Abstract
The decision aid called “Adjuvant Online” (Adjuvant! for short) helps breast cancer patients make treatment decisions by providing numerical estimates of treatment efficacy (e.g., 10-y relapse or survival). Studies exploring how patients’ numeracy interacts with the estimates provided by Adjuvant! are lacking. Pooling across 2 studies totaling 105 women with estrogen receptor–positive, early-stage breast cancer, the authors explored patients’ treatment expectations, perceived benefit from treatments, and confidence of personal benefit from treatments. Patients who were more numerate were more likely to provide estimates of cancer-free survival that matched the estimates provided by Adjuvant! for each treatment option compared with patients with lower numeracy (odds ratios of 1.6 to 2.4). As estimates of treatment efficacy provided by Adjuvant! increased, so did patients’ estimates of cancer-free survival (0.37 > rs > 0.48) and their perceptions of treatment benefit from hormonal therapy (rs = 0.28) and combined therapy (rs = 0.27). These relationships were significantly more pronounced for those with higher numeracy, especially for perceived benefit of combined therapy. Results suggest that numeracy influences a patient’s ability to interpret numerical estimates of treatment efficacy from decision aids such as Adjuvant!.
Keywords: patient decision making, risk communication or risk perception, numeracy, breast cancer/mammography, health literacy
Many women with early-stage, estrogen receptor–positive (ER+) breast cancers will need to decide whether to undergo adjuvant therapy to prevent cancer recurrence and which form of treatment, if any, to choose: hormonal therapy, chemotherapy, or both. They will need to know treatment risk/benefit tradeoffs to derive an accurate understanding of treatment expectations1–4; this responsibility often falls to the oncologist. To this end, oncologists often share data from decision aids with patients to help facilitate clinical discussions about breast cancer adjuvant therapy.5–10 A decision aid called Adjuvant! is used frequently in practice; it provides numerical estimates, accompanied by graphical displays, of being cancer free in 10 y under 4 treatment options: no further treatment, chemotherapy only, hormonal therapy only, and combined chemotherapy and hormonal therapy (http://www.adjuvantonline.com).6 Adjuvant! has been shown to affect the proportion of women desiring adjuvant therapy.9,10 For example, Siminoff and colleagues10 found that among breast cancer patients with stage I to III disease (N = 405), patients who received an Adjuvant! Decision Guide were less likely to choose adjuvant therapy compared with patients who received an educational pamphlet on adjuvant treatments.
Very little data exist as to how patients with varying numeracy skills (i.e., facility with understanding and applying mathematical concepts11) extract and assign degree of benefit to numerical estimates provided by Adjuvant!. Indeed, many breast cancer patients overestimate the benefit of treatment.12–14 Individual differences in numeracy may account partially for why misunderstandings occur. For example, Zikmund-Fisher and colleagues15 enlisted the aid of women aged 40 to 74 y as part of a Web-based study to explore how different graphical displays (e.g., pictographs) of conveying data from Adjuvant! interacted with a measure of subjective numeracy16 to affect comprehension. Although no interactions emerged between graphical format and subjective numeracy, those with higher subjective numeracy scores had better comprehension of the benefit of adding chemotherapy to hormonal therapy than those participants with lower numeracy. Whether their results generalize to the context of actual treatment decisions that involve breast cancer patients and the use of an objective numeracy measure is unknown. The main focus of this report is to ascertain how early-stage breast cancer patients’ objective numeracy skills influence their ability to extract statistical data on the likelihood of cancer-free survival based on different treatment options and how they apply this information to affect their perceptions of personal treatment benefit and treatment decision.
METHODS
Study Participants and Recruitment
This report is based on data from 2 pilot studies exploring how breast cancer patients understand treatment expectations using Adjuvant! to inform treatment decisions—called pilot study 1 and pilot study 2, respectively. For both studies, women who received oncologic care at Duke University Medical Center after definitive surgery for removal of early breast cancer and who were pathologically staged (T1-T3, N0–1 [< 9 + LN]) were eligible to participate. Patients were identified before or at their initial clinic visit to discuss adjuvant therapy, sent a letter signed by their oncologist describing the study, and then called to assess interest in participation. If we were unable to reach a patient before the visit, she was approached in the waiting room about participation by either a clinical study oncology nurse or the oncologist. After explaining the purpose of each study, patients who gave written consent took part in the study procedures (discussed below). Participants were paid $30 (pilot 1) or $40 (pilot 2) for their participation. For both studies, final treatment decisions up to 1 y after initial clinic discussions were obtained based on medical chart audits. For 17 patients who were seen at Duke only once, final treatment delivered was unknown.
