Abstract
The bacterial Type VI secretion system (T6SS) is dynamic organelle that bacteria use to target prey cells for inhibition via translocation of effector proteins. Time-lapse fluorescence microscopy has documented striking dynamics of opposed T6SS organelles in adjacent sister cells of Pseudomonas aeruginosa. Such cell-cell interactions have been termed "T6SS dueling" and likely reflect a biological process that is driven by T6SS antibacterial attack. Here we show that T6SS dueling behavior strongly influences the ability of P. aeruginosa to prey upon heterologous bacterial species. We show that in the case of P. aeruginosa, T6SS-dependent killing of either Vibrio cholerae or Acinetobacter baylyi is greatly stimulated by T6SS activity occurring in those prey species. Our data suggest that in P. aeruginosa, T6SS organelle assembly and lethal counterattack are regulated by a signal that corresponds to the point of attack of the T6SS apparatus elaborated by a second aggressive T6SS+ bacterial cell.
Introduction
The ecological interactions between bacterial species range from cooperative (e.g., mutualism and commensalism) to competitive (e.g., parasitism and predation). Contact-dependent, cooperative interactions involving adherence and nutrient scavenging within biofilms have been demonstrated (Kolenbrander et al., 2010; Peters et al., 2012). Several types of contact-dependent, antagonistic interactions have also been described. For example, contact-dependent growth inhibition (CDI) in E. coli involves the interaction of outer membrane protein CdiA on cdi+ cells with BamA receptors on cdi− target cells, a process that triggers growth inhibition when cdi− cells lack the cognate immunity protein CdiI (Aoki et al., 2008; Aoki et al., 2005; Aoki et al., 2009). Proteus mirabilis strains display a self vs. non-self discrimination that has been recently genetically defined but is not well understood mechanistically (Gibbs et al., 2008; Gibbs et al., 2011).
One of the most widely distributed examples of contact-dependent antagonistic behavior involves the Type VI secretion system (T6SS) (Pukatzki et al., 2006). This secretion system is functionally analogous to a bacteriophage tail and corresponds to a dynamic organelle located in the cytosol and attached to the cell envelope by a base plate structure (Basler et al., 2012; Leiman et al., 2009; Pukatzki et al., 2007). The T6SS apparatus can power secretion of proteins between cells by utilizing a contractile phage sheath-like structure (Basler et al., 2012; Bonemann et al., 2009; Leiman et al., 2009). 'T6SS activity' (i.e., T6SS sheath extension, contraction, and disassembly cycles) can be readily visualized by time-lapse microscopy utilizing fluorescent fusion proteins to orthologs of either of two V. cholerae T6SS gene products, VipA or ClpV (Basler and Mekalanos, 2012; Basler et al., 2012). This dynamic activity leads to the translocation of proteins that comprise the T6SS spike/tube complex, VgrG and Hcp, out of the cell (Basler et al., 2012; Leiman et al., 2009).
Approximately 25% of all sequenced Gram-negative bacteria, including members of the genera Vibrio, Pseudomonas, and Acinetobacter encode T6SS gene clusters (Boyer et al., 2009). In several of these species, T6SS have been associated with either antagonistic (Hood et al., 2010; Schwarz et al., 2010b) or outright bacteriocidal (Chou et al., 2012; MacIntyre et al., 2010; Murdoch et al., 2011; Zheng et al., 2011) activity toward heterologous bacterial species. For example, P. aeruginosa can outcompete P. putida in mixed culture through the translocation of one or more of three different T6SS effector proteins termed Tse1, Tse2 and Tse3 (Russell et al., 2011). P. aeruginosa sister cells avoid inhibiting each other by encoding three immunity proteins Tsi1, Tsi2, and Tsi3, which bind to and presumably neutralize the activity of their cognate effectors (Ding et al., 2012; Li et al., 2012). However, despite having this immunity, P. aeruginosa cells respond to T6SS activity directed at them by adjacent sister cells with their own T6SS activity (Basler and Mekalanos, 2012). The spatial and temporal coincidence of T6SS activity between adjacent P. aeruginosa sister cells suggests that contact-dependent, protein translocation produces a signal that triggers T6SS activity in the adjacent cell. The dynamic T6SS activity that occurs between pairs of interacting cells was termed "T6SS dueling" and proposed to reflect a biologically significant process that occurred between heterologous T6SS+ species (Basler and Mekalanos, 2012).
In order to characterize the contact-dependent signal that triggers T6SS dueling behavior, we have explored the ability of P. aeruginosa to prey upon T6SS+ and T6SS− V. cholerae and A. baylyi. We found that P. aeruginosa does not efficiently kill T6SS− V. cholerae or T6SS− A. baylyi but readily attacks these species if they express a functional T6SS. The TagQRST-PpkA-PppA-Fha1 regulatory system is essential for P. aeruginosa T6SS dueling and prey selection indicating it is likely responsible for sensing a T6SS-mediated attack on P. aeruginosa cells by heterologous T6SS+ predatory species. These results provide evidence for a bacterial "tit-for-tat" evolutionary strategy that controls the social interaction between different bacterial species (Axelrod and Hamilton, 1981).
Results
P. aeruginosa specifically targets T6SS+ V. cholerae cells for T6SS-mediated counterattack
Previously, we proposed that T6SS dueling behavior specifically marks the location of T6SS effector delivery between sister cells of P. aeruginosa (Basler and Mekalanos, 2012). We first considered the possibility that P. aeruginosa T6SS dueling activity might respond to the penetration of the outer membrane by the T6SS spike/tube complex injected by sister cells. Because the VgrG and Hcp proteins that comprise this complex are highly conserved among different bacterial species (Leiman et al., 2009), we hypothesized that the T6SS spike/tube complex of heterologous organisms might also induce a T6SS dueling response in P. aeruginosa. V. cholerae has been reported to effectively kill E. coli using its T6SS (MacIntyre et al., 2010; Zheng et al., 2011), and its T6SS apparatus has been structurally characterized (Basler et al., 2012). Thus, the T6SS from V. cholerae was a logical candidate for testing this hypothesis.
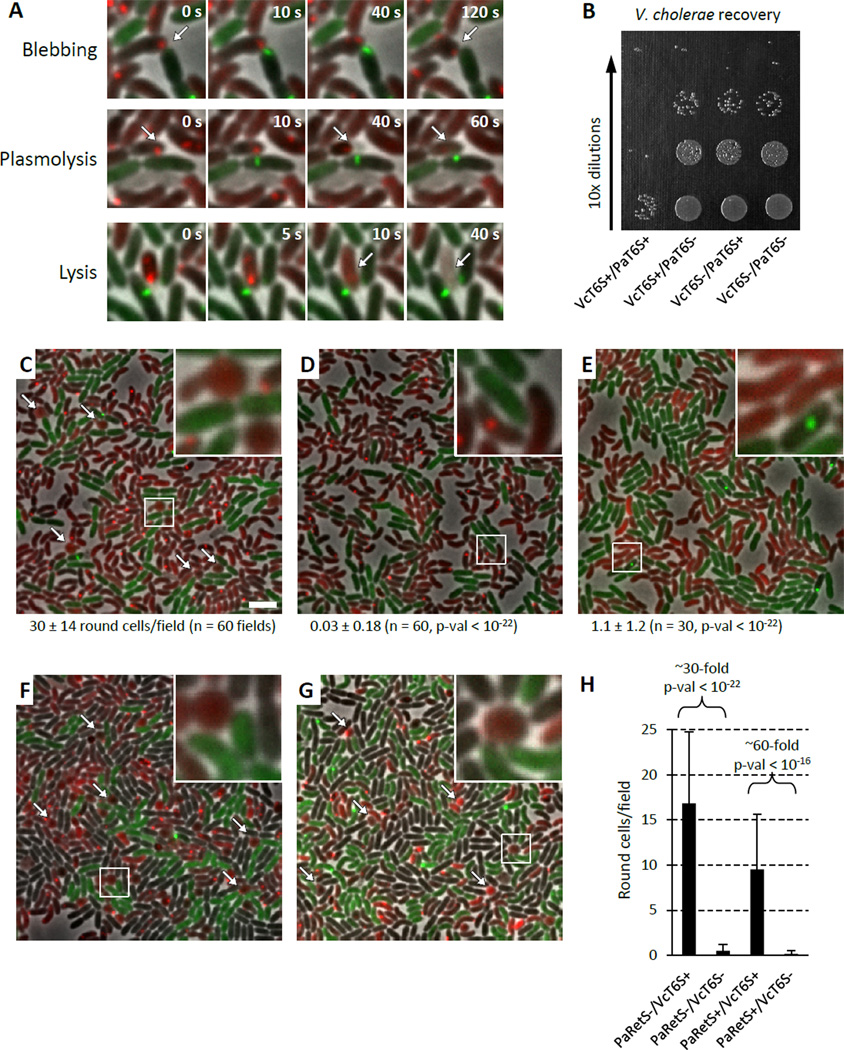
To determine whether V. cholerae T6SS could induce T6SS activity in P. aeruginosa, we observed mixtures of P. aeruginosa PAO1 with V. cholerae 2740-80 by time-lapse fluorescence microscopy. As in previous studies, we used retS derivatives of P. aeruginosa that are known to over express the H1-T6SS locus at the transcriptional level (Basler and Mekalanos, 2012; Mougous et al., 2006). T6SS activity was monitored with ClpV1-GFP and ClpV-mCherry2 fusion proteins in P. aeruginosa and V. cholerae, respectively. This experiment revealed that P. aeruginosa induced striking morphological changes in V. cholerae cells that could be differentiated into categories including cell rounding, cell blebbing, plasmolysis, and overt lysis (Figure 1A, Movie S1 and S2). Rounding of V. cholerae cells in these mixtures was dependent on the functionality of H1-T6SS locus of P. aeruginosa (Figure 1C, D, Table S1) and occurred predominantly in V. cholerae cells directly contacting P. aeruginosa cells. Remarkably, in accordance with our hypothesis that the activity of the T6SS of a heterologous organism might trigger the dueling response of P. aeruginosa, rounding of V. cholerae cells was virtually absent in mixtures containing T6SS− V. cholerae (Figure 1E and Movie S1, Table S1).
