Heron MP, Murphy SL, et al. for the Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System. Deaths: Final data for 2004. Natl Vital Stat Rep. 2007;55:1–119. [PubMed] [Google Scholar]
- 2.Henriques A, Dickson N. Menopause: The Woman’s View. 2nd rev ed. London, United Kingdom: Quartet Books Ltd; 1992. [Google Scholar]
- 3.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, et al. for the Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Sherwin BB. The critical period hypothesis: Can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 6.Shumaker SA, Legault C, Rapp SR, et al. for the WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 7.Clemons M, Goss P. Estrogen and the risk of breast cancer [published correction appears in N Engl J Med. 2001;344:1804] N Engl J Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 8.Hulka BS. Epidemiology of susceptibility to breast cancer. Prog Clin Biol Res. 1996;395:159–174. [PubMed] [Google Scholar]
- 9.Lambe M, Hsieh CC, Chan HW, et al. Parity, age at first and last birth, and risk of breast cancer: A population-based study in Sweden. Breast Cancer Res Treat. 1996;38:305–311. doi: 10.1007/BF01806150. [DOI] [PubMed] [Google Scholar]
- 10.Madigan MP, Ziegler RG, Benichou J, et al. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87:1681–1685. doi: 10.1093/jnci/87.22.1681. [DOI] [PubMed] [Google Scholar]
- 11.Ramon JM, Escriba JM, Casas I, et al. Age at first full-term pregnancy, lactation and parity and risk of breast cancer: A case-control study in Spain. Eur J Epidemiol. 1996;12:449–453. doi: 10.1007/BF00143995. [DOI] [PubMed] [Google Scholar]
- 12.Thomas HV, Key TJ, Allen DS, et al. A prospective study of endogenous serum hormone concentrations and breast cancer risk in post-menopausal women on the island of Guernsey. Br J Cancer. 1997;76:401–405. doi: 10.1038/bjc.1997.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cauley JA, Lucas FL, Kuller LH, et al. for the Study of Osteoporotic Fractures Research Group. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Ann Intern Med. 1999;130:270–277. doi: 10.7326/0003-4819-130-4_part_1-199902160-00004. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson SE, Willett WC, Manson JE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 15.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87:190–197. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 16.Meijer WJ, van Lindert AC. Prophylactic oophorectomy. Eur J Obstet Gynecol Reprod Biol. 1992;47:59–65. doi: 10.1016/0028-2243(92)90215-k. [DOI] [PubMed] [Google Scholar]
- 17.Schairer C, Persson I, Falkeborn M, et al. Breast cancer risk associated with gynecologic surgery and indications for such surgery. Int J Cancer. 1997;70:150–154. doi: 10.1002/(sici)1097-0215(19970117)70:2<150::aid-ijc2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 19.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer [published correction appears in Lancet. 1997;350:1484] Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 20.Magnusson C, Baron JA, Correia N, et al. Breast-cancer risk following long-term oestrogen- and oestrogen-progestin-replacement therapy. Int J Cancer. 1999;81:339–344. doi: 10.1002/(sici)1097-0215(19990505)81:3<339::aid-ijc5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Seeman MV. Psychopathology in women and men: Focus on female hormones. Am J Psychiatry. 1997;154:1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- 22.Harsh V, Schmidt PJ, Rubinow DR. The menopause transition: The next neuroendocrine frontier. Expert Rev Neurother. 2007;7(Suppl 11):S7–S10. doi: 10.1586/14737175.7.11s.S7. [DOI] [PubMed] [Google Scholar]
- 23.Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 24.Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: The lessons of premenstrual syndrome. Front Neuroendocrinol. 2006;27:210–216. doi: 10.1016/j.yfrne.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Bebbington P, Hurry J, Tennant C, et al. Epidemiology of mental disorders in Camberwell. Psychol Med. 1981;11:561–579. doi: 10.1017/s0033291700052879. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins R. Sex differences in depression. Br J Hosp Med. 1987;38:485–486. [PubMed] [Google Scholar]
- 27.Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch Gen Psychiatry. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- 28.Wittchen HU, Hoyer J. Generalized anxiety disorder: Nature and course. J Clin Psychiatry. 2001;62(Suppl 11):15–19. discussion 20–21. [PubMed] [Google Scholar]
- 29.Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: A preliminary report. Am J Obstet Gynecol. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 30.Almeida OP, Lautenschlager N, Vasikaram S, et al. Association between physiological serum concentration of estrogen and the mental health of community-dwelling postmenopausal women age 70 years and over. Am J Geriatr Psychiatry. 2005;13:142–149. doi: 10.1176/appi.ajgp.13.2.142. [DOI] [PubMed] [Google Scholar]
- 31.Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: A preliminary report. Am J Psychiatry. 2003;160:1519–1522. doi: 10.1176/appi.ajp.160.8.1519. [DOI] [PubMed] [Google Scholar]
- 32.Ditkoff EC, Crary WG, Cristo M, Lobo RA. Estrogen improves psychological function in asymptomatic postmenopausal women. Obstet Gynecol. 1991;78:991–995. [PubMed] [Google Scholar]