Pilot 1 Procedure
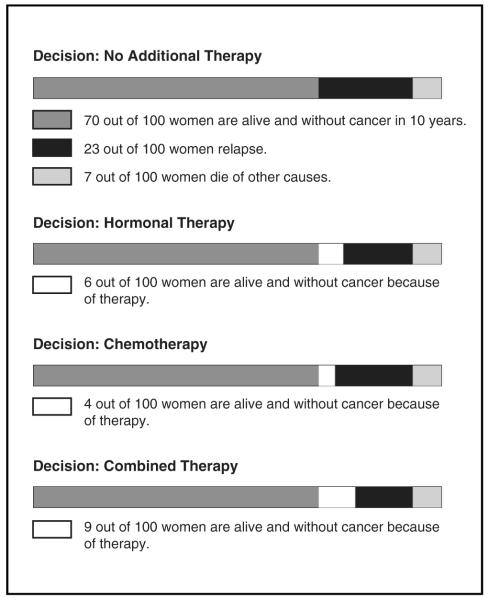
The study involved patients completing a survey before seeing their oncologist (preclinic survey), then having discussions of adjuvant therapy with their oncologist, followed by completing a 2nd survey (postclinic survey) immediately after the discussion. Oncologists presented patients with their chances of being cancer free during the next 10 y using the printout accompanying Adjuvant! (see Figure 1). As shown in Figure 1, specific for this patient with ER+ disease, the estimates of being cancer free during the next 10 y without treatment, with hormonal therapy only, chemotherapy only, and hormonal plus chemotherapy are 70, 76, 74, and 79 out of 100, respectively. For example, the patient would be told that without further treatment after surgery, we would expect 70 women out of 100 women like her to be cancer free in 10 y.
Figure 1.
Example of Adjuvant! printout showing cancer-free survival under different treatment options.
All patients were given the printout to help, as needed, answer questions on the postclinic survey. The completed survey was sealed in a manila envelope and collected by the oncology nurse; patients were told their physician would not see their responses. Because the nurse did not review the survey prior to its being placed in the envelope, several questions were left blank, reducing the sample size for some analyses. Seventy-three patients consented, of which 60 had ER+ disease.
Pilot 2 Procedure
This pilot examined how providing patients a videotape discussing adjuvant treatment influenced treatment expectations. After following the recruitment and consenting procedures discussed previously, patients on the day of their clinic visit were randomized to watch a video or not. The video, reviewed by patients in the waiting room prior to seeing their oncologist, was a decision aid developed by the Foundation for Informed Medical Decision Making titled “Early Breast Cancer: Hormone Therapy and Chemotherapy—Are They Right for You?” (version BCA001 v.03). It covered adjuvant treatment options and when a treatment might be more effective, and it provided narratives of women discussing why they selected a treatment. All patients completed a preclinic survey, then discussed adjuvant treatment with their oncologist, and then completed the postclinic survey, which included a printout of Adjuvant!. Sixty-one of the 89 patients (68%) approached consented to participatea; we obtained complete data on 59 (1 patient did not complete the postclinic survey, and for 1 patient, the Adjuvant! Web site was unavailable). Among these patients, 45 had ER+ disease. Of these, 20 watched the video and 25 did not.
Preclinic Measures
Patients in both pilot studies completed an 11-item numeracy scale11 that assesses the ability to convert percentages to proportions, proportions to percentages, and probabilities to proportions and to perform tasks on risk magnitudes and proportions. Items left blank and incorrect answers were scored 0; correct answers were scored as 1.
Postclinic Measures
The postclinic survey assessed patients’ treatment expectations and evaluations of the numerical information. The measures common to both pilots are described below.
Factual knowledge of treatment benefit
Patients chose which option afforded them the best chance of remaining cancer free in the next 10 y: 1) have no additional treatment, 2) take some form of hormonal therapy only, 3) take some form of chemotherapy only, and 4) take some form of hormonal therapy with chemotherapy (combined therapy). The correct answer was always combined therapy.
Expectations of treatment benefit
This was assessed 3 ways. First, patients were asked what their chance was out of 100 of being cancer free in the next 10 y if they 1) had no additional treatment, 2) took some form of hormonal therapy only, 3) took some form of chemotherapy only, and 4) took some form of hormonal therapy with chemotherapy. Second, they were asked, as it applied to them only, the degree of benefit they would receive by taking hormonal therapy or chemotherapy only as well as combined therapy (has no benefit, has little benefit, has a moderate amount of benefit, and has a lot of benefit). This was followed by asking patients how confident they felt they would be one of the women who would benefit from taking each of the 3 treatment options (not at all confident, slightly confident, somewhat confident, very confident, and completely confident).
Statistical Methods
Tests of association were conducted via Spearman correlations and χ2 tests. Mean comparisons between 2 groups were tested via 2-group independent t-tests; mean differences between more than 2 groups were tested by analysis of variance followed by tests of simple effects (i.e., Tukey). To test the main effect of numeracy, estimates from Adjuvant! and their interaction on patients’ estimates of cancer-free survival (scored 0–100), perceived treatment benefit (scored 1–4), and confidence that one would benefit from treatment (scored 1–5), we used least squares regression analyses. We further examined which variables predicted choosing combined therapy. We postulated that patients would be more likely to choose combined therapy if they 1) accurately identified combined therapy as the treatment most likely to maximize cancer-free survival by Adjuvant! statistical estimates, 2) provided higher estimates of benefit (0–100), 3) perceived greater treatment benefit, 4) felt more confident in being one of the women who would benefit from treatment, and 5) were more numerate. We also explored the influence of age, race, and education on this decision. To test these associations, we ran logistic regression models examining each of these 8 variables separately while controlling for actual degree of benefit based on Adjuvant! estimates and study design.
To account for variability due to difference in study designs, in all regression models we created and entered a 3-level dummy variable such that one group represented patients who could not be randomized to view the video (pilot 1 participants), a 2nd group who could be randomized but did not watch the video, and a 3rd group who could be randomized and viewed the video (the latter 2 groups were from the 2nd pilot). Two patients did not complete the numeracy scale; analyses involving numeracy excluded these patients. Patients in the top and bottom quartile of the distribution of numeracy scores were grouped as high and low numeracy groups, respectively; the remaining patients constituted the middle numeracy group.