Figure 1. P. aeruginosa T6SS preferentially targets T6SS positive V. cholerae.
See also Movie S1, S2, Table S1, S2. VcT6S+ indicates V. cholerae clpV-mCherry2, VcT6S− indicates V. cholerae ΔvipA clpV-mCherry2, PaT6S+ indicates P. aeruginosa ΔretS clpV1-gfp, PaT6S− indicates P. aeruginosa ΔretS ΔvipA1 clpV1-gfp. PaRetS− indicates P. aeruginosa ΔretS, PaRetS+ indicates P. aeruginosa. (A) Examples of morphological changes of V. cholerae seen in mixtures of P. aeruginosa ΔretS clpV1-gfp (T6SS+, green) and V. cholerae clpV-mCherry2 (T6SS+, red). 4.5x4.5 µm fields are shown. (B) Example of a dilution series used to enumerate V. cholerae recovery from a competition with P. aeruginosa. (C−G) 30x30 µm representative field of cells with a 4x magnified 3x3 µm inset (marked by box). Bar in C is 3 µm and applies to C–G. Arrows point to examples of round V. cholerae cells. (C−E) Average number of round V. cholerae cells per 30x30 µm field (± standard deviation) is shown for each mixture (n fields were analyzed), p-val compared to mixture in C. (C) P. aeruginosa ΔretS clpV1-gfp (T6SS+, green) mixed with V. cholerae clpV-mCherry2 (T6SS+, red), (D) P. aeruginosa ΔretS ΔvipA1 clpV1-gfp (T6SS−, green) mixed with V. cholerae clpV-mCherry2 (T6SS+, red), (E) P. aeruginosa ΔretS clpV1-gfp (T6SS+, green) mixed with V. cholerae ΔvipA clpV-mCherry2 (T6SS−, red). (F, G) V. cholerae clpV-mCherry2 (T6SS+, red), and V. cholerae ΔvipA clpV-gfp (T6SS−, green) strains were mixed at equal ratios with (F) P. aeruginosa ΔretS (T6SS+, unlabeled), (G) P. aeruginosa (T6SS+, unlabeled). (H) Quantification of number of round V. cholerae cells detected per 30x30 µm field (n = 60) for mixtures shown in F and G.
To ascertain if preferential rounding of T6SS+ V. cholerae under microscopic conditions also reflected preferential killing of T6SS+ cells by P. aeruginosa, we performed quantitative competition assays. In agreement with the observed microscopy data, when competed against T6SS+ P. aeruginosa, we recovered approximately 100-fold fewer surviving T6SS+ V. cholerae cells than T6SS− V. cholerae cells (Figure 1B). T6SS+ and T6SS− P. aeruginosa were only marginally inhibited by T6SS+ V. cholerae and thus the difference seen in prey sensitivity does not reflect a difference in P. aeruginosa survival in these quantitative assays (Figure S1A). These data suggest that an antibacterial P. aeruginosa T6SS dueling response was likely directed specifically at T6SS+ V. cholerae cells that had attacked P. aeruginosa cells first.
We sought to confirm the target specificity of the P. aeruginosa heterologous dueling/antibacterial response and that this induced response did not cause collateral damage to nearby cells that had not attacked the retaliating P. aeruginosa cell. Accordingly, we designed a mixture experiment involving three strains that would allow the specificity of the P. aeruginosa dueling/antibacterial response to be assessed at the microscopic level. We mixed P. aeruginosa with differentially, fluorescently-labeled T6SS+ (red) and T6SS− V. cholerae (green) and scored the relative proportion of red or green cells that showed rounding in the assay. Strikingly, V. cholerae cells exhibiting rounded morphologies were predominantly T6SS+, while T6SS− V. cholerae cells remained largely unaffected (Figure 1F, H, Movie S1, Table S2). This selective targeting of T6SS+ V. cholerae was also observed in wild type P. aeruginosa PAO1 (Figure 1G, H, Table S2) and is thus independent of H1-T6SS transcriptional expression level. Collectively, these data suggest that P. aeruginosa cells were precisely directing their T6SS-dependent antibacterial activity to specifically target only V. cholerae prey cells that had attacked first with their own heterologous T6SS apparatus.
P. aeruginosa T6SS delivers Tse1 effector into V. cholerae cells but Tse effectors are dispensable for dueling and V. cholerae killing
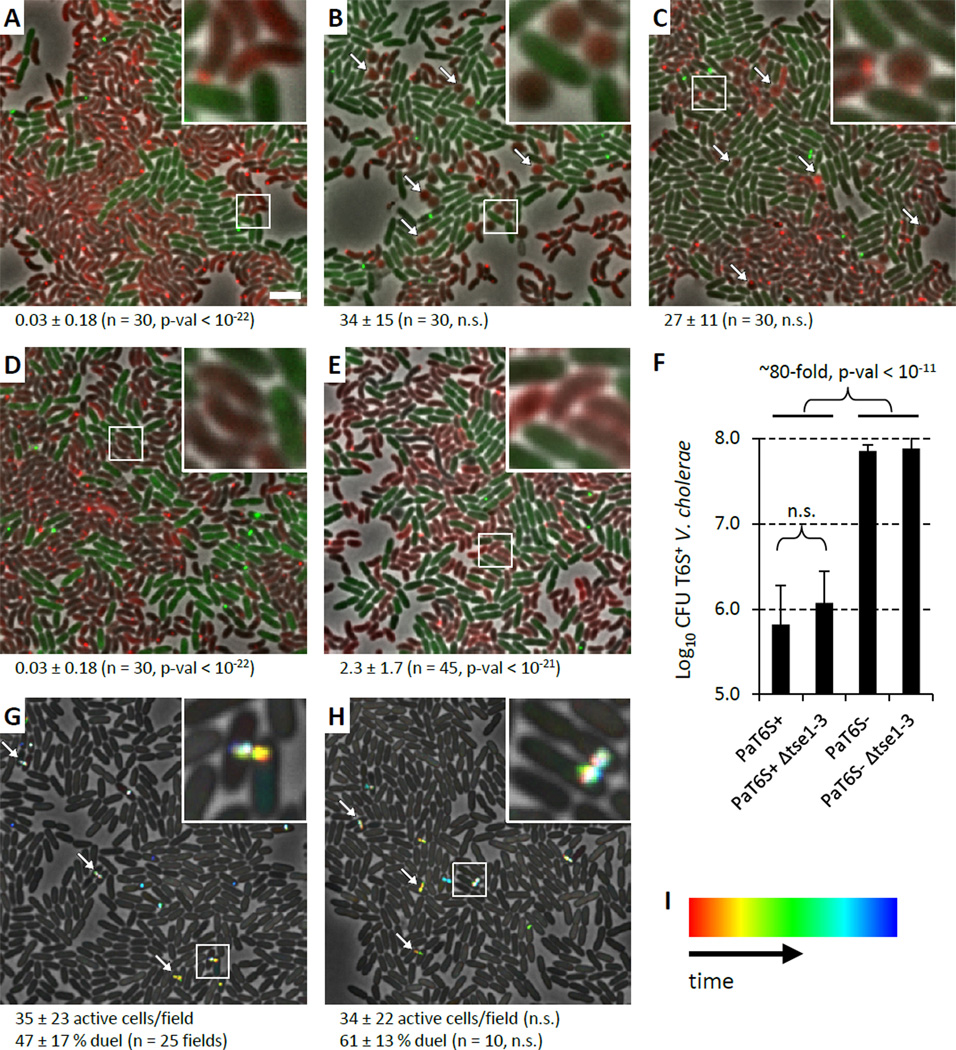
When mixed with T6SS+ P. aeruginosa, the cell rounding morphological response of T6SS+ V. cholerae was reminiscent of one observed in E. coli cells engineered to express the P. aeruginosa T6SS effector Tse1 in their periplasm in the absence of the cognate immunity protein Tsi1 (Russell et al., 2011). Therefore, we next asked whether the rounding morphology exhibited by V. cholerae could be specifically attributed to Tse1 activity. Although knockouts of tse2 and tse3 in P. aeruginosa still caused V. cholerae cell rounding, knocking out all three effectors or just tse1 alone eliminated the cell rounding activity (Figure 2A–D, Table S1). Furthermore, expression of Tsi1 (the cognate immunity protein of Tse1), in the periplasm of V. cholerae significantly decreased cell rounding when mixed with P. aeruginosa (Figure 2E, Table S1). These data provide clear visual evidence (P. aeruginosa T6SS-dependent V. cholerae cell rounding) of the delivery of a specific T6SS effector (Tse1) into a bacterial target cell by a functional T6SS organelle.