- 33.Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–343. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- 34.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vesco KK, Haney EM, Humphrey L, et al. Influence of menopause on mood: A systematic review of cohort studies. Climacteric. 2007;10:448–465. doi: 10.1080/13697130701611267. [DOI] [PubMed] [Google Scholar]
- 36.Voytko ML, Tinkler GP. Cognitive function and its neural mechanisms in nonhuman primate models of aging, Alzheimer disease, and menopause. Front Biosci. 2004;9:1899–1914. doi: 10.2741/1370. [DOI] [PubMed] [Google Scholar]
- 37.Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- 39.Nomikos GG, Spyraki C. Influence of oestrogen on spontaneous and diazepam-induced exploration of rats in an elevated plus maze. Neuropharmacology. 1988;27:691–696. doi: 10.1016/0028-3908(88)90077-9. [DOI] [PubMed] [Google Scholar]
- 40.Slater J, Blizard DA. A reevaluation of the relation between estrogen and emotionality in female rats. J Comp Physiol Psychol. 1976;90:755–764. doi: 10.1037/h0077248. [DOI] [PubMed] [Google Scholar]
- 41.Guide for the Care and Use of Laboratory Animals. Bethesda, Md: Offices of Science and Health Reports, DRR/National Institutes of Health; 1985. DHEW Publication 80-23. [Google Scholar]
- 42.Walf AA, Frye CA. Estradiol enhances sociosexual behavior and augments carcinogen-induced tumorigenesis in ovariectomized rats. Age. doi: 10.1007/s11357-008-9079-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3alpha,5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- 44.Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 45.Hardy DF, Debold JF. Effects of mounts without intromission upon the behavior of female rats during the onset of estrogen-induced heat. Physiol Behav. 1971;7:643–645. doi: 10.1016/0031-9384(71)90120-x. [DOI] [PubMed] [Google Scholar]
- 46.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 47.Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3alpha, 5alpha-THP and 3alpha-Diol. J Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- 48.Rodbard D, Hutt DM. Symposium on Radioimmunoassay and Related Procedures in Medicine. New York, NY: Uniput; 1974. Statistical analysis of radioimmunoassay and immunoradiometric assays: A generalized, weighted iterative, least squares method for logistic curve fitting. In International Atomic Energy Agency, ed; pp. 209–233. [Google Scholar]
- 49.Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- 50.Leung G, Tsao SW, Wong YC. Sex hormone-induced mammary carcinogenesis in female Noble rats: Detection of differentially expressed genes. Breast Cancer Res Treat. 2003;77:49–63. doi: 10.1023/a:1021123914339. [DOI] [PubMed] [Google Scholar]
- 51.Cheung SY, Yuen MT, Choi HL, et al. An expression study of hormone receptors in spontaneously developed, carcinogen-induced and hormone-induced mammary tumors in female Noble rats. Int J Oncol. 2003;22:1383–1395. [PubMed] [Google Scholar]
- 52.Russo IH, Koszalka M, Gimotty PA, Russo J. Protective effect of chorionic gonadotropin on DMBA-induced mammary carcinogenesis. Br J Cancer. 1990;62:243–247. doi: 10.1038/bjc.1990.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russo J, Russo IH. Experimentally induced mammary tumors in rats. Breast Cancer Res Treat. 1996;39:7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- 54.Stewart SL, Querec TD, Ochman AR, et al. Characterization of a carcinogenesis rat model of ovarian preneoplasia and neoplasia. Cancer Res. 2004;64:8177–8183. doi: 10.1158/0008-5472.CAN-04-1702. [DOI] [PubMed] [Google Scholar]
- 55.Razavi D, Farvacques C, Delvaux N, et al. Psychosocial correlates of oestrogen and progesterone receptors in breast cancer. Lancet. 1990;335:931–933. doi: 10.1016/0140-6736(90)90996-i. [DOI] [PubMed] [Google Scholar]
- 56.Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: Estrogenic compounds with selective properties—the next generation of therapeutics. Endocrine. 2005;28:235–242. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- 57.Gustafsson JA. What pharmacologists can learn from recent advances in estrogen signalling. Trends Pharmacol Sci. 2003;24:479–485. doi: 10.1016/S0165-6147(03)00229-3. [DOI] [PubMed] [Google Scholar]
- 58.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 59.Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Krezel W, Dupont S, Krust A, et al. Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rocha BA, Fleischer R, Schaeffer JM, et al. 17 Betaestradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology (Berl) 2005;179:637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- 62.Walf AA, Ciriza I, Garcia-Segura LM, Frye CA. Antisense oligodeoxynucleotides for estrogen receptor-beta and alpha attenuate estradiol’s modulation of affective and sexual behavior, respectively. Neuropsychopharmacology. 2008;33:431–440. doi: 10.1038/sj.npp.1301416. [DOI] [PubMed] [Google Scholar]
- 63.Mazzucco CA, Walker HA, Pawluski JL, et al. ERalpha, but not ERbeta, mediates the expression of sexual behavior in the female rat. Behav Brain Res. 2008;191:111–117. doi: 10.1016/j.bbr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 65.Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]