RESULTS
Sample Characteristics
One-hundred five patients with ER+ disease completed a preclinic and postclinic survey. Demographic characteristics, clinical parameters, and distributions of numeracy scores for each sample and their combination are presented in Table 1. Compared with other study samples testing patient understanding and use of Adjuvant!, our sample has comparable racial distributions, is slightly younger, and is more educated.9,10,17 There were no statistically significant differences between the 2 samples on any demographic and clinical parameters, including comparisons between participants who watched the video or not.
Table 1.
Characteristics of Participants
| Variable | Sample 1 |
Control | Sample 2 Video |
Total | Combined Sample |
|---|---|---|---|---|---|
| Age, y | |||||
| 55.2 | 55.5 | 58.9 | 57.0 | 56.0 | |
| s | 10.3 | 13.3 | 11.0 | 12.3 | 11.2 |
| Race (%) | |||||
| Caucasian | 63.3 | 72.0 | 80.0 | 75.6 | 68.6 |
| African | 13.3 | 28.0 | 10.0 | 20.0 | 16.2 |
| American | |||||
| Asian | 0.0 | 0.0 | 5.0 | 2.2 | 0.9 |
| Other | 13.3 | 0.0 | 5.0 | 2.2 | 8.6 |
| Unknown | 10.0 | 0.0 | 0.0 | 0.0 | 5.7 |
| Education (%) | |||||
| High school graduate or less |
15.0 | 28.0 | 30.0 | 28.9 | 21.0 |
| Technical/trade | 3.3 | 0.0 | 0.0 | 0.0 | 1.9 |
| Some college | 26.7 | 12.0 | 20.0 | 15.6 | 21.9 |
| College graduate | 23.3 | 36.0 | 40.0 | 37.8 | 29.5 |
| Some graduate education |
0.0 | 8.0 | 0.0 | 4.4 | 1.9 |
| Postgraduate | 21.7 | 16.0 | 10.0 | 13.0 | 18.1 |
| Unknown | 10.0 | 0.0 | 0.0 | 0.0 | 5.7 |
| Numeracy scores | |||||
| Scale mean | 7.4 | 7.0 | 6.5 | 6.8 | 7.2 |
| s | 2.6 | 2.5 | 2.1 | 2.3 | 2.5 |
| Upper quartile | 10.0 | 9.0 | 8.0 | 8.0 | 9.0 |
| Median | 8.0 | 7.0 | 7.0 | 7.0 | 8.0 |
| Lower quartiles | 5.0 | 5.0 | 4.0 | 5.0 | 5.0 |
| Tumor grade (%) | |||||
| 1 | 35.0 | 32.0 | 35.0 | 33.3 | 34.3 |
| 2 | 48.3 | 64.0 | 40.0 | 53.3 | 50.5 |
| 3 | 13.3 | 4.0 | 15.0 | 8.9 | 11.4 |
| Undefined | 3.3 | 0.0 | 10.0 | 4.4 | 3.8 |
| Tumor size (%) | |||||
| 0.1 to 1.0 | 23.3 | 24.0 | 15.0 | 20.0 | 21.9 |
| 1.1 to 2.0 | 43.3 | 52.0 | 50.0 | 51.1 | 46.7 |
| 2.1 to 3.0 | 21.7 | 16.0 | 25.0 | 20.0 | 21.0 |
| 3.1 to 5.0 | 10.0 | 8.0 | 10.0 | 8.9 | 9.5 |
| >5.0 | 1.7 | 0.0 | 0.0 | 0.0 | 1.0 |
| Nodes (%) | |||||
| 0 | 78.3 | 76.0 | 70.0 | 73.3 | 76.2 |
| 1 to 3 | 21.7 | 20.0 | 30.0 | 24.4 | 22.9 |
| 4 to 9 | 0.0 | 4.0 | 0.0 | 2.2 | 0.9 |
Note: Numbers have been rounded
The mean numeracy of 7.2 fell within the lower range found in other studies with adults (range 7.0–8.6; Zikmund-Fisher, personal communication, 3 June 2009).18,19 Numeracy scores differed by race. African Americans had lower numeracy scores compared with Caucasians or Asians (; P < 0.05 for each comparison); the latter 2 groups did not differ. Patients with at least some college, including a trade/technical school education, were more numerate than patients with a high school education or less ( v. 6.4, t(101) = 2.0 P = 0.044). Numeracy scores decreased with advancing age (rs(103)= −0.28, P = 0.004).
Factual Knowledge of Treatment Expectations
Sixty-six percent of patients correctly indicated that combined therapy maximized cancer-free survival. More numerate patients were significantly more likely to identify combined therapy as yielding the highest chance of cancer-free survival (n = 101, Wald’s χ2 = 12.1, P = 0.007; odds ratio [OR] = 1.36; 95% confidence interval [CI] = 1.12, 1.64). Overall, 78%, 72%, and 48% of patients in the high, middle, and low numeracy groups, respectively, identified combined therapy as yielding the highest chance of cancer free survival.