Figure 2. P. aeruginosa T6SS effector Tse1 responsible for V. cholerae cell rounding but Tse effectors dispensable for dueling and V. cholerae growth inhibition.
See also Movie S3, Table S1, S3. 30x30 µm representative field of cells with a 4x magnified 3x3 µm inset (marked by box) is shown for A–E, G, H. Bar in A is 3 µm and applies to A–E, G, H. Strain abbreviations were as used in Figure 1. n.s. – not statistically significant (p-val > 0.01). (A−E) Arrows point to examples of round V. cholerae cells. Average number of round V. cholerae cells per 30x30 µm field (± standard deviation) is shown for each mixture (n fields were analyzed), p-val compared to mixture in Figure 1C. For A–D V. cholerae clpV-mCherry2 strain was mixed with (A) P. aeruginosa ΔretS Δtse1 clpV1-gfp, (B) P. aeruginosa ΔretS Δtse2 clpV1-gfp, (C) P. aeruginosa ΔretS Δtse3 clpV1-gfp, (D) P. aeruginosa ΔretS Δtse1-3 clpV1-gfp. (E) P. aeruginosa ΔretS clpV1-gfp was mixed with V. cholerae / pBAD24-Tsi1-mCherry2 strain. (F) Summary of competition assays for P. aeruginosa and V. cholerae mixtures. Data are presented as mean of Log10CFU of recovered V. cholerae with error bar representing standard deviation (n = 8 – 19). (G, H) ClpV1-GFP localization was followed for 3 minutes and temporally color coded. Arrows point to examples of dueling P. aeruginosa cells. Average number of active cells and dueling cells per 30x30 µm field (± standard deviation) is shown (n fields were analyzed), p-val compared to strain in G. (G) P. aeruginosa ΔretS clpV1-gfp, (H) P. aeruginosa ΔretS Δtse1-3 clpV1-gfp. (I) Color scale used to temporal-color code ClpV1-GFP signal.
Interestingly, when a competition experiment was performed using a tse1-3 triple knockout of P. aeruginosa, wild-type levels of T6SS-dependent killing were observed (Figure 2F). Thus, in the case of V. cholerae, even though T6SS-dependent delivery of Tse1 is detected by microscopy, prey cell killing occurs independent of the three known Tse effector proteins of P. aeruginosa. Additionally, the tse1-3 effector knockout strain exhibited dueling activity between sister cells similar to wild-type (Figure 2G, H, Movie S3, Table S3) indicating that T6SS-mediated translocation of these three Tse effectors into target cells is also not required for the T6SS dueling response.
Inactivation of signaling cascade results in loss of P. aeruginosa T6SS dueling
We next sought to define the signaling pathway regulating T6SS dueling and the recognition of homologous or heterologous T6SS attack. The kinase PpkA is known to be required for T6SS function (Mougous et al., 2007). It phosphorylates the essential T6SS apparatus component Fha1, which then associates with the T6SS apparatus visualized with ClpV1-GFP (Mougous et al., 2007). PpkA activity is counteracted by phosphatase PppA, which deactivates the P. aeruginosa T6SS apparatus by dephosphorylating Fha1 (Mougous et al., 2007). Moreover, the cell envelope-associated TagQRST regulatory system controls PpkA phosphorylation of Fha1 (Casabona et al., 2012). Thus, TagQRST-PpkA-Fha1-PppA has been a proposed to control assembly or function of the T6SS apparatus post-transcriptionally in response to undefined environmental signals (Casabona et al., 2012; Hsu et al., 2009; Mougous et al., 2007; Silverman et al., 2011). However, this post-translational regulatory loop has not been previously evaluated for its effect on the dynamics of T6SS organelles.
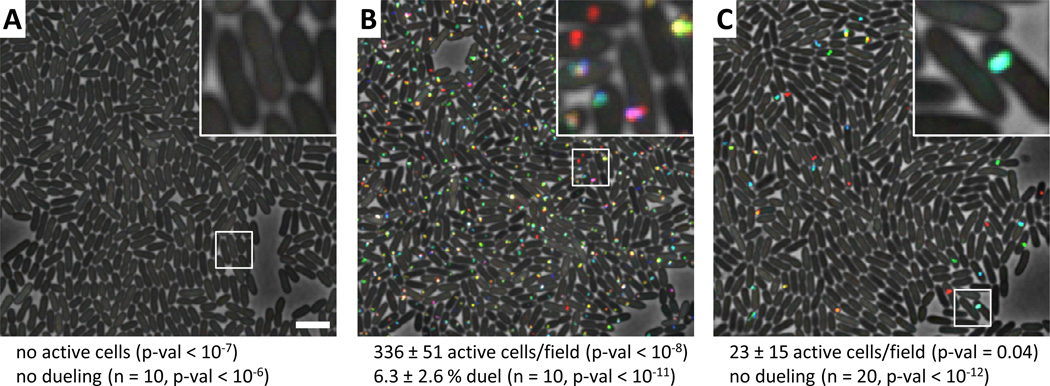
Accordingly, we tested whether inactivation of PpkA (kinase), PppA (phosphatase), or TagT (ATP-binding cassette transporter) affect the level of T6SS activity and dueling behavior as measured by ClpV1-GFP dynamics (Basler and Mekalanos, 2012). Inactivation of ppkA resulted in a complete block of T6SS dynamics (Figure 3A, Movie S3, Table S3), while inactivation of pppA resulted in a dramatic increase in the number of cells showing T6SS activity compared to the PppA+ parental strain (Figure 3B, Movie S3, Table S3). Like V. cholerae, this T6SS activity occurred spontaneously in most cells and irrespective of neighboring cell T6SS activity. However, unlike V. cholerae (Basler and Mekalanos, 2012), the T6SS activity visualized with ClpV1-GFP remained localized to a given subcellular site in each P. aeruginosa pppA mutant cell (Figure 3B, Movie S3). T6SS activity of this sort has been previously hypothesized to reflect either the re-cycling of the T6SS baseplate complex through multiple rounds of T6SS organelle sheath extension/contraction/disassembly cycles or clustering of multiple dynamic T6SS organelles in close proximity to each other (Basler and Mekalanos, 2012). For simplicity we refer to such cycles of T6SS activity as "firing" in that such activity likely also marks the location of extracellular secretion events that could attack a correctly positioned neighboring prey cell. This positional restriction of T6SS organelle firing was characteristic of a majority of the T6SS activity observed in the pppA knockout mutant. Thus, despite its overall increase in T6SS organelle assembly and firing activity, the pppA mutant is defective in the T6SS dueling response (Figure 3B, Movie S3, Table S3). This result suggests that the PppA phosphatase is required to dephosphorylate Fha1 and allow the T6SS organelle to be targeted for disassembly rather than re-cycling in the same location. Lastly, knocking out tagT resulted in loss of dueling activity without loss of T6SS activity in that cells continue to fire their T6SS organelles at the same location but not in temporal or spatial register with an active sister cell (Figure 3C, Movie S3, Table S3). This result indicates that the TagQRST signaling cascade is required for sensing T6SS activity occurring in nearby sister cells that are under attack by adjacent sister cell T6SS organelles. This conclusion is also consistent with the observed stimulation of T6SS organelle formation by the TagQRST system on solid medium compared to liquid medium, where such interactions are more likely to occur (Casabona et al., 2012).
Figure 3. T6SS dueling depends on PpkA, PppA and TagT.
See also Movie S3, Table S3. ClpV1-GFP localization was followed for 3 minutes and temporally color coded (color scale in Figure 2I). 30x30 µm representative field of cells with a 4x magnified 3x3 µm inset (marked by box) is shown for A–C. Bar in A is 3 µm and applies to A–C. Average number of active cells and dueling cells per 30x30 µm field (± standard deviation) is shown (n fields were analyzed), p-val compared to parental strain in Figure 2G. (A) P. aeruginosa ΔretS ΔppkA clpV1-gfp, (B) P. aeruginosa ΔretS ΔpppA ΔclpV1-gfp, (C) P. aeruginosa ΔretS ΔtagT clpV1-gfp.
Loss of T6SS dueling behavior blocks targeting of T6SS+ prey cells
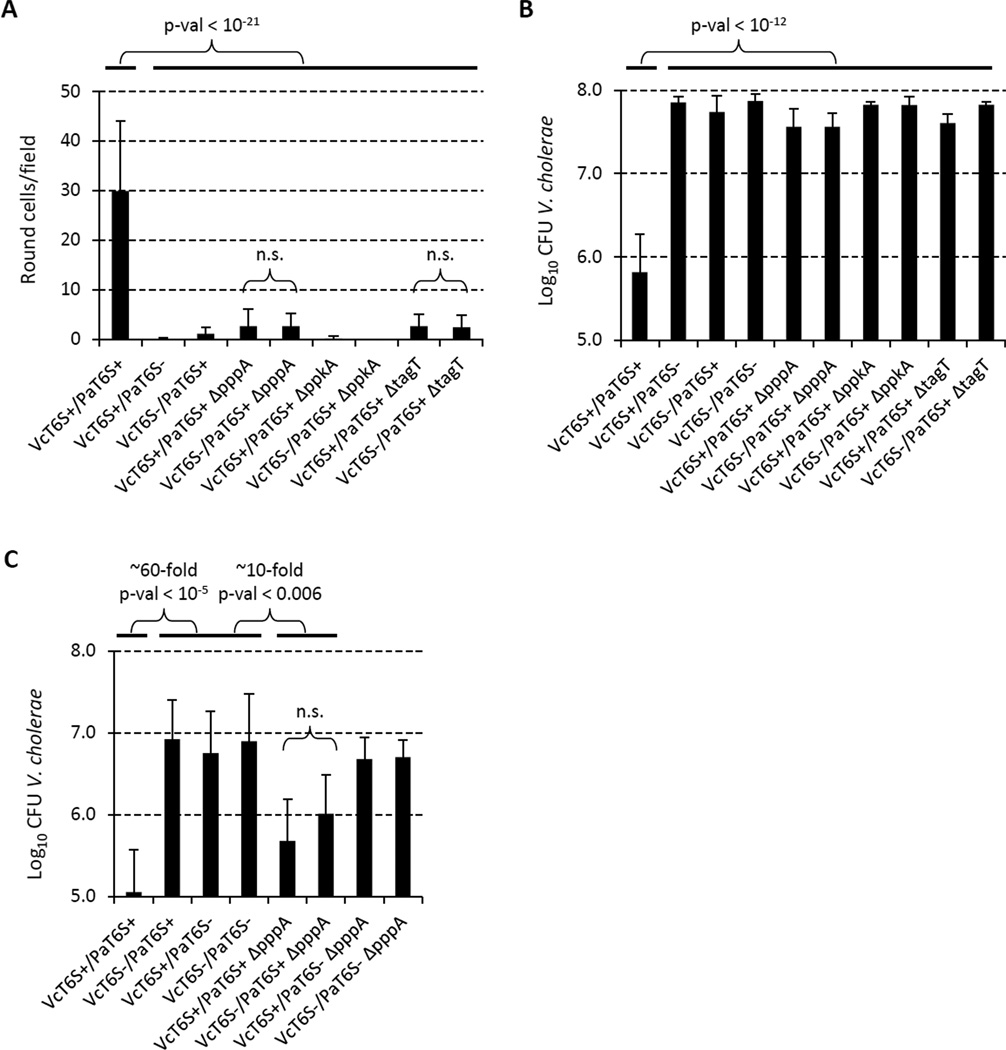
We further tested whether mutations in the TagQRST-PpkA-Fha1-PppA regulatory cascade affected P. aeruginosa targeting of V. cholerae cells by counting round V. cholerae cells and measuring inhibition of V. cholerae growth. P. aeruginosa ppkA mutant cells, which exhibited no T6SS firing activity, did not target V. cholerae for either cell rounding or growth inhibition (Figure 4A, B, Table S1). Similarly, the dueling-defective tagT mutant did not target V. cholerae for cell rounding and growth inhibition (Figure 4A, B, Table S1) consistent with the notion that the TagQRST signaling cascade is required for detecting the T6SS attack by V. cholerae.
Figure 4. PpkA, PppA and TagT important for P. aeruginosa targeting of the prey.
See also Table S1. Strain abbreviations were as used in Figure 1. n.s. – not statistically significant (p-val > 0.01). (A) Quantification of number of round V. cholerae cells per 30x30 µm field for indicated mixtures (n = 60 for VcT6S+/PaT6S+ and VcT6S+/PaT6S− mixtures, n = 30 for all other indicated mixtures). (B) Summary of competition assays for P. aeruginosa and V. cholerae mixtures. Data are presented as mean of Log10CFU of recovered V. cholerae with error bar representing standard deviation (n = 6 – 19). (C) Competition assays for P. aeruginosa and V. cholerae mixtures at 10:1 ratio. Data are presented as mean of Log10CFU of recovered V. cholerae with error bars representing standard deviation (n = 5 – 10).
Given that the P. aeruginosa pppA mutant cells fire their T6SS apparatus repeatedly in a specific arbitrary direction but are unable to respond to external T6SS assault (i.e., they are T6SS dueling defective), we made three predictions regarding how effectively the pppA knockout mutant would target V. cholerae in competition assays. First, since the T6SS of the pppA knockout continually fires in a single unchanging direction, these bacteria should be limited in targeting prey cells in a majority of the surrounding space over a unit of time. Thus, a pppA mutant cell should attack a target cell only if its T6SS apparatus happened to be in the proper orientation relative to the point of contact between predator and prey cells, which would lead to a decreased frequency of V. cholerae prey cell rounding in mixtures with the pppA mutant relative to its PppA+ parent. Second, we should be able to compensate for this targeting restriction of pppA mutant cells by increasing the ratio of P. aeruginosa predator cells to V. cholerae prey cells. Increasing the number of P. aeruginosa to V. cholerae contacts would increase the likelihood that a P. aeruginosa T6SS apparatus would happen to be in the correct orientation to fire directly at the V. cholerae cell. Thirdly, if dueling behavior reflects an "aiming" process for directing T6SS firing at aggressive T6SS+ prey, any residual killing activity displayed by the pppA mutant should not be selective for killing T6SS+ prey cells compared to T6SS− prey. Indeed, all three of the above predictions were supported by microscopic and quantitative competition analysis. When the pppA knockout mutant was mixed with T6SS+ V. cholerae in a 1-to-1 ratio, there were very few rounded cells (Figure 4A, Table S1) and virtually no detectable killing of V. cholerae in competition assays (Figure 4B), however, increasing the relative number of P. aeruginosa to V. cholerae by 10-fold partially restored the observable killing activity of the pppA mutant (Figure 4C). This killing activity by the pppA mutant was still dependent on P. aeruginosa T6SS and the observable cell rounding and killing activity of the pppA mutant exhibited no preference for T6SS+ prey V. cholerae cells versus T6SS− prey cells (Figure 4, Table S1). Additionally, the absence of target specificity by the pppA mutant also confirms that T6SS+ V. cholerae are not inherently more sensitive to P. aeruginosa T6SS attack.