Relationship between Adjuvant! Estimates and Self-Estimates of Cancer-Free Survival
The mean estimates provided by Adjuvant! for being cancer free under the treatment and no-treatment options were compared with patient estimates (see Table 2). Compared with estimates provided by Adjuvant!, patients significantly underestimated their chances of remaining cancer free, regardless of treatment option (Ps < 0.006). Nonetheless, correlations between Adjuvant! and patient estimates across treatments were positive. Overall, 43%, 17%, 17%, and 19% of participants gave the exact estimate as Adjuvant! for no further treatment, hormonal therapy, chemotherapy, and combined therapy, respectively. Patients with higher numeracy scores were more likely to give personal estimates that matched the estimates from Adjuvant! for hormonal therapy (OR = 2.4, 95% CI = 1.5, 4.0; P = 0.0004; among those with an exact match, 83%, 17%, and 0% of patients were in the high, middle, and low numeracy groups, respectively), chemotherapy (OR = 1.7, 95% CI = 1.2, 2.4; P = 0.002; among those with an exact match, 78%, 17%, and 5% were patients in the high, middle, and low numeracy groups, respectively), and combined therapy (OR = 1.6, 95% CI = 1.2, 2.2; P = 0.002; among those with an exact match, 70%, 25%, and 5% of patients were in the high, middle, and low numeracy groups, respectively), but not with estimates of no further treatment (OR = 1.1, 95% CI = 1.2, 0.9; P = 0.178). Numeracy was unrelated to mean differences between Adjuvant! and self-estimates of benefit.
Table 2.
Mean Estimates of Being Cancer Free in the Next 10 Y under Different Treatment Options as Provided by Adjuvant! and Patients’ Estimates for Self
| Treatment | Adjuvant Estimate |
Range | Additional Benefit |
Self-Estimates out of 100 |
Combined Samples |
Correlation between Self and Adjuvant Estimate |
|||
|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Video | Control | Sample 2 | ||||||
| No further | 61.3 (16.7) | 10–90 | — | 52.4 (26.9) | 59.3 (22.7) | 54.8 (24.7) | 56.8 (23.6) | 54.3 (25.6) | 0.48; P = 0.0001 |
| Chemotherapy | 69.8 (15.2) | 12–92 | 8.4 (6.6) | 43.5 (35.1) | 50.6 (35.7) | 46.3 (39.3) | 48.2 (37.4) | 45.5 (36.0) | 0.37; P = 0.0001 |
| Hormonal | 72.0 (14.5) | 14–92 | 10.7 (5.3) | 46.9 (35.1) | 56.2 (35.0) | 48.4 (35.3) | 52.0 (35.0) | 49.0 (35.0) | 0.39; P = 0.0001 |
| Combined | 77.7 | 15–94 | 16.3 (9.3) | 53.5 (36.1) | 62.2 (36.3) | 53.2 (39.9) | 57.3 (38.1) | 55.1 (36.8) | 0.43; P = 0.0001 |
Note: Sample sizes ranged from 96 to 101. Sample 1 and Sample 2 are total sample means. Numbers in parentheses represent standard deviations. Additional benefit is the additional women alive by taking a specific treatment relative to no further treatment. Estimates for self and others differed significantly from Adjuvant! across all options.
We tested whether patient estimates of cancer-free survival would parallel those of Adjuvant! as numeracy scores increased. The numeracy by Adjuvant! estimate interaction was significant for estimates under no further treatment, F(5, 92) = 8.6, P < 0.0001, adjusted R2 = 28, β = 0.20, P < 0.001 for interaction; chemotherapy only, F(5, 88) = 4.3, P = 0.001, adjusted R2 = 0.15, β = 0.21, P = 0.022 for interaction; hormonal therapy only, F(5, 93) = 3.7, P = 0.004, adjusted R2 = 0.12, β = 0.23, P = 0.019 for interaction; and combined therapy, F(5, 89) = 4.0, P = 0.004, adjusted R2 = 0.14, β = 0.27, P = 0.012 for interaction. Figure 2 displays for combined therapy how expectations of personal estimates of cancer-free survival differ among participants 1 standard deviation above and below the mean for numeracy and at the mean in relation to Adjuvant! estimates—at the mean and at 1 standard deviation above and below the mean. As Adjuvant!’s estimates increased, so did patients’ estimates, especially among participants at and 1 standard deviation above the mean for numeracy; at low levels of numeracy, there was little relation between patients’ estimates and Adjuvant! estimates. The same general pattern held for the other 3 treatment options.
Figure 2.
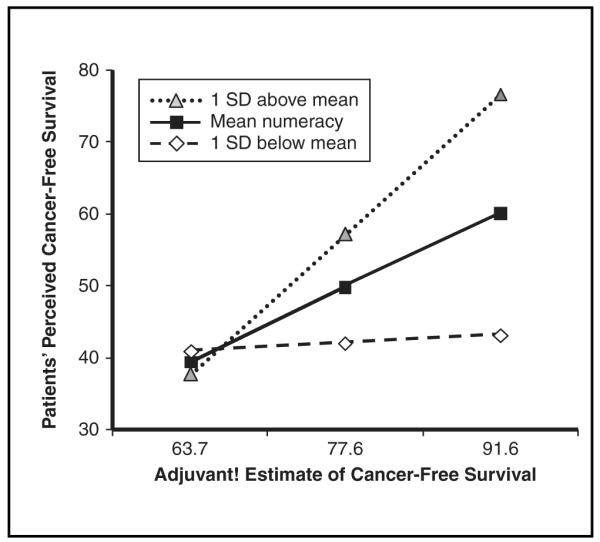
Numeracy by Adjuvant! estimate interaction predicting patients’ estimates of perceptions of cancer-free survival (0 to 100) based on combined hormonal therapy and chemotherapy.
Relationship between Adjuvant! Estimates and Subjective Evaluation of Benefit for Self and Confidence in Benefit for Self
Patients’ ratings of personal treatment benefit and of their confidence that they will personally benefit from treatment are shown in Table 3: Correlations between perceived benefit and confidence ranged from 0.41 to 0.59, Ps < 0.0001. As Adjuvant!’s estimates for being cancer free under hormonal therapy only and combined therapy increased, so did patients’ perceived benefit. No significant correlations were found between the estimates provided by Adjuvant! and participants’ confidence in being one of the women who would personally benefit.
Table 3.
Relationships between Adjuvant! Treatment Estimates and Patients’ Perceived Degree of Treatment Benefit and Confidence That They Would Be the One Who Benefits from Treatment
|
|
Combined Sample |
Correlations with Estimates from Adjuvant |
||||||
|---|---|---|---|---|---|---|---|---|
| Patient Estimate | Sample 1 | Video | Control | Sample 2 | Hormonal | Chemotherapy | Combined Therapy | |
| Degree of benefit | ||||||||
| Hormonal | 3.5 (0.62) | 3.2 (0.55) | 3.4 (0.53) | 3.3 (0.57) | 3.4 (0.60) | 0.28; P = 0.003 | ||
| Chemotherapy | 3.0 (0.92) | 2.5 (0.77) | 3.0 (0.77) | 2.8 (0.79) | 2.9 (0.87) | 0.15; P = 0.113 | ||
| Combined | 3.4 (0.82) | 3.2 (0.86) | 3.8 (0.53) | 3.5 (0.74) | 3.5 (0.79) | 0.27; P = 0.006 | ||
| Confidence in benefit |
||||||||
| Hormonal | 3.8 (0.89) | 3.4 (1.03) | 3.8 (1.02) | 3.5 (1.01) | 3.6 (0.95) | 0.11l P = 0.266 | ||
| Chemotherapy | 3.0 (1.17) | 2.7 (0.90) | 3.0 (1.18) | 2.8 (1.07) | 2.9 (1.13) | 0.01; P = 0.888 | ||
| Combined | 3.4 (1.18) | 3.3 (1.07) | 4.0 (0.95) | 3.7 (1.06) | 3.5 (1.14) | 0.07; P = 0.494 | ||
Note: Sample sizes ranged from 99 to 102. Sample 1 and Sample 2 are total sample means. Numbers in parentheses represent standard deviations. Degree of benefit ranged from 1 (has no benefit) to 4 (has a lot of benefit). Perceived confidence ranged from 1 (not at all confident) to 5 (completely confident)
We tested whether patient estimates of perceived treatment benefit and confidence that they will benefit from treatment would increase as estimates provided by Adjuvant! increased and whether these relationships would be affected by patient numeracy. As shown in Figure 3, numeracy interacted significantly with the estimates provided by Adjuvant!. Those with average or higher numeracy showed the expected relationship between perceived and estimated treatment benefit, whereas those with lower numeracy demonstrated no differences in perceived benefit across different levels of estimated benefit, F(5, 91) = 2.7, P = 0.026, adjusted R2 = 0.08, β = 0.005, P = 0.038 for interaction. The interaction with confidence was not significant but displayed the same pattern (β = 0.005, P = 0.118 for interaction).
Figure 3.
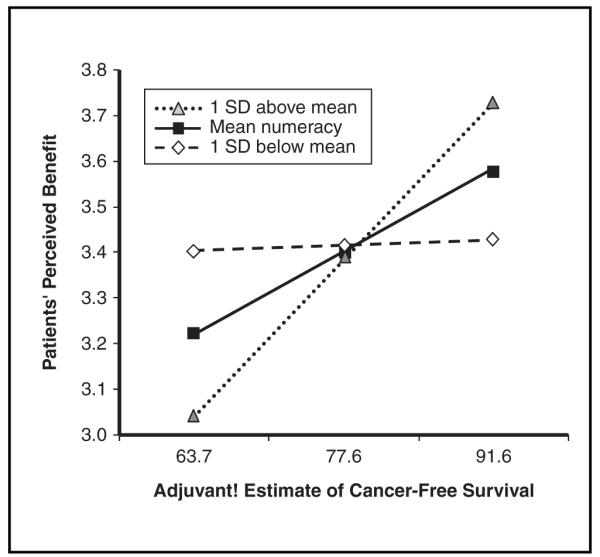
Numeracy by Adjuvant! estimate interaction predicting patients’ estimates of perceived personal benefit of combined hormonal therapy and chemotherapy.
Treatment Decisions
Among the 88 patients with data, 2 opted for no further treatment, 2 opted for chemotherapy only, 1 did not make a decision, 48 chose hormonal therapy only, and 35 chose combined chemotherapy and hormonal therapy. The logistic regression results testing 8 separate predictors of choosing combined therapy are presented in Table 4.
Table 4.
Logistic Regression Results Predicting Selecting Combined Hormonal and Chemotherapy
| Predictor | Odds Ratio |
95% Confidence Interval |
P |
|---|---|---|---|
| Age | 0.83 | 0.76–0.91 | 0.0001 |
| Race | |||
| Caucasian | 1.00 | ||
| African American | 1.17 | 0.34–4.05 | 0.81 |
| Other | 0.78 | 0.19–3.16 | 0.434 |
| Educationa | |||
| High school graduate or less |
1.00 | ||
| Some college or greater | 1.08 | 0.38–3.10 | 0.88 |
| Numeracy | 1.08 | 0.90–1.30 | 0.42 |
| Estimate of treatment benefit |
0.99 | 0.98–1.01 | 0.26 |
| Perceived treatment benefit |
6.45 | 2.25–18.46 | 0.0005 |
| Confidence that one will benefit from treatment |
3.81 | 1.85–7.86 | 0.0003 |
| Correctly identified combined treatment as option with greatest chance of cancer free survival |
81.60 | 9.09–732.55 | 0.0001 |
Some college or greater includes trade school. Analyses control for study design and estimates provided by Adjuvant! of cancer-free survival based on combined therapy.