Altogether, these results suggest that exogenous T6SS attack on a P. aeruginosa cell produces a signal that is perceived by the TagQRST system, which then promotes local, anatomically correct, phosphorylation of Fha1 and thus the assembly of a T6SS organelle at the site of the attack followed by its firing in a T6SS “counterattack” directed precisely at the contact point of the attacker.
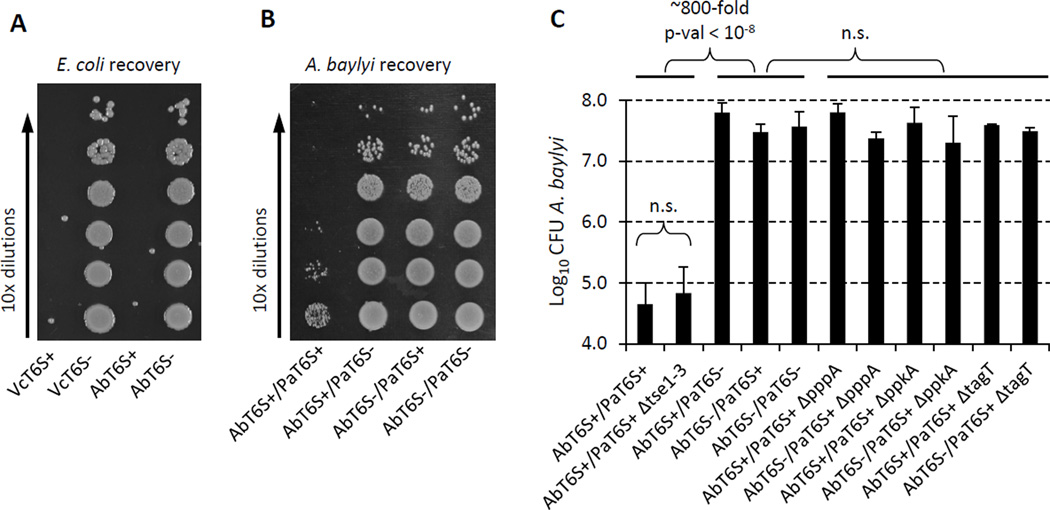
Acinetobacter baylyi T6SS also induces P. aeruginosa T6SS-dependent killing while T6SS− E. coli does not
Given that P. aeruginosa can respond T6SS attack by V. cholerae, we wondered if other heterologous T6SS systems could also induce P. aeruginosa T6SS attack. Acinetobacter baylyi has been shown to have a T6SS dependent growth phenotype on solid media suggesting that it has active T6SS (de Berardinis et al., 2008). We confirmed that its T6SS is functional and could effectively target and kill E. coli at least as efficiently as V. cholerae (Figure 5A). When mixed with P. aeruginosa in competition, approximately 1000-fold fewer A. baylyi T6SS+ cells were recovered compared to T6SS– cells (Figure 5B, C). Neither T6SS+ nor T6SS− P. aeruginosa were killed by A. baylyi T6SS (Figure S1B). Like V. cholerae, T6SS+ A. baylyi was still killed by P. aeruginosa tse1-3 null mutant (Figure 5C). Furthermore, the TagQRST-PpkA-Fha1 regulatory cascade was required for sensing A. baylyi T6SS attack, as mutants altered in PpkA, PppA or TagT no longer killed T6SS+ A. baylyi (Figure 5C). These data suggest that the regulatory cascade activating P. aeruginosa T6SS can be triggered in response to attack by any arbitrary T6SS+ organism.
Figure 5. A. baylyi has functional T6SS and is targeted by P. aeruginosa.
Strain abbreviations were as used in Figure 1. AbT6S+ indicates A. baylyi parental strain, AbT6Sindicates A. baylyi ΔT6SS. (A) Example of a dilution series used to enumerate E. coli survival in mixtures with A. baylyi or V. cholerae. (B) Example of a dilution series used to enumerate A. baylyi survival in mixtures with P. aeruginosa. (C) Summary of competition assays for P. aeruginosa and A. baylyi mixtures. Data are presented as mean of Log10CFU of recovered A. baylyi with error bar representing standard deviation (n = 3 – 8).
If the dueling response of P. aeruginosa is indeed an evolutionary adaption to counterattack aggressive T6SS+ heterologous species, we reasoned that P. aeruginosa should have little or no ability to kill T6SS− species such as E. coli K12. Indeed, retS mutants of P. aeruginosa cannot kill this species efficiently under conditions that lead to 2–3 orders of magnitude more efficient killing or inhibition of T6SS+ species (Figure S1C).
Discussion
In this study we explored the biological activity of the bacterial Type VI secretion system (T6SS) when four different bacterial species (P. aeruginosa, V. cholerae, A. baylyi and E. coli) interact on solid culture media. In addition to quantitative killing/growth inhibition assays, we utilized time-lapse fluorescence microscopy to reveal cellular and subcellular morphological changes that specifically correlated with the genetic phenotypes of the interacting heterologous species. Our logic for performing these studies stemmed in part from our recent discovery that T6SS+ P. aeruginosa sister cells respond to the T6SS activity of adjacent sister cells with a dramatic increases in their own spatial and temporal T6SS activity (Basler and Mekalanos, 2012). We termed this phenomenon, "T6SS dueling" and reasoned that it might reflect a natural process occurring between heterologous T6SS+ species that co-exist in the same ecological niche.
The results presented here document the striking ability of T6SS+ P. aeruginosa to attack heterologous T6SS+ organisms much more efficiently than T6SS− organisms. We observed that T6SS+ V. cholerae were typically killed about 100-fold more efficiently than isogenic T6SS− V. cholerae (Figure 1B, Figure 4B). We also observed a statistically significant difference in the ability of T6SS+ P. aeruginosa to cause rounding of V. cholerae cells, a morphological change attributable to the peptidoglycan degrading T6SS effector Tse1 (Figure 1C–H, Figure 2A–E, Table S1). Furthermore, in mixtures of T6SS+ P. aeruginosa, T6SS− V. cholerae, and isogenic T6SS+ V. cholerae, only the latter were targeted for attack (Figure 1F and 1G, Movie S1, Table S2). The T6SS-dependent morphological changes could not be directly correlated with killing activity under microscopic conditions because these conditions are not optimal for detecting T6SS-mediated killing; the latter typically requires longer time periods of cellular interaction and aerobic conditions. Nonetheless, both assays yielded the same conclusion that T6SS-dependent events (i.e., killing or morphological changes in prey cells) were strikingly dependent on the T6SS+ activity displayed by V. cholerae prey cells. The T6SS of V. cholerae was not unique in this regard, as the T6SS of A. baylyi induced a similar counter attack by P. aeruginosa (Figure 5B, C). In contrast, P. aeruginosa did not efficiently kill T6SS− E. coli (Figure S1C) despite the fact that this species is sensitive to Tse1, Tse-2 and Tse-3 effectors when expressed inside intact cells (Hood et al., 2010; Russell et al., 2011). Although T6SS+ P. aeruginosa have been reported to cause the release of about 4-fold more β-galactosidase from E. coli than Tse1-negative P. aeruginosa (Chou et al., 2012), we view such activity as modest given that P. aeruginosa T6SS antibacterial activity directed against either T6SS+ V. cholerae or T6SS+ A. baylyi appears to be 2–3 orders of magnitude greater under the conditions we employed in our analysis.
The data presented in this report provide a new view of prey selection by P. aeruginosa. Our results suggest that in P. aeruginosa, T6SS-mediated killing activity is regulated by a signal that corresponds to detection of the point of attack of the T6SS apparatus elaborated by a T6SS+ cell be it V. cholerae, A. baylyi, P. aeruginosa or likely other T6SS+ species. The P. aeruginosa T6SS counterattack is finely directed with spatial and temporal accuracy so as to engage the T6SS+ attacker within seconds of its initial attack. In this way, precise killing of aggressive neighboring T6SS+ cells can be efficiently achieved by P. aeruginosa while sparing 'peaceful by-standers' despite their close proximity. This strategy makes ecological sense in that biofilms composed of communities of diverse but cooperative bacterial species likely have more biodegradative (and thus growth) potential than biofilms composed of single bacterial species (Flemming and Wingender, 2010; Wintermute and Silver, 2010). Thus, regulation of T6SS activity by P. aeruginosa may be an evolutionary reflection of the old adage "Don't bite the hand that feeds you" and that P. aeruginosa might prefer to co-exist and cooperate with other bacterial species as long as they are not aggressive predators. On the other hand, the ability of P. aeruginosa to counterattack an aggressive T6SS+ species provides a bacterial example of a “tit-for-tat” evolutionary strategy predicted by Axelrod and Hamilton in their quantitative analysis of gaming strategies that win the "Prisoner Dilemma" challenge (Axelrod and Hamilton, 1981). Our results on induced aggressive behavior between competing bacterial species should be of interest to evolutionary biologists in this context.
In this report we describe the first genetic analysis to provide mechanistic detail and biological context for T6SS dueling between heterologous bacterial species. We showed that a null mutation in pppA dramatically increases P. aeruginosa T6SS dynamic activity on a per cell basis but loss of T6SS dueling behavior (Figure 3B, 4B, Table S1, S3) and failure to selectively kill or induce rounding of T6SS+ V. cholerae (Figure 3B, Table S3). The observed increase in T6SS activity is consistent with the known activities of PppA, a phosphatase that regulates T6SS secretion through the dephosphorylation of Fha1, a scaffold protein that promotes T6SS apparatus assembly in P. aeruginosa specifically after its phosphorylation by the kinase PpkA (Mougous et al., 2007). Our results suggest that the PpkA-Fha1-PppA cycle may well play a role in 1) suppressing random formation of T6SS organelles within the cell and 2) inducing their formation precisely at the point of exogenous T6SS attack, and 3) targeting the disassembly of T6SS organelles once exogenous attack signals are no longer perceived.
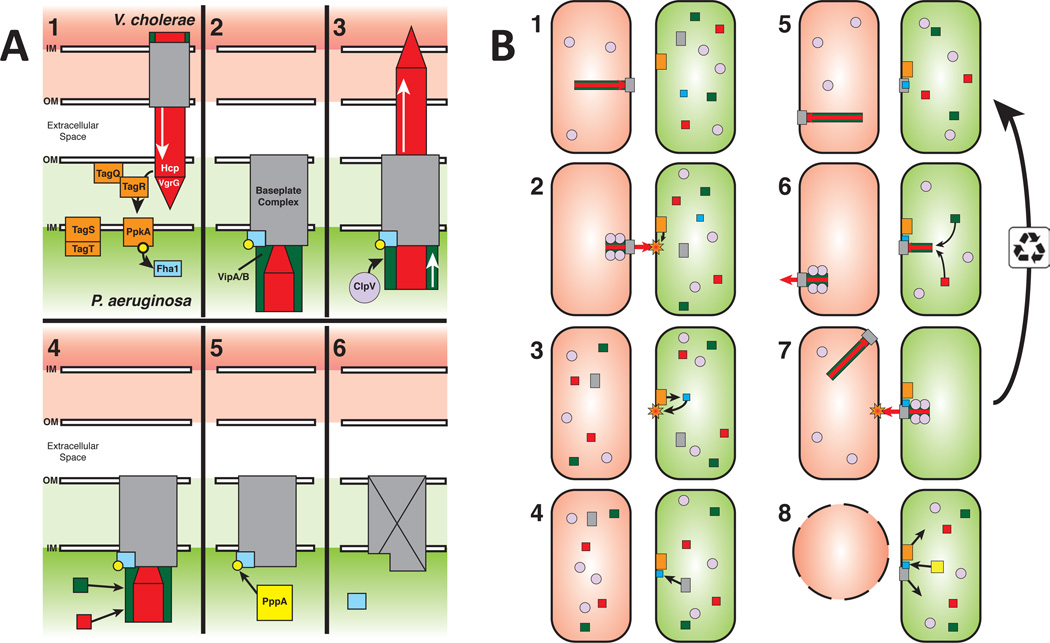
Recently, Casabona et al. (Casabona et al., 2012) have reported that an outer membrane lipoprotein (TagQ) and a set of periplasmic and inner membrane proteins (TagR, TagT, and TagS) control the activation of PpkA and thus phosphorylation of Fha1 and assembly of the T6SS apparatus. The fact that TagQRST acts upstream of the PpkA-Fha1-PppA (Casabona et al., 2012; Hsu et al., 2009), together with our new data suggests that this protein complex may play a direct role in sensing envelope perturbations (e.g., outer membrane breach, peptidoglycan disruption, inner membrane perforation) caused by exogenous T6SS attack. Thus, T6SS dueling may be the manifestation of a signal transduction cascade that starts with recognition by the TagQRST system of a subcellularly localized signal associated with exogenous T6SS attack, dimerization and autophosphorylation of PpkA, trans-phosphorylation of Fha1, and finally phosphorylated Fha1-directed assembly of a T6SS organelle precisely at the point corresponding to the initial exogenous T6SS attack (Figure 6). Repeated firing of the newly assembled T6SS organelle results in a counterattack aimed precisely at the point of initial attack by heterologous T6SS+ cell. If no further attacks are sensed, then dephosphorylation of Fha1 would allow the T6SS organelle to be disassembled and thus primed (by establishing a pool of T6SS organelle precursors) for a quick response (i.e., de novo organelle assembly) to a new attack at a different anatomical site within the cell (Figure 6). It is also worth noting that phosphorylated Fha1 might promote the formation of multiple, clustered T6SS organelles in the vicinity of the initial T6SS exogenous attack as well.
Figure 6. Model for TagQRST-mediated T6SS aiming.
(A) Regulation of the T6SS response in P. aeruginosa. (A1) T6SS assault from V. cholerae is sensed by the TagQRST/PpkA signal cascade (orange) to phosphorylate Fha1 (blue). (A2) Phosphorylated Fha1 interacts with the baseplate complex (grey) locking it in place and allowing assembly of the Hcp/VgrG tube/spike (red) and VipA/B sheath (green). (A3) Sheath contraction fires the retaliatory P. aeruginosa tube/spike complex at V. cholerae. ClpV (light purple) then disassembles the contracted sheath. (A4) The baseplate can then be reused to assemble an additional tube/spike/sheath complex, or (A5) PppA (yellow) can dephosphorylate Fha1, (A6) inactivating or disassembling the baseplate complex. The P. aeruginosa T6SS is now ready to respond to new T6SS assaults. (B) T6SS interactions between P. aeruginosa and V. cholerae. (B1) V. cholerae T6SS will spontaneously fire occasionally hitting a nearby P. aeruginosa cell. (B2-6) P. aeruginosa sense the assault and builds its T6SS organelle at the location of the assault. (B7) P. aeruginosa fires its T6SS organelle back at the V. cholerae cell. The baseplate is then recycled to allow for multiple firing events or (B8) the Fha1 complex is dephosphorylated by PppA and the baseplate complex is disassembled and free to reform at a new location. (B5) Meanwhile, V. cholerae continues to fire its T6SS organelle arbitrarily in a different location and direction. (B8) After the retaliatory attack from P. aeruginosa, the V. cholerae cell dies.
In contrast, the T6SS+ V. cholerae strains studied here are able to kill E. coli nearly 1000-fold more efficiently than P. aeruginosa. We propose that the difference observed in killing activity reflects the dynamics of the V. cholerae T6SS apparatus, which forms, fires and reforms constantly and in different locations within the cell (Basler et al., 2012). In this way, V. cholerae cells protect their surrounding space and attack all encroaching invaders. However, this strategy is not without an energy cost as most V. cholerae cells show high levels of T6SS activity with no benefit gained, while P. aeruginosa displays only minor levels until it encounters a T6SS+ threat. These two different strategies may also reflect the key underlying evolutionary adaption that is characteristic of two distinct uses for the T6SS organelle: V. cholerae uses the apparatus as an offensive weapon while P. aeruginosa uses the organelle as a defensive weapon. The ability of P. aeruginosa to detect the attack of another T6SS+ cell and to respond with its own T6SS counter-attack represents a fascinating new example of highly selective, antagonistic bacterial interactions.