Patients were more likely to choose combined therapy if they were younger, perceived greater personal treatment benefit, felt more confident in being one of the women who would personally benefit, and accurately picked combined therapy as the treatment that statistically maximized the likelihood of cancer-free survival. Although numeracy was not a significant independent predictor of choices of combined therapy, we explored whether it would interact with each of the 4 aforementioned significant predictors, controlling for actual degree of benefit based on Adjuvant! estimates and study design. Numeracy interacted only with age (OR = 1.05, 95% CI = 1.01, 1.09; P = 0.022). As shown in Figure 4, the predicted probability of choosing combined therapy decreased significantly with increasing age but decreased less among patients with higher numeracy scores compared with patients with average or lower numeracy scores.
Figure 4.
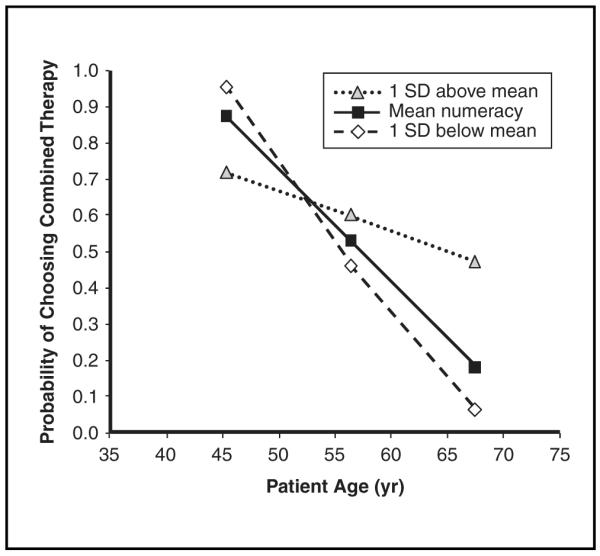
Numeracy by age interaction predicting probability of selecting combined hormonal therapy and chemotherapy.
DISCUSSION
We found, consistent with other reports,16 that most participants did not provide a personal estimate of cancer-free survival that matched the estimates provided by Adjuvant!. However, matches were more likely among patients who were more numerate. Furthermore, the outcomes of perceived cancer-free survival and perceived treatment benefit were affected by the interaction between numeracy and the estimates provided by Adjuvant! As Adjuvant! cancer-free survival scores increased, more numerate patients provided higher estimates of cancer-free survival and perceived greater personal treatment benefit, especially for combined therapy, but those with lower numeracy did not. As such, this study goes beyond the study of comprehension to demonstrate a lack of sensitivity in benefit ratings to different numeric levels of risk in the less numerate whereas the highly numerate demonstrated the expected sensitivity.20
Disconcertingly, 30% to 40% of women did not identify correctly the treatment that statistically maximized cancer-free survival. Patients with higher numeracy scores more often identified which treatment maximized cancer-free survival. Although this effect may be attributable to numeracy, some patients logically may have thought that 2 treatments, chemotherapy and hormonal therapy, are better than 1. Factual understanding of the latter was significantly related to whether they chose combined therapy. Although numeracy did not directly influence treatment choice, it may have had a pronounced effect in this process by helping women extract meaning from numerical estimates that they applied later to treatment selection. The same holds true of the relationship between perceived treatment benefit and treatment selection. Women who perceived greater benefit of combined therapy were also more likely to select this option.
Based on these findings, one may conclude that physicians should not provide numerical information such as Adjuvant! estimates because patients do not consistently use the information. We disagree. Numerical data might serve better, however, than the current format of Adjuvant! if presented in a more user-friendly manner. As shown in Figure 1, data on treatment benefit as presented by Adjuvant! are complex and potentially confusing. Patients have to add numbers together from different locations (e.g., add estimates from additional number of women helped under a treatment option to the no-treatment option to derive total benefit); furthermore, the shaded aspects of the horizontal stacked bars and the redundancy across the stacked bars likely add to the confusion. Reduced comprehension and lower-quality choices are related to decisions that require greater cognitive effort in processing numeric information, particularly among less numerate consumers.21
Other potentially more effective strategies might be to present the total number of women expected to be cancer free by summing the additional number of women expected to be cancer free under a specific treatment with the number expected with no additional treatment, as well as to order treatments from least to most effective.22 Zikmund-Fisher and colleagues15 found that a 2-option pictograph format that compared hormonal therapy to that of hormonal therapy and chemotherapy (e.g., how many more women were alive due to chemotherapy) was significantly more effective at helping women understand the level of risk reduction associated with adding chemotherapy to hormonal therapy than the 4-option horizontal bar format used by Adjuvant!. Such simplified graphics that focus attention on the most crucial information may help patients retain the gist of which treatment option offers the best hope of remaining cancer free.23 If true, formats of the presentation should focus on helping patients accurately understand and remember the essential meaning of treatment benefits and risks.24
We were surprised to find that patients’ feelings of confidence that they would be one of the women to benefit from various treatments were uncorrelated with the estimates provided by Adjuvant!. It is unclear whether patients were extracting the essential meaning of what these estimates implied or whether the implicit consideration of being one who benefitted versus one who did not might have altered processing in some way. Studies are warranted that explore the meaning patients attach to estimates and how they translate population-based statistics into feelings about whether they themselves will benefit. With respect to the above, it is unclear why numeracy interacted with estimates from Adjuvant! to predict treatment benefit but not with confidence ratings (although the results were in the predicted direction), especially given that perceived benefit and confidence were highly correlated (r(s) = 0.59). A patient may take into account different factors when judging confidence that she will be one of the women who benefits than when estimating amount of personal benefit. This explanation is consistent with the greater statistical variability associated with the confidence than the benefit ratings.