In this study, we also show that although T6SS− E. coli were efficiently killed by T6SS+ V. cholerae or A. baylyi T6SS+ strains, neither T6SS+ and nor T6SS− P. aeruginosa were killed by the V. cholerae T6SS+ or A. baylyi T6SS+ strains (Figure S1A, B). Because P. aeruginosa is sensitive to its own T6SS effectors in the absence of its cognate immunity proteins (Hood et al., 2010), it seems highly likely that the resistance of P. aeruginosa to T6SS+ V. cholerae may be more intrinsic than specific. The "T6SS armor" that P. aeruginosa deploys against the killing activity of the V. cholerae T6SS may be related to its notorious outer membrane impermeability (Nikaido, 1994) or perhaps alterations in its peptidoglycan structure. However, because T6SS+ P. aeruginosa detect both T6SS+ sister cells (Basler and Mekalanos, 2012) as well as the T6SS+ heterologous species V. cholerae and A. baylyi, it is clear P. aeruginosa detects T6SS-associated attack signals even if they have no lethal consequence. Understanding in more detail the parameters involved in T6SS+ prey detection as well as prey sensitivity and resistance to T6SS-mediated attacks will be a fruitful area for future investigations. Given that the P. aeruginosa T6SS is likely also a mammalian virulence factor (Mougous et al., 2006; Schwarz et al., 2010a), it will also be of interest to determine if eukaryotic cell-derived signals can also induce a P. aeruginosa T6SS counterattacks.
A key question that has remained unanswered until the present study was how important the best characterized non-VgrG related antibacterial effectors, the Tse proteins of P. aeruginosa (Hood et al., 2010; Russell et al., 2011; Russell et al., 2012), were to the T6SS-dependent killing of sensitive heterologous target cells. Here we show that this category of T6SS effector is of little importance to the ability of P. aeruginosa to kill T6SS+ V. cholerae (Figure 2F) or T6SS+ A. baylyi (Figure 5C). Thus, other yet to be discovered P. aeruginosa antibacterial effectors may play a role in killing these T6SS+ prey species. Alternatively, Tse-independent T6SS-dependent killing could be attributed to the dynamic activity of the T6SS apparatus alone. Our data support the hypothesis that the T6SS phage tail-like spike/tube complex of P. aeruginosa may kill some target cells after T6SS-mediated envelope insertion without the need for enzymatically active accessory effector delivery. The breech of the outer membrane and/or peptidoglycan and inner membrane of susceptible prey cells with the T6SS VgrG spike/Hcp tube complex might be sufficient to initiate a lethal event in prey cell targets due to, for example, depolarization of the inner membrane, activation of autolytic pathways, or other secondary metabolic responses to this damage (Kohanski et al., 2007; Lewis, 2000; Uratani and Hoshino, 1984).
In this report, we also presented microscopic evidence for delivery of an antibacterial effector to a target prey cell by a native T6SS apparatus. Previous evidence presented for such delivery included the observation that Tsi immunity proteins protect cells from antibacterial Tse effectors secreted by sister cells (Russell et al., 2011) and that Tse effectors are toxic when expressed in heterologous bacterial cells such as E. coli (Russell et al., 2011). In our studies, T6SS+ V. cholerae cells exposed to T6SS+ P. aeruginosa exhibited a rounded morphology that could be specifically attributed to Tse1 activity. While a Tse1-dependent morphological change in T6SS+ V. cholerae could be clearly demonstrated in our studies, as noted earlier, we were unable to attribute a significant contribution of this effector (or indeed any of the known Tse effectors) to the total T6SS-dependent bacteriocidal activity of P. aeruginosa directed against T6SS+ V. cholerae or T6SS+ A. baylyi. We are currently exploring the hypothesis that Tse effectors may be more important to lysing some target species and thus releasing cytoplasmic contents that could serve as growth substrates, than for killing target cells per se. Because there are T6SS+ bacterial species that utilize other bacteria as growth substrates (i.e., Myxococcus xanthus), the concept that T6SS effectors may play a role in nutrient scavenging rather than simply being the mediators of lethality, is a new insight that has emerged from the studies presented here.
Experimental procedures
Bacterial strains
V. cholerae 2740-80 and P. aeruginosa PAO1 strains used in this study were described previously (Basler and Mekalanos, 2012; Basler et al., 2012; Mougous et al., 2006). Gentamicin resistant E. coli MG1655 strain was used for bacterial competition assays. A. baylyi ADP1 was obtained from ATCC (#33305) and spontaneous streptomycin resistant mutant was used as a parental strain. Antibiotic concentrations were: streptomycin - 100 µg/mL, gentamicin - 15 µg/mL, and irgasan - 20 µg/mL. Luria-Bertani (LB) broth (5 g/L NaCl) was used for all growth conditions. Liquid cultures were grown aerobically at 37 °C.
DNA manipulations
Genes pa1844, pa2702, and pa3484 (tse1, tse2, and tse3) in P. aeruginosa were replaced using the pEXG2 suicide plasmid (Rietsch et al., 2005) by in-frame deleted genes encoding following peptides: pa1844 – MDSLDQCPRAS, pa2702 – MSYDGL, pa3484 – MTTFLDPGMRFP. In-frame deletions of pa0073, pa0074, pa0075, and pa0083 (tagT1, ppkA, pppA, and vipA1) in P. aeruginosa were described previously or prepared by the same approach (Basler and Mekalanos, 2012; Mougous et al., 2006; Mougous et al., 2007). Gene pa1845 (tsi1) was cloned in-frame with mcherry2 (separated by DNA linker encoding 3Ala-3Gly) to pBAD24 plasmid as described previously (Basler et al., 2012) to allow for arabinose inducible expression of Tsi1-mCherry2 fusion protein in V. cholerae. A. baylyi T6SS genes aciad2688 to aciad2694 (including homologs of V. cholerae T6SS genes: gp25-like, hcp, vipA, vipB, clpV) were replaced with KanR cassette from pRSFDuet-1 plasmid (Novagen) as described previously (Metzgar et al., 2004). All cloning products were sequence-verified. Chromosomal mutations were verified by PCR using primers outside of the replaced region.
Fluorescence microscopy
Procedures similar to procedures described previously (Basler and Mekalanos, 2012; Basler et al., 2012) were used to detect fluorescence signal in V. cholerae and P. aeruginosa. Overnight cultures of V. cholerae or P. aeruginosa were washed by LB and diluted 50x – 200x into fresh LB and cultivated for 2.5 – 3.5 hours to OD about 0.5 – 1.0. For V. cholerae 2740-80 + pBAD24-Tsi1-mCherry2 expression of Tsi1-mCherry2 in V. cholerae was induced by 0.01% arabinose. Cells from 100 µL of the culture were re-suspended in 5 – 10 µL of fresh LB (to OD ~10), mixed as indicated, spotted on a thin pad of 1% agarose in 0.5x PBS (pH 7.4, Invitrogen), and covered with a glass cover slip. Cells were imaged at 25 – 30 °C after 20 to 60 minutes (for 3 minutes for detection of morphological changes or dueling), or after 40 to 90 minutes (for detection of round cells). Cells close to the edge of the agarose pad were imaged. Multiple 30x30 µm fields of cells (30–60 for detection of round cells and 10–25 for detection of dueling, indicated as n in figures) were analyzed for at least four biological replicates. Microscope configuration was described previously (Basler and Mekalanos, 2012): Nikon Ti-E inverted motorized microscope with Perfect Focus System and Plan Apo 100x Oil Ph3 DM (NA 1.4) objective lens. SPECTRA X light engine (Lumencore), ET-GFP (Chroma #49002) and ET-mCherry (Chroma #49008) filter sets were used to excite and filter fluorescence. Photometrics CoolSNAP HQ2 camera (pixel-size was 60 nm) and NIS Elements 4.0 was used to record images.