Based on our findings, oncologists should spend more time assessing patient understanding of treatment benefits. At a minimum, they should probe whether patients understand which treatment option statistically maximizes the chance of remaining cancer free; such an understanding is related to treatment choice. As a potential barrier to this process, oncologists who believe their patients comprehend the information may probe less for understanding. Indeed, physicians often overestimate patient knowledge.25,26 An interesting question is whether informing oncologists of their patients’ numeracy skills would alter both the amount and the format in which information about treatment risks and benefits is presented. Would they convey risk information using a greater number of verbal probability statements (e.g., unlikely)? A problem with using verbal probability statements is that such terms have substantial variability in interpretations, potentially leading to more confusion.27
The study has several limitations. First, it is not clear how representative this sample was of breast cancer patients in general. Second, no data were obtained as to what transpired during the conversations. For example, we do not know what similarities and differences existed among the oncologists in their use of Adjuvant!, how the estimates were reviewed, or the type of questions patients posed about the Adjuvant! estimates that might have influenced understanding and decisions. One might assume an age bias in conversations with older persons such that the provider gave less emphasis to the benefit of chemotherapy or combined therapy than what was given to younger women in similar clinical situations. Our data suggest that such discussions are justifiable given that age was negatively correlated with the Adjuvant! estimates of cancer-free survival across all treatment (−0.48 < rs(105) < 0.71, Ps < 0.0001). Of import, numeracy interacted with age: Patients with higher numeracy scores are less likely to take combined therapy at younger ages and more likely to take combined therapy with increasing age compared with women with average or lower numeracy scores. This suggests that numeracy may have a deleterious effect by dissuading women at younger ages to select combined therapy when it is more advantageous than at older ages. An alternative interpretation, however, is that greater numeracy assisted women of all ages to appropriately take into account their numerical odds of survival along with other relevant information, whereas less numerate women reacted more to preconceived age biases in the need for aggressive care. Further study of this age by numeracy interaction is needed.
Fourth, many patients left questions unanswered, reducing statistical power. Fifth, we assume our questions about treatment benefit and confidence in benefit corresponded closely with estimates provided by Adjuvant!. However, patients may have thought of benefit in other terms (e.g., fewer side effects, less relapse risk, etc.). Sixth, we pooled together 2 studies that used different designs. Although we accounted for design differences in our analyses, some unmeasured between-study factors may have revealed more differences between these study samples than observed herein. Clearly, these findings merit replication.
Despite these weaknesses, our data suggest more work is needed to probe how early-stage breast cancer patients extract, understand, and use numerical estimates of adjuvant treatment benefit based on existing decision aids and how those estimates can be presented to improve clinical care. In this multistep decision-making process, numeracy seems to play a significant role and should be investigated further in the critical processes of breast cancer treatment decisions. Indeed, with the availability of 1st-generation genomic tests such as the Oncotype DX and Mammaprint,28 patients will continue to face quantitative risk information to guide treatment decisions and individualized care.
ACKNOWLEDGMENT
This study was supported by the Foundation for Informed Medical Decision Making and pilot funding from Duke University Medical Center’s Breast Cancer SPORE grant 5 P50 CA068438–09. We thank Shelly Clark for serving as the project coordinator and Dr. John Feaganes for his statistical support.
Footnotes
With respect to demographic characteristics, patients with ER+ disease who declined to participate were older than those who consented ( v. respectively, P < 0.005) but did not differ significantly with respect to distributions of race or education. Furthermore, there were no differences on the distributions of any of the clinical parameters (e.g., tumor size, number of nodes). Data on decliners were not assessed in the 1st pilot study.