Image analysis
Fiji was used for all image analysis and manipulations (Schindelin et al., 2012). The individual fluorescence images from a time series were corrected for photobleaching by normalizing the intensity of a region containing mostly cells to the same mean intensity. Image contrast was set to clearly show localization of signal within cells and is set to the same level when direct comparison between strains are presented. Small movement of whole field in time was corrected by registering individual frames using StackReg plugin for Fiji (“Rigid Body” transformation). Fiji macro “Temporal-Color Code” was used to visualize localization of fluorescent foci in time. Merged image of the phase contrast and fluorescence images are presented.
Bacterial competition assay
Cells were prepared as for fluorescence microscopy analysis. Cells were mixed at OD ~10 in 1:1 or 10:1 ratio as specified and 5 µL of the mixture was spotted on a dry LB agar plate. After 2 hours, bacterial spots were cut out and the cells were re-suspended in 1 mL LB. The cellular suspension was serially diluted in LB and 5 µL of the suspensions was spotted on selective plates (irgasan for P. aeruginosa, streptomycin for V. cholerae and A. baylyi, and gentamicin for E. coli). Colonies were counted after ~16 h incubation at 30 °C. At least three biological replicates were analyzed.
Statistics
Student’s t-test was used to determine significance between indicated groups of numbers.
Supplementary Material
highlights.
P. aeruginosa targets T6SS positive bacteria for T6SS-mediated counterattack.
Phosphorylation signaling cascade regulates P. aeruginosa response to attack.
P. aeruginosa T6SS delivers Tse-1 effector to target cells.
Tit-for-tat evolutionary strategy controls interactions between bacterial species.
Acknowledgements
We thank Casey Gifford for constructing pEXG2-ΔtagT plasmid. We also thank Russell Vance and Stephen Lory for helpful discussions and Tom Bernhardt and David Rudner for access to microscopic resources and tips for optimal use. This work was supported by NIAID grants AI-018045 and AI-26289 to J.J.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki SK, Malinverni JC, Jacoby K, Thomas B, Pamma R, Trinh BN, Remers S, Webb J, Braaten BA, Silhavy TJ, et al. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol Microbiol. 2008;70:323–340. doi: 10.1111/j.1365-2958.2008.06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309:1245–1248. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- Aoki SK, Webb JS, Braaten BA, Low DA. Contact-dependent growth inhibition causes reversible metabolic downregulation in Escherichia coli. J Bacteriol. 2009;191:1777–1786. doi: 10.1128/JB.01437-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- Basler M, Mekalanos JJ. Type 6 secretion dynamics within and between bacterial cells. Science. 2012;337:815. doi: 10.1126/science.1222901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature. 2012;483:182–186. doi: 10.1038/nature10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonemann G, Pietrosiuk A, Diemand A, Zentgraf H, Mogk A. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. Embo J. 2009;28:315–325. doi: 10.1038/emboj.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer F, Fichant G, Berthod J, Vandenbrouck Y, Attree I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics. 2009;10:104. doi: 10.1186/1471-2164-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casabona MG, Silverman JM, Sall KM, Boyer F, Coute Y, Poirel J, Grunwald D, Mougous JD, Elsen S, Attree I. An ABC transporter and an outer membrane lipoprotein participate in posttranslational activation of type VI secretion in Pseudomonas aeruginosa. Environ Microbiol. 2012 doi: 10.1111/j.1462-2920.2012.02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Bui NK, Russell AB, Lexa KW, Gardiner TE, Leroux M, Vollmer W, Mougous JD. Structure of a Peptidoglycan Amidase Effector Targeted to Gram-Negative Bacteria by the Type VI Secretion System. Cell Rep. 2012;1:656–664. doi: 10.1016/j.celrep.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Berardinis V, Vallenet D, Castelli V, Besnard M, Pinet A, Cruaud C, Samair S, Lechaplais C, Gyapay G, Richez C, et al. A complete collection of single-gene deletion mutants of Acinetobacter baylyi ADP1. Mol Syst Biol. 2008;4:174. doi: 10.1038/msb.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang W, Feng H, Zhang Y, Wang DC. Structural Insights into the Pseudomonas aeruginosa Type VI Virulence Effector Tse1 Bacteriolysis and Self-protection Mechanisms. J Biol Chem. 2012;287:26911–26920. doi: 10.1074/jbc.M112.368043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Gibbs KA, Urbanowski ML, Greenberg EP. Genetic determinants of self identity and social recognition in bacteria. Science. 2008;321:256–259. doi: 10.1126/science.1160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs KA, Wenren LM, Greenberg EP. Identity gene expression in Proteus mirabilis. J Bacteriol. 2011;193:3286–3292. doi: 10.1128/JB.01167-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 2010;7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F, Schwarz S, Mougous JD. TagR promotes PpkA-catalysed type VI secretion activation in Pseudomonas aeruginosa. Mol Microbiol. 2009;72:1111–1125. doi: 10.1111/j.1365-2958.2009.06701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Palmer RJ, Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8:471–480. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- Leiman PG, Basler M, Ramagopal UA, Bonanno JB, Sauder JM, Pukatzki S, Burley SK, Almo SC, Mekalanos JJ. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci U S A. 2009;106:4154–4159. doi: 10.1073/pnas.0813360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. Programmed death in bacteria. Microbiol Mol Biol Rev. 2000;64:503–514. doi: 10.1128/mmbr.64.3.503-514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Le Trong I, Carl MA, Larson ET, Chou S, De Leon JA, Dove SL, Stenkamp RE, Mougous JD. Structural basis for type VI secretion effector recognition by a cognate immunity protein. PLoS Pathog. 2012;8:e1002613. doi: 10.1371/journal.ppat.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A. 2010;107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzgar D, Bacher JM, Pezo V, Reader J, Doring V, Schimmel P, Marliere P, de Crecy-Lagard V. Acinetobacter sp ADP1: an ideal model organism for genetic analysis and genome engineering. Nucleic Acids Res. 2004;32:5780–5790. doi: 10.1093/nar/gkh881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordonez CL, Lory S, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougous JD, Gifford CA, Ramsdell TL, Mekalanos JJ. Threonine phosphorylation post-translationally regulates protein secretion in Pseudomonas aeruginosa. Nat Cell Biol. 2007;9:797–803. doi: 10.1038/ncb1605. [DOI] [PubMed] [Google Scholar]
- Murdoch SL, Trunk K, English G, Fritsch MJ, Pourkarimi E, Coulthurst SJ. The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J Bacteriol. 2011;193:6057–6069. doi: 10.1128/JB.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev. 2012;25:193–213. doi: 10.1128/CMR.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci U S A. 2007;104:15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A. 2006;103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietsch A, Vallet-Gely I, Dove SL, Mekalanos JJ. ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2005;102:8006–8011. doi: 10.1073/pnas.0503005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, Mougous JD. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Singh P, Brittnacher M, Bui NK, Hood RD, Carl MA, Agnello DM, Schwarz S, Goodlett DR, Vollmer W, et al. A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell Host Microbe. 2012;11:538–549. doi: 10.1016/j.chom.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Hood RD, Mougous JD. What is type VI secretion doing in all those bugs? Trends Microbiol. 2010a;18:531–537. doi: 10.1016/j.tim.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, West TE, Boyer F, Chiang WC, Carl MA, Hood RD, Rohmer L, Tolker-Nielsen T, Skerrett SJ, Mougous JD. Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions. PLoS Pathog. 2010b;6:e1001068. doi: 10.1371/journal.ppat.1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Austin LS, Hsu F, Hicks KG, Hood RD, Mougous JD. Separate inputs modulate phosphorylation-dependent and -independent type VI secretion activation. Mol Microbiol. 2011;82:1277–1290. doi: 10.1111/j.1365-2958.2011.07889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uratani Y, Hoshino T. Pyocin R1 inhibits active transport in Pseudomonas aeruginosa and depolarizes membrane potential. J Bacteriol. 1984;157:632–636. doi: 10.1128/jb.157.2.632-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermute EH, Silver PA. Dynamics in the mixed microbial concourse. Genes Dev. 2010;24:2603–2614. doi: 10.1101/gad.1985210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Ho B, Mekalanos JJ. Genetic Analysis of Anti-Amoebae and Anti-Bacterial Activities of the Type VI Secretion System in Vibrio cholerae. PLoS One. 2011;6:e23876. doi: 10.1371/journal.pone.0023876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.