REFERENCES
- 1.Hembroff LA, Holmes-Rovner M, Wills CE. Treatment decision-making and the form of risk communication: results of a factorial survey. BMC Med Inform Dec Making. 2004;4:20. doi: 10.1186/1472-6947-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Studts JL, Abell TD, Roetzer LM, Albers AN, McMasters KM, Chao C. Preferences for different methods of communicating information regarding adjuvant chemotherapy for breast cancer. Psycho-Oncol. 2005;14(8):647–60. doi: 10.1002/pon.886. [DOI] [PubMed] [Google Scholar]
- 3.Takasugi M, Iwamoto E, Akashi-Tanaka S, Kinoshita T, Fukutomi T, Kubouchi K. General aspects and specific issues of informed consent on breast cancer treatments. Breast Cancer. 2005;12(1):39–44. doi: 10.2325/jbcs.12.39. [DOI] [PubMed] [Google Scholar]
- 4.Woloshin S, Schwartz LM. How can we help people make sense of medical data? Effect Clin Pract. 1999;2(4):176–83. [PubMed] [Google Scholar]
- 5.Levine MN, Gafni A, Markham B, MacFarlane D. A bedside decision instrument to elicit a patient’s preference concerning adjuvant chemotherapy for breast cancer. Ann Intern Med. 1992;117(1):53–8. doi: 10.7326/0003-4819-117-1-53. [DOI] [PubMed] [Google Scholar]
- 6.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19(4):980–91. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 7.Irwin E, Arnold A, Whelan TJ, Reyno LM, Cranton P. Offering a choice between two adjuvant chemotherapy regimens: a pilot study to develop a decision aid for women with breast cancer. Patient Educ Couns. 1999;37(3):283–91. doi: 10.1016/s0738-3991(98)00117-7. [DOI] [PubMed] [Google Scholar]
- 8.Whelan T, Sawka C, Levine M, et al. Helping patients make informed choices: a randomized trial of a decision aid for adjuvant chemotherapy in lymph node-negative breast cancer. J Natl Cancer Inst. 2003;95(8):581–7. doi: 10.1093/jnci/95.8.581. [DOI] [PubMed] [Google Scholar]
- 9.Peele PB, Siminoff LA, Xu Y, Ravdin PM. Decreased use of adjuvant breast cancer therapy in a randomized controlled trial of a decision aid with individualized risk information. Med Decis Making. 2005;25(3):301–7. doi: 10.1177/0272989X05276851. [DOI] [PubMed] [Google Scholar]
- 10.Siminoff LA, Gordon NH, Silverman P, Budd T, Ravdin PM. A decision aid to assist in adjuvant therapy choices for breast cancer. Psychol Oncol. 2006;15:1001–13. doi: 10.1002/pon.1040. [DOI] [PubMed] [Google Scholar]
- 11.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 12.Mackillop WJ, Stewart WE, Ginsburg AD, Stewart SS. Cancer patients’ perceptions of their disease and its treatment. Br J Cancer. 1988;58(3):355–8. doi: 10.1038/bjc.1988.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eidinger RN, Schapira DV. Cancer patients’ insight into their treatment, prognosis, and unconventional therapies. Cancer. 1984;53(12):2736–40. doi: 10.1002/1097-0142(19840615)53:12<2736::aid-cncr2820531233>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Siminoff LA, Fetting JH, Abeloff MD. Doctor-patient communication about breast cancer adjuvant therapy. J Clin Oncol. 1989;7(9):1192–200. doi: 10.1200/JCO.1989.7.9.1192. [DOI] [PubMed] [Google Scholar]
- 15.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. improving understanding of adjuvant therapy options via simpler risk graphics. Cancer. 2008;113(12):3382–90. doi: 10.1002/cncr.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry H, Smith DM. Measuring numeracy without a math test: development of the subjective numeracy scale (SNS) Med Decis Making. 2007;27(5):672–80. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 17.Hutton DW, Belkora JK, Shachter RD, Moore DH. Are patients getting the “gist” in risk communication? Patient understanding of prognosis in breast cancer treatment. J Cancer Educ. 2009;24(3):194–9. doi: 10.1080/08858190902876452. [DOI] [PubMed] [Google Scholar]
- 18.Peters E, Slovic P, Västfjäll D, Mertz CK. Intuitive numbers guide decisions. Judgm Decis Mak. 2008;3(8):619–35. [Google Scholar]
- 19.Galesic M, Garcia-Retamero R, Gigerenzer G. Using icon arrays to communication medical risks: overcoming low numeracy. Health Psychol. 2009;28(2):210–16. doi: 10.1037/a0014474. [DOI] [PubMed] [Google Scholar]
- 20.Dieckmann NF, Slovic P, Peters E. The use of narrative evidence and explicit probability by decision makers varying in numeracy. Risk Anal. 2009;29(10):1473–88. doi: 10.1111/j.1539-6924.2009.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters E, Dieckmann N, Dixon A, Hibbard JH, Mertz CK. Less is more in presenting quality information to consumers. Med Care Res Rev. 2007;64(2):169–90. doi: 10.1177/10775587070640020301. [DOI] [PubMed] [Google Scholar]
- 22.Peters E, Hibbard JH, Slovic P, et al. Numeracy skill and the communication, comprehension, and use of risk and benefit information. Health Aff. 2007;26(3):741–8. doi: 10.1377/hlthaff.26.3.741. [DOI] [PubMed] [Google Scholar]
- 23.Reyna VF. How people make decisions that involve risk: a dual-processes approach. Curr Dir Psychol Sci. 2004;13(2):60–6. [Google Scholar]
- 24.Peters E, Dieckmann NF, Västfjäll D, Mertz CK, Slovic P, Hibbard J. Bringing meaning to numbers: the impact of evaluative categories on decisions. J Exp Psychol Appl. 2009;15(3):213–27. doi: 10.1037/a0016978. [DOI] [PubMed] [Google Scholar]
- 25.Nickerson RS. How we know—and sometimes misjudge—what others know: imputing one’s own knowledge to others. Psychol Bull. 1999;125(6):737–59. [Google Scholar]
- 26.Nickerson RS. The projective way of knowing: a useful heuristic that sometimes misleads. Curr Dir Psychol Sci. 2001;10(5):168–72. [Google Scholar]
- 27.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27(5):696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 28.Buyse M, Loi S, van’t Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with nodenegative breast cancer. J Natl Cancer Inst. 2006;98(17):1183–92. